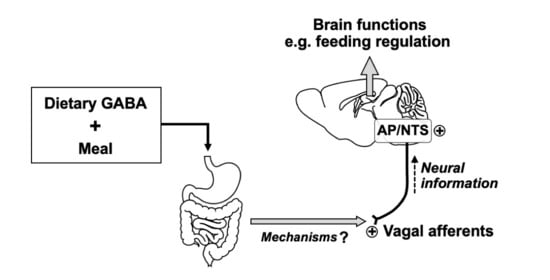
Dietary Gamma-Aminobutyric Acid (GABA) Induces Satiation by Enhancing the Postprandial Activation of Vagal Afferent Nerves
Abstract
:1. Introduction
2. Materials and methods
2.1. Materials
2.2. Animals
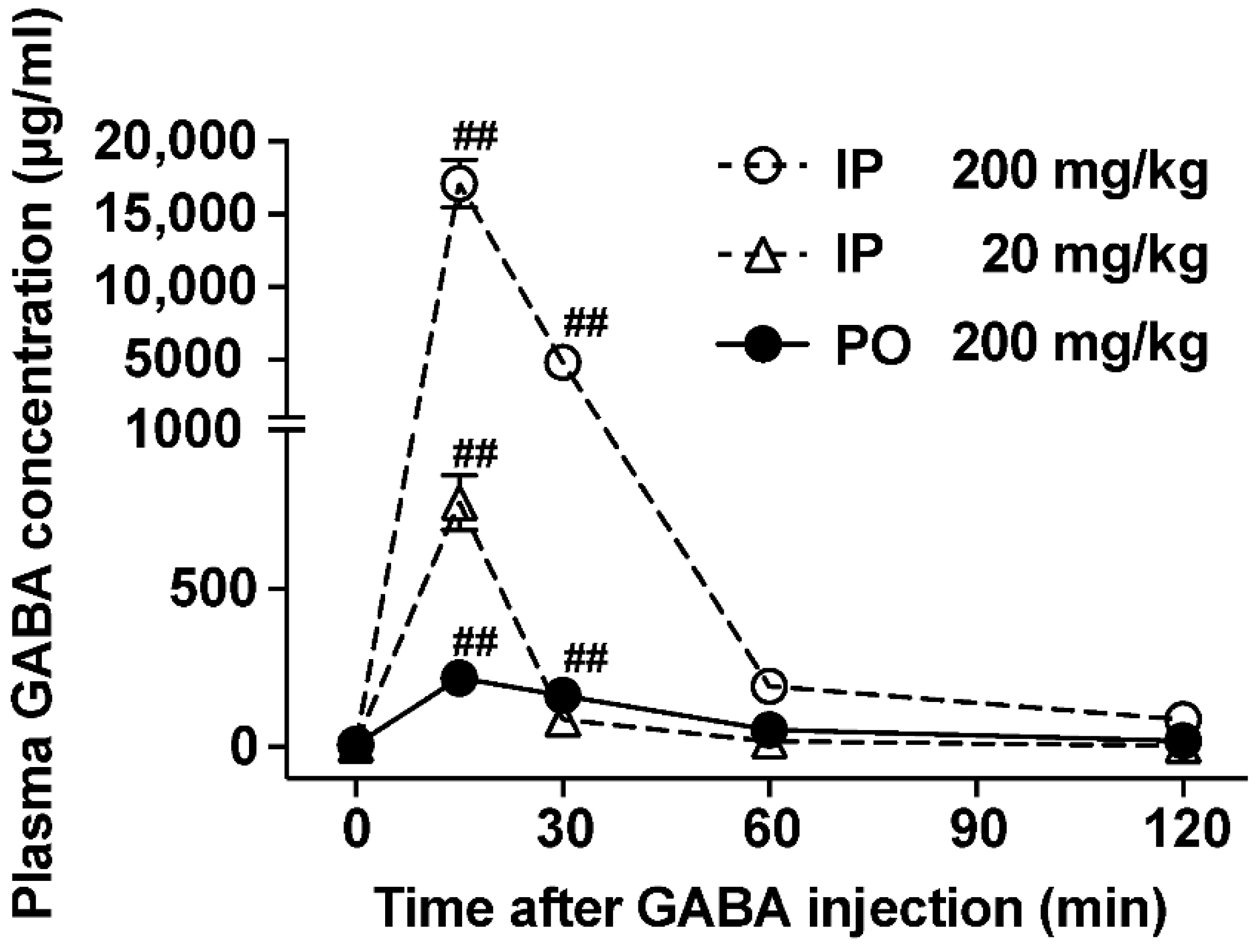
2.3. Measurement of Plasma GABA Concentration in the Postcaval Vein
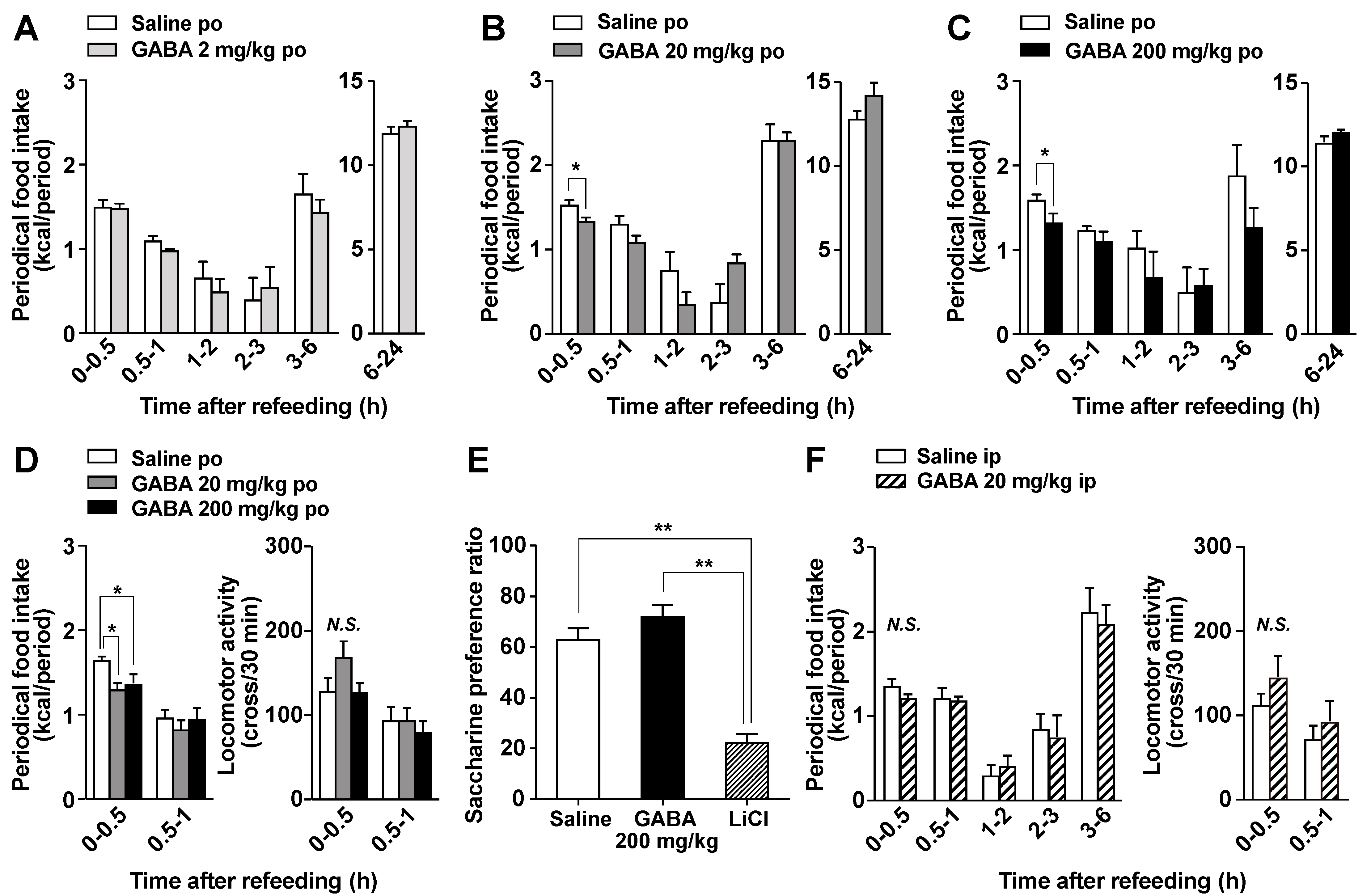
2.4. Measurements of Food Intake and Locomotor Activities
2.5. Conditioned Taste Aversion Test
2.6. Systemic Capsaicin Treatment
2.7. Subdiaphragmatic Vagotomy
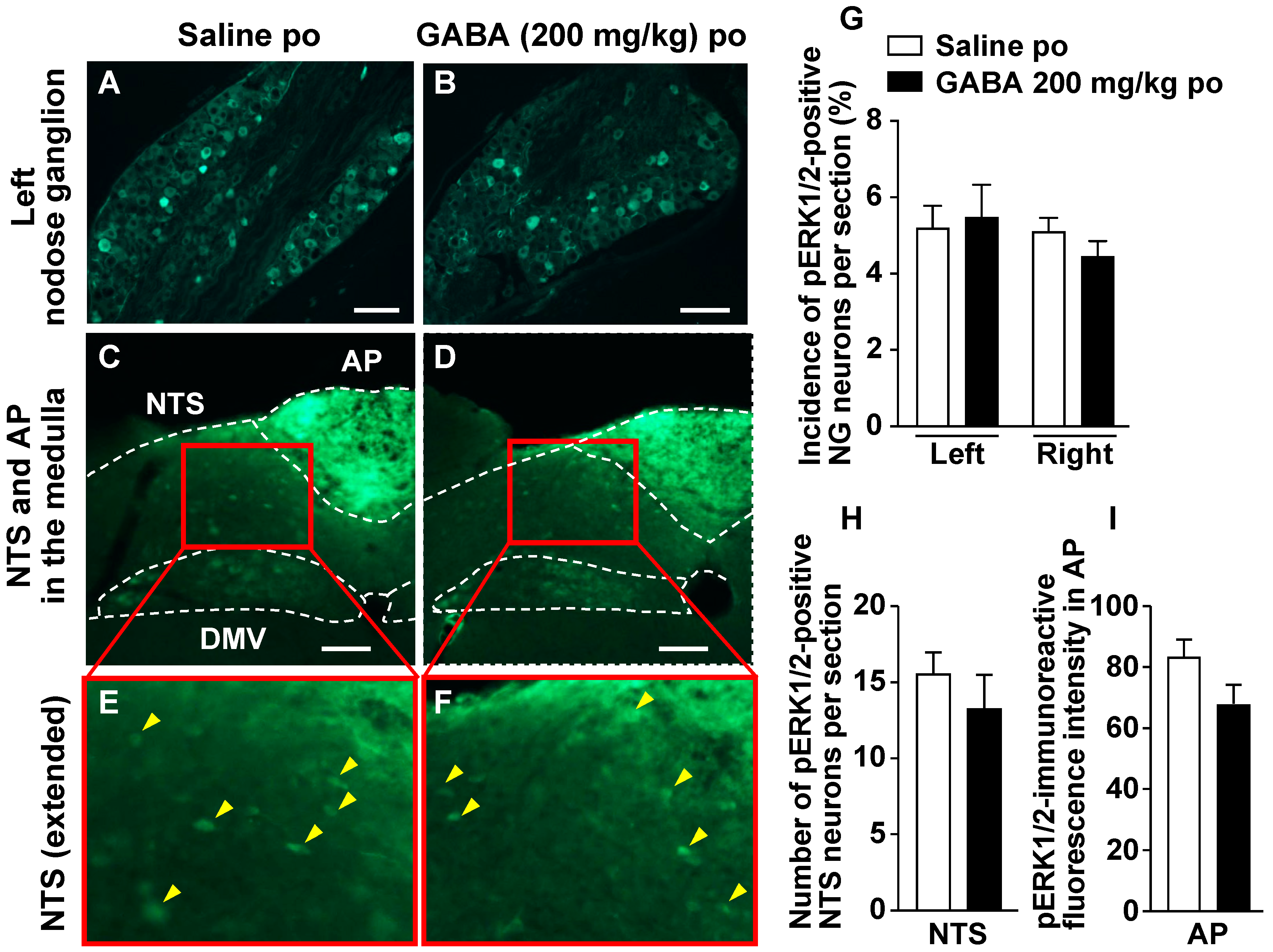
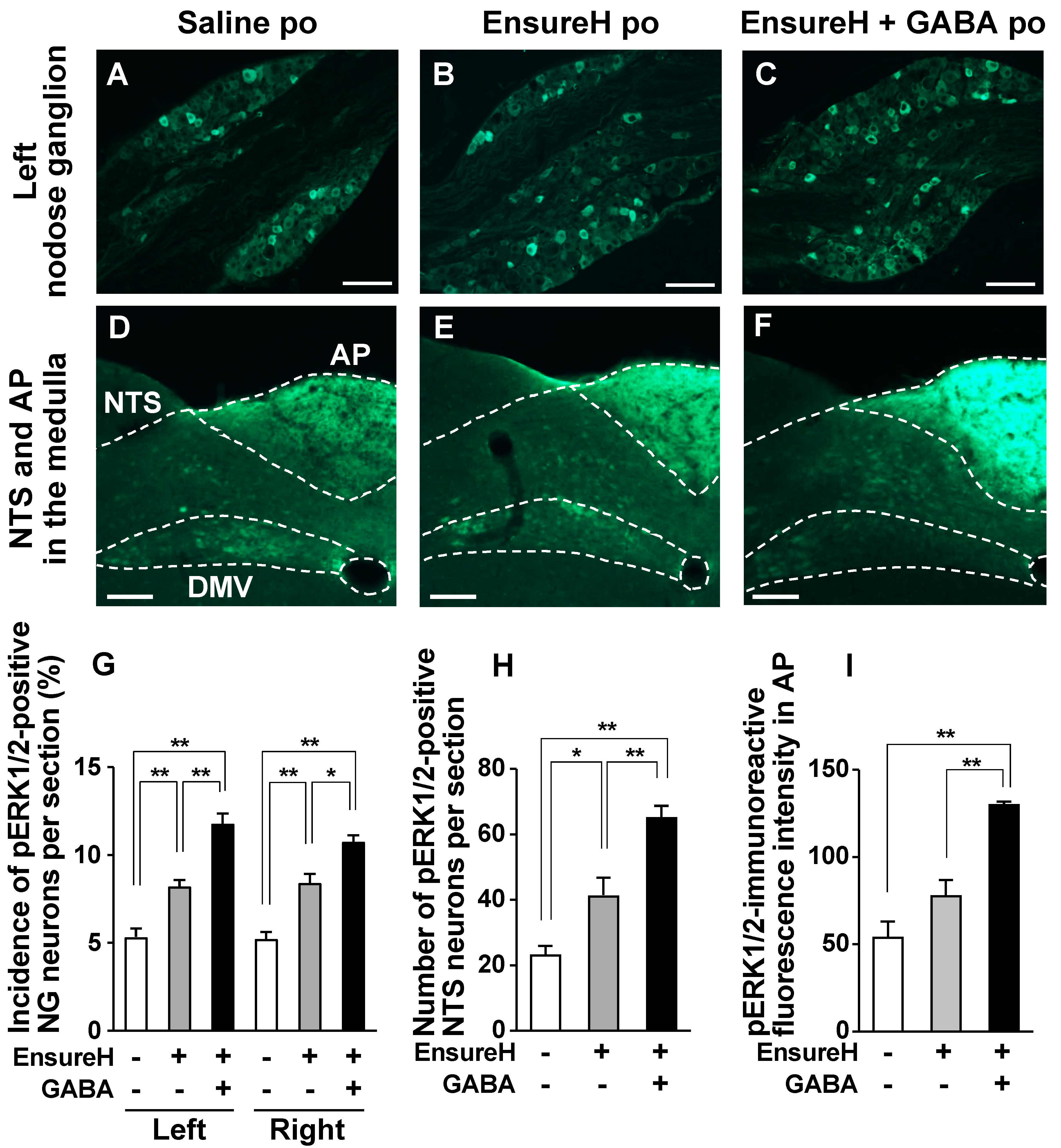
2.8. Immunohistochemical Detection of pERK1/2 in the Nodose Ganglion, Medial Nucleus Tractus Solitarius, and Area Postrema
2.9. Statistical Analysis
3. Results
3.1. The Ability to Increase Plasma GABA Concentration after po GABA Administration Is Markedly Lower Than That after Ip Administration
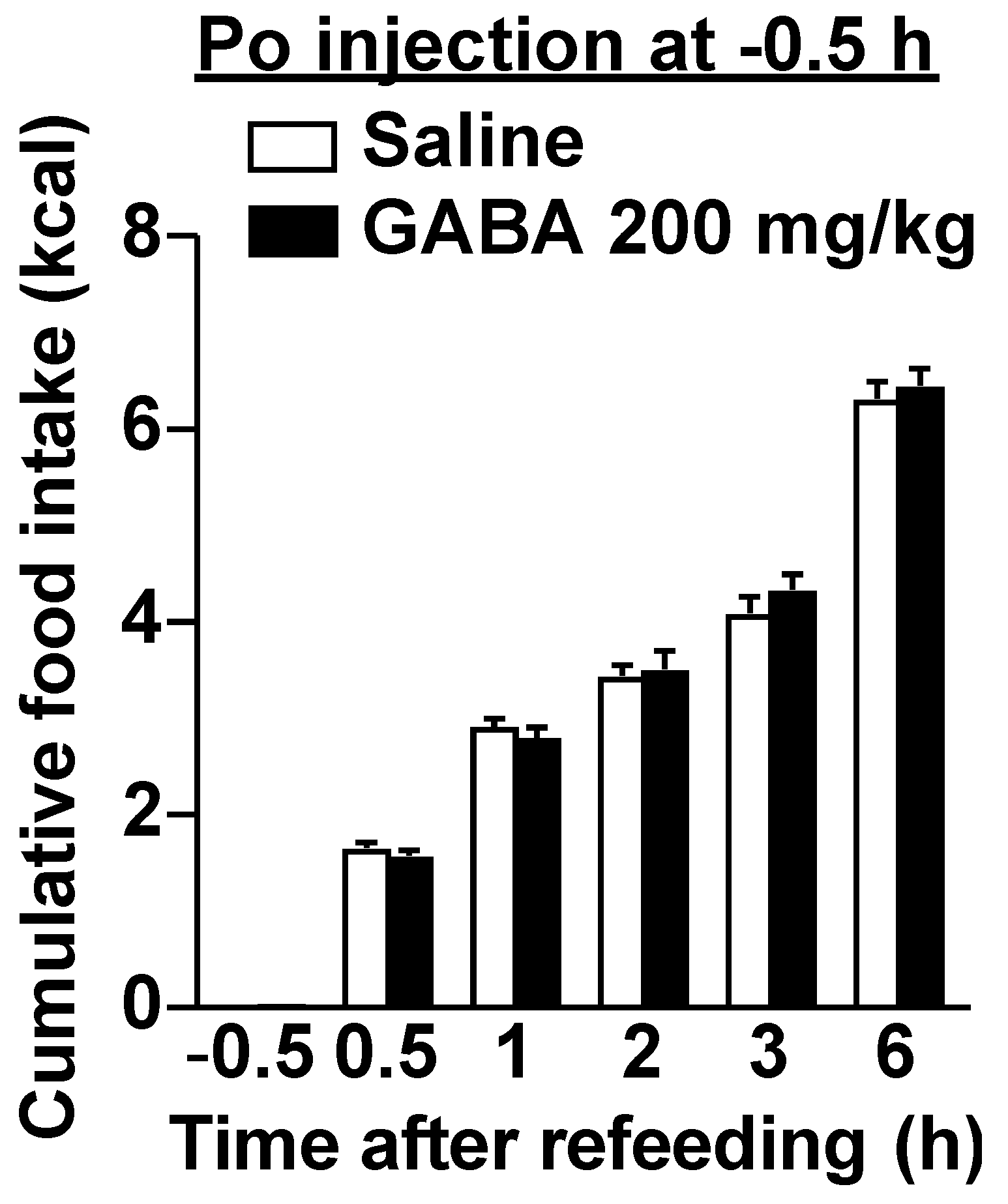
3.2. Po GABA Administration Immediately before Refeeding Suppresses Food Intake without Aversive Behavior
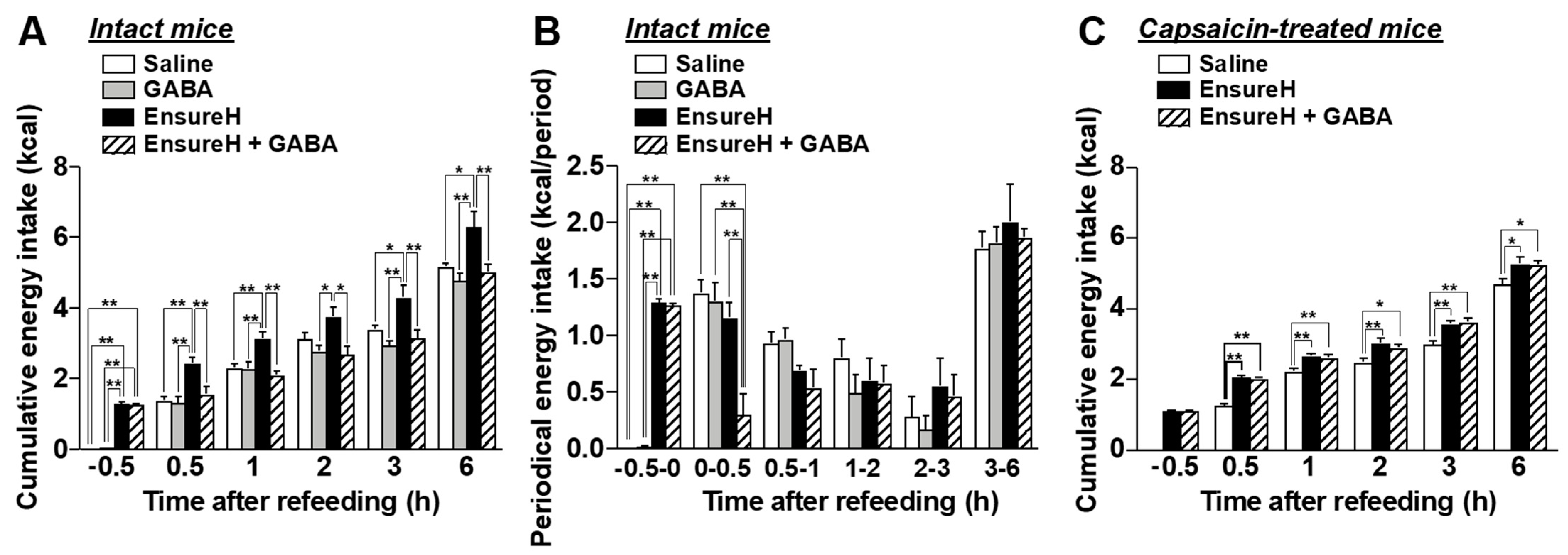
3.3. Feeding Suppression by Dietary GABA Supplementation Is Attenuated by the Chemical and Surgical Denervation of Vagal Afferent Nerves
3.4. Po Administration of GABA Only without Feeding Does Not Alter Neural Activities in Vagal Afferent Neurons
3.5. GABA-Supplemented Liquid Diet Enhances the Postprandial Activation of Vagal Afferent Neurons
3.6. The Coadministration of GABA and Liquid Diet Prevents Overeating via Capsaicin-Sensitive Sensory Nerves Including Vagal Afferents
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, E.; Frankel, S. Gamma-Aminobutyric acid in brain: Its formation from glutamic acid. J. Biol. Chem. 1950, 187, 55–63. [Google Scholar] [CrossRef]
- Krnjevic, K.; Schwartz, S. The action of gamma-aminobutyric acid on cortical neurones. Exp. Brain Res. 1967, 3, 320–336. [Google Scholar] [CrossRef] [PubMed]
- Diana, M.; Quílez, J.; Rafecas, M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Func. Foods 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Rashmi, D.; Zanan, R.; John, S.; Khandagale, K.; Nadaf, A. Chapter 13-γ-aminobutyric acid (GABA): Biosynthesis, role, commercial production, and applications. Stud. Nat. Products Chem. 2018, 57, 413–452. [Google Scholar]
- Briguglio, M.; Dell’Osso, B.; Panzica, G.; Malgaroli, A.; Banfi, G.; Zanaboni Dina, C.; Galentino, R.; Porta, M. Dietary neurotransmitters: A narrative review on current knowledge. Nutrients 2018, 10, 591. [Google Scholar] [CrossRef] [Green Version]
- Shimada, M.; Hasegawa, T.; Nishimura, C.; Kan, H.; Kanno, T.; Nakamura, T.; Matsubayashi, T. Anti-hypertensive effect of gamma-aminobutyric acid (GABA)-rich Chlorella on high-normal blood pressure and borderline hypertension in placebo-controlled double blind study. Clin. Exp. Hypertens. 2009, 31, 342–354. [Google Scholar] [CrossRef]
- Abdou, A.M.; Higashiguchi, S.; Horie, K.; Kim, M.; Hatta, H.; Yokogoshi, H. Relaxation and immunity enhancement effects of gamma-aminobutyric acid (GABA) administration in humans. Biofactors 2006, 26, 201–208. [Google Scholar] [CrossRef]
- Yamatsu, A.; Yamashita, Y.; Pandharipande, T.; Maru, I.; Kim, M. Effect of oral gamma-aminobutyric acid (GABA) administration on sleep and its absorption in humans. Food Sci. Biotechnol. 2016, 25, 547–551. [Google Scholar] [CrossRef]
- Yamatsu, A.; Nakamura, U.; Saddam, H.M.; Horie, N.; Kaneko, T.; Kim, M. Improvement of memory and spatial cognitive function by continuous ingestion of 100mg/day of γ—aminobutyric acid (GABA)—a randomized, double—blind, placebo—controlled parallel—group clinical trial—. Jpn. Phamacol. Ther. 2020, 48, 475–486. [Google Scholar]
- Yamatsu, A.; Nakamura, U.; Saddam, H.M.; Horie, N.; Kaneko, T.; Kim, M. Intake of 200 mg/day of γ—aminobutyric acid (GABA) improves a wide range of cognitive functions—A randomized, double—blind, placebo—controlled parallel—group clinical trial—. Jpn. Phamacol. Ther. 2020, 48, 461–474. [Google Scholar]
- Roberts, E.; Lowe, I.P.; Guth, L.; Jelinek, B. Distribution of γ-aminobutyric acid and other amino acids in nervous tissue of various species. J. Exp. Zool. 1958, 138, 313–328. [Google Scholar] [CrossRef]
- Van Gelder, N.M.; Elliott, K.A.C. Disposition of γ-aminobutyric acid administered to mammals. J. Neurochem. 1958, 3, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, K.; Sze, P.Y. Blood–brain barrier to h3-γ-aminobutyric acid in normal and amino oxyacetic acid-treated animals. Neuropharmacology 1971, 10, 103–108. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Yada, T. Vagal afferents sense meal-associated gastrointestinal and pancreatic hormones: Mechanism and physiological role. Neuropeptides 2012, 46, 291–297. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Neuhuber, W.L. Vagal mechanisms as neuromodulatory targets for the treatment of metabolic disease. Ann. NY Acad. Sci. 2019, 1454, 42–55. [Google Scholar] [CrossRef] [Green Version]
- Williams, E.K.; Chang, R.B.; Strochlic, D.E.; Umans, B.D.; Lowell, B.B.; Liberles, S.D. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 2016, 166, 209–221. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Tellez, L.A.; Perkins, M.H.; Perez, I.O.; Qu, T.; Ferreira, J.; Ferreira, T.L.; Quinn, D.; Liu, Z.W.; Gao, X.B.; et al. A neural circuit for gut-induced reward. Cell 2018, 175, 665–678.e623. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Mesgarzadeh, S.; Ramesh, K.S.; Huey, E.L.; Liu, Y.; Gray, L.A.; Aitken, T.J.; Chen, Y.; Beutler, L.R.; Ahn, J.S.; et al. Genetic identification of vagal sensory neurons that control feeding. Cell 2019, 179, 1129–1143. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Sendo, M.; Dezaki, K.; Hira, T.; Sato, T.; Nakata, M.; Goswami, C.; Aoki, R.; Arai, T.; Kumari, P.; et al. GLP-1 release and vagal afferent activation mediate the beneficial metabolic and chronotherapeutic effects of D-allulose. Nat. Commun 2018, 9, 113. [Google Scholar] [CrossRef]
- Rosen, L.B.; Ginty, D.D.; Weber, M.J.; Greenberg, M.E. Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron 1994, 12, 1207–1221. [Google Scholar] [CrossRef]
- Chandler, L.J.; Sutton, G.; Dorairaj, N.R.; Norwood, D. N-methyl D-aspartate receptor-mediated bidirectional control of extracellular signal-regulated kinase activity in cortical neuronal cultures. J. Biol. Chem. 2001, 276, 2627–2636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindgren, N.; Goiny, M.; Herrera-Marschitz, M.; Haycock, J.W.; Hokfelt, T.; Fisone, G. Activation of extracellular signal-regulated kinases 1 and 2 by depolarization stimulates tyrosine hydroxylase phosphorylation and dopamine synthesis in rat brain. Eur. J. Neurosci. 2002, 15, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Baldassa, S.; Zippel, R.; Sturani, E. Depolarization-induced signaling to Ras, Rap1 and MAPKs in cortical neurons. Brain Res. Mol. Brain. Res. 2003, 119, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Iwata, K.; Fukuoka, T.; Kondo, E.; Tokunaga, A.; Yamanaka, H.; Tachibana, T.; Liu, Y.; Noguchi, K. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J. Neurosci. 2002, 22, 7737–7745. [Google Scholar] [CrossRef] [PubMed]
- Ayush, E.A.; Iwasaki, Y.; Iwamoto, S.; Nakabayashi, H.; Kakei, M.; Yada, T. Glucagon directly interacts with vagal afferent nodose ganglion neurons to induce Ca2+ signaling via glucagon receptors. Biochem. Biophys. Res. Commun. 2015, 456, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Goswami, C.; Iwasaki, Y.; Yada, T. Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J. Nutr. Biochem. 2018, 57, 130–135. [Google Scholar] [CrossRef]
- Meleine, M.; Mounien, L.; Atmani, K.; Ouelaa, W.; Bole-Feysot, C.; Guerin, C.; Depoortere, I.; Gourcerol, G. Ghrelin inhibits autonomic response to gastric distension in rats by acting on vagal pathway. Sci. Rep. 2020, 10, 9986. [Google Scholar] [CrossRef]
- Sakurai, J.; Obata, K.; Ozaki, N.; Tokunaga, A.; Kobayashi, K.; Yamanaka, H.; Dai, Y.; Kondo, T.; Miyoshi, K.; Sugiura, Y.; et al. Activation of extracellular signal-regulated protein kinase in sensory neurons after noxious gastric distention and its involvement in acute visceral pain in rats. Gastroenterology 2008, 134, 1094–1103. [Google Scholar] [CrossRef]
- Kondo, T.; Obata, K.; Miyoshi, K.; Sakurai, J.; Tanaka, J.; Miwa, H.; Noguchi, K. Transient receptor potential A1 mediates gastric distention-induced visceral pain in rats. Gut 2009, 58, 1342–1352. [Google Scholar] [CrossRef]
- Teratani, T.; Mikami, Y.; Nakamoto, N.; Suzuki, T.; Harada, Y.; Okabayashi, K.; Hagihara, Y.; Taniki, N.; Kohno, K.; Shibata, S.; et al. The liver-brain-gut neural arc maintains the Treg cell niche in the gut. Nature 2020, 585, 591–596. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Maejima, Y.; Suyama, S.; Yoshida, M.; Arai, T.; Katsurada, K.; Kumari, P.; Nakabayashi, H.; Kakei, M.; Yada, T. Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin-resistant mice: A route for ameliorating hyperphagia and obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R360–R369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, A.; Okuma, Y.; Hosoi, T.; Nomura, Y. Effect of subdiaphragmatic vagotomy on bacterial DNA-induced IL-1beta expression in the mouse hypothalamus. Brain Res. 2004, 1028, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of oral gamma-aminobutyric acid (GABA) administration on stress and sleep in humans: A systematic review. Front. Neurosci. 2020, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Fedele, S.; Arnold, M.; Krieger, J.P.; Wolfstadter, B.; Meyer, U.; Langhans, W.; Mansouri, A. Oleoylethanolamide-induced anorexia in rats is associated with locomotor impairment. Physiol. Rep. 2018, 6, e13517. [Google Scholar] [CrossRef]
- Buck, S.H.; Burks, T.F. The neuropharmacology of capsaicin: Review of some recent observations. Pharmacol. Rev. 1986, 38, 179–226. [Google Scholar]
- Krieger, J.P. Intestinal glucagon-like peptide-1 effects on food intake: Physiological relevance and emerging mechanisms. Peptides 2020, 131, 170342. [Google Scholar] [CrossRef]
- Wu, G. Intestinal mucosal amino acid catabolism. J. Nutr. 1998, 128, 1249–1252. [Google Scholar] [CrossRef] [Green Version]
- Tome, D. The roles of dietary glutamate in the intestine. Ann. Nutr. Metab. 2018, 73 (Suppl. 5), 15–20. [Google Scholar] [CrossRef]
- Takahashi, H.; Sumi, M.; Koshino, F. Effect of gamma-aminobutyric acid (GABA) on normotensive or hypertensive rats and men. Jpn. J. Physiol. 1961, 11, 89–95. [Google Scholar] [CrossRef]
- Cavagnini, F.; Benetti, G.; Invitti, C.; Ramella, G.; Pinto, M.; Lazza, M.; Dubini, A.; Marelli, A.; Muller, E.E. Effect of gamma-aminobutyric acid on growth hormone and prolactin secretion in man: Influence of pimozide and domperidone. J. Clin. Endocrinol. Metab. 1980, 51, 789–792. [Google Scholar] [CrossRef]
- Sakashita, M.; Horie, K.; Iwaki, K. Safety evaluation of excessive intake of γ-aminobutyric acid in healthy subjects -a randomized, placebo-controlled, double-blind study-. Jpn. Phamacol. Ther. 2016, 44, 1639–1644. [Google Scholar]
- Puschner, B. CHAPTER 72-Mushroom toxins. Vet. Toxicol. Basic Clin. Princ. 2007, 915–925. [Google Scholar]
- Chebib, M.; Johnston, G.A. The ‘ABC’ of GABA receptors: A brief review. Clin. Exp. Pharmacol. Physiol. 1999, 26, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Kupari, J.; Haring, M.; Agirre, E.; Castelo-Branco, G.; Ernfors, P. An atlas of vagal sensory neurons and their molecular specialization. Cell Rep. 2019, 27, 2508–2523.e2504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smid, S.D.; Young, R.L.; Cooper, N.J.; Blackshaw, L.A. GABA(B)R expressed on vagal afferent neurones inhibit gastric mechanosensitivity in ferret proximal stomach. Am. J. Physiol. Gastrointest. Liver. Physiol. 2001, 281, G1494–G1501. [Google Scholar] [CrossRef]
- Ashworth-Preece, M.; Krstew, E.; Jarrott, B.; Lawrence, A.J. Functional GABAA receptors on rat vagal afferent neurones. Br. J. Pharmacol. 1997, 120, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Wallis, D.I.; Stansfeld, C.E.; Nash, H.L. Depolarizing responses recorded from nodose ganglion cells of the rabbit evoked by 5-hydroxytryptamine and other substances. Neuropharmacology 1982, 21, 31–40. [Google Scholar] [CrossRef]
- Krieger, J.P.; Arnold, M.; Pettersen, K.G.; Lossel, P.; Langhans, W.; Lee, S.J. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes 2016, 65, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Gameiro, A.; Reimann, F.; Habib, A.M.; O’Malley, D.; Williams, L.; Simpson, A.K.; Gribble, F.M. The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line. J. Physiol. 2005, 569, 761–772. [Google Scholar] [CrossRef]
- Tian, L.; Jin, T. The incretin hormone GLP-1 and mechanisms underlying its secretion. J. Diabetes 2016, 8, 753–765. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhan, G.; Cai, Z.; Jiao, B.; Zhao, Y.; Li, S.; Luo, A. Vagus nerve stimulation in brain diseases: Therapeutic applications and biological mechanisms. Neurosci. Biobehav. Rev. 2021, 127, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Rong, C.; Wang, F.; Liu, X.; Sun, Y.; Zhang, H.T. GABAergic system in stress: Implications of GABAergic neuron subpopulations and the gut-vagus-brain pathway. Neural. Plast. 2020, 2020, 8858415. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kwon, S.H.; Kam, T.I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal propagation of pathologic alpha-synuclein from the gut to the brain models Parkinson’s disease. Neuron 2019, 103, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [Green Version]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron 2019, 101, 246–259. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, U.; Nohmi, T.; Sagane, R.; Hai, J.; Ohbayashi, K.; Miyazaki, M.; Yamatsu, A.; Kim, M.; Iwasaki, Y. Dietary Gamma-Aminobutyric Acid (GABA) Induces Satiation by Enhancing the Postprandial Activation of Vagal Afferent Nerves. Nutrients 2022, 14, 2492. https://doi.org/10.3390/nu14122492
Nakamura U, Nohmi T, Sagane R, Hai J, Ohbayashi K, Miyazaki M, Yamatsu A, Kim M, Iwasaki Y. Dietary Gamma-Aminobutyric Acid (GABA) Induces Satiation by Enhancing the Postprandial Activation of Vagal Afferent Nerves. Nutrients. 2022; 14(12):2492. https://doi.org/10.3390/nu14122492
Chicago/Turabian StyleNakamura, Utano, Taichi Nohmi, Riho Sagane, Jun Hai, Kento Ohbayashi, Maiko Miyazaki, Atsushi Yamatsu, Mujo Kim, and Yusaku Iwasaki. 2022. "Dietary Gamma-Aminobutyric Acid (GABA) Induces Satiation by Enhancing the Postprandial Activation of Vagal Afferent Nerves" Nutrients 14, no. 12: 2492. https://doi.org/10.3390/nu14122492
APA StyleNakamura, U., Nohmi, T., Sagane, R., Hai, J., Ohbayashi, K., Miyazaki, M., Yamatsu, A., Kim, M., & Iwasaki, Y. (2022). Dietary Gamma-Aminobutyric Acid (GABA) Induces Satiation by Enhancing the Postprandial Activation of Vagal Afferent Nerves. Nutrients, 14(12), 2492. https://doi.org/10.3390/nu14122492