The Effect of Weaning with Adult Food Typical of the Mediterranean Diet on Taste Development and Eating Habits of Children: A Randomized Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Interventions
2.2. Inclusion and Exclusion Criteria
2.3. Ethical Approval and Informed Consent
2.4. Study Objectives and Endpoints
2.5. Sample Size
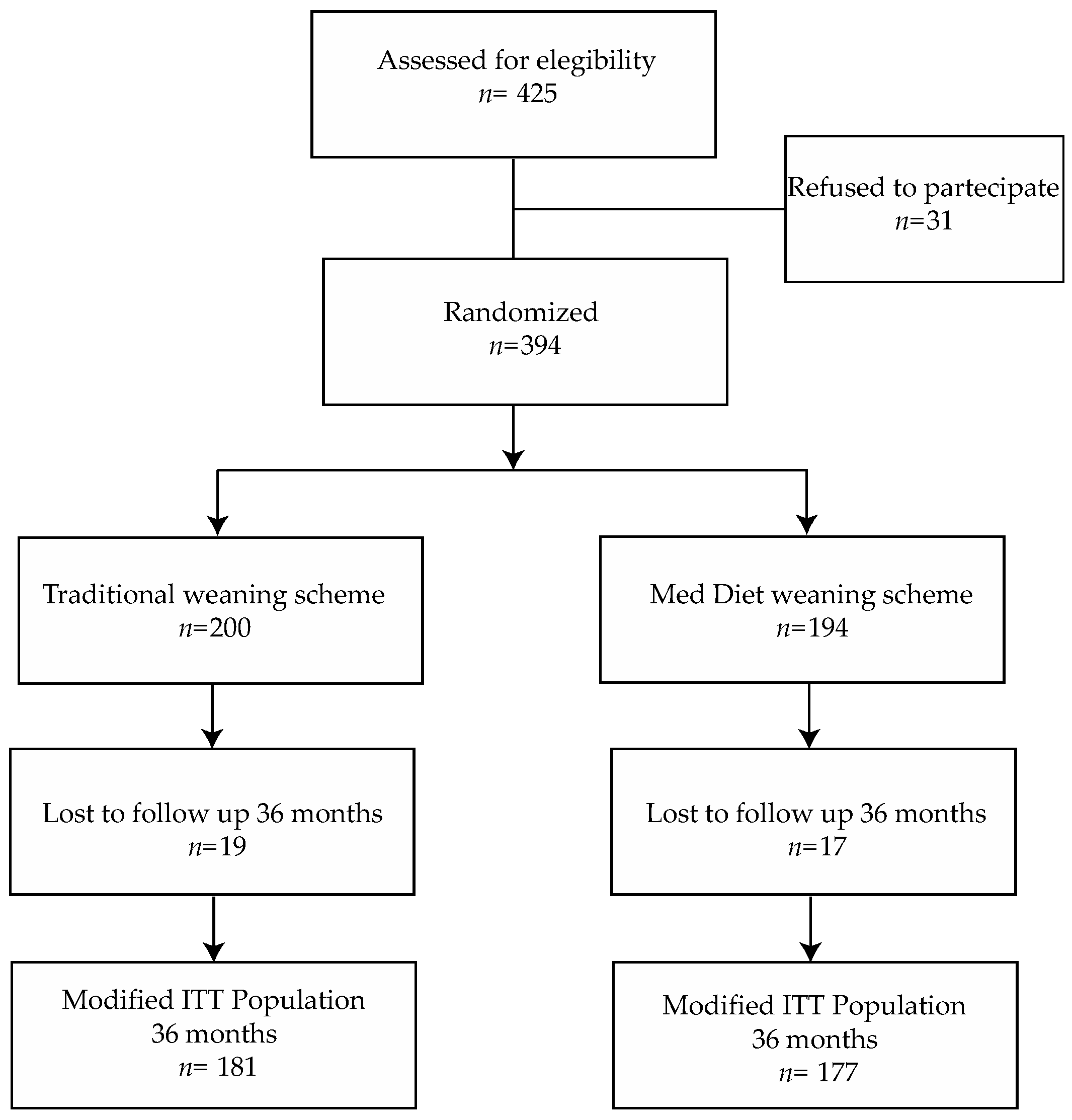
2.6. Randomization
2.7. DNA Extraction and Fecal Microbiota Analysis
2.8. Statistical Analysis
2.9. Fecal Microbiota Analysis
3. Results
3.1. Baseline Characteristics
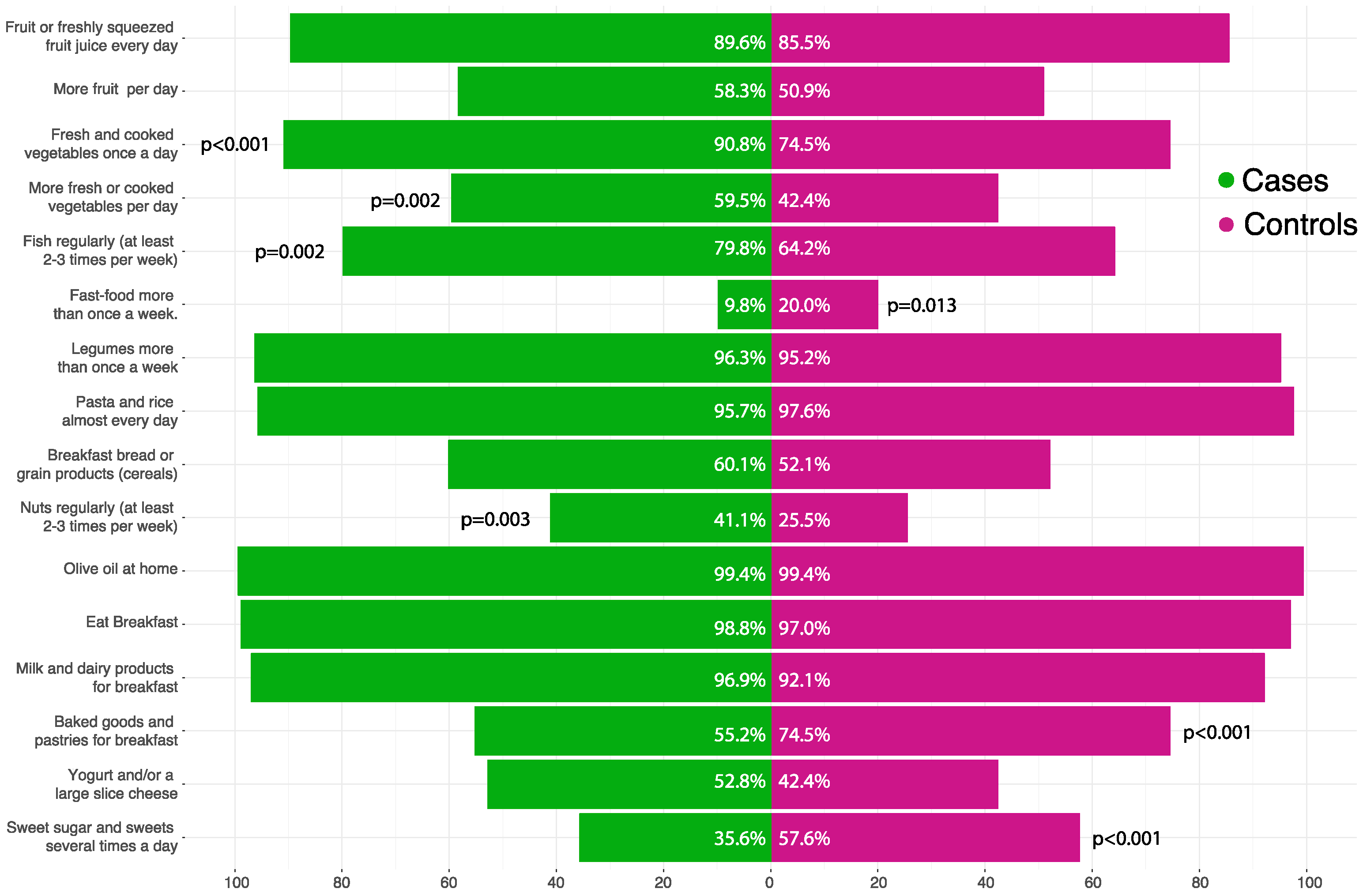
3.2. Mediterranean Diet Adherence in Children
3.3. Children’s BMI
3.4. Mediterranean Diet Adherence in Mothers
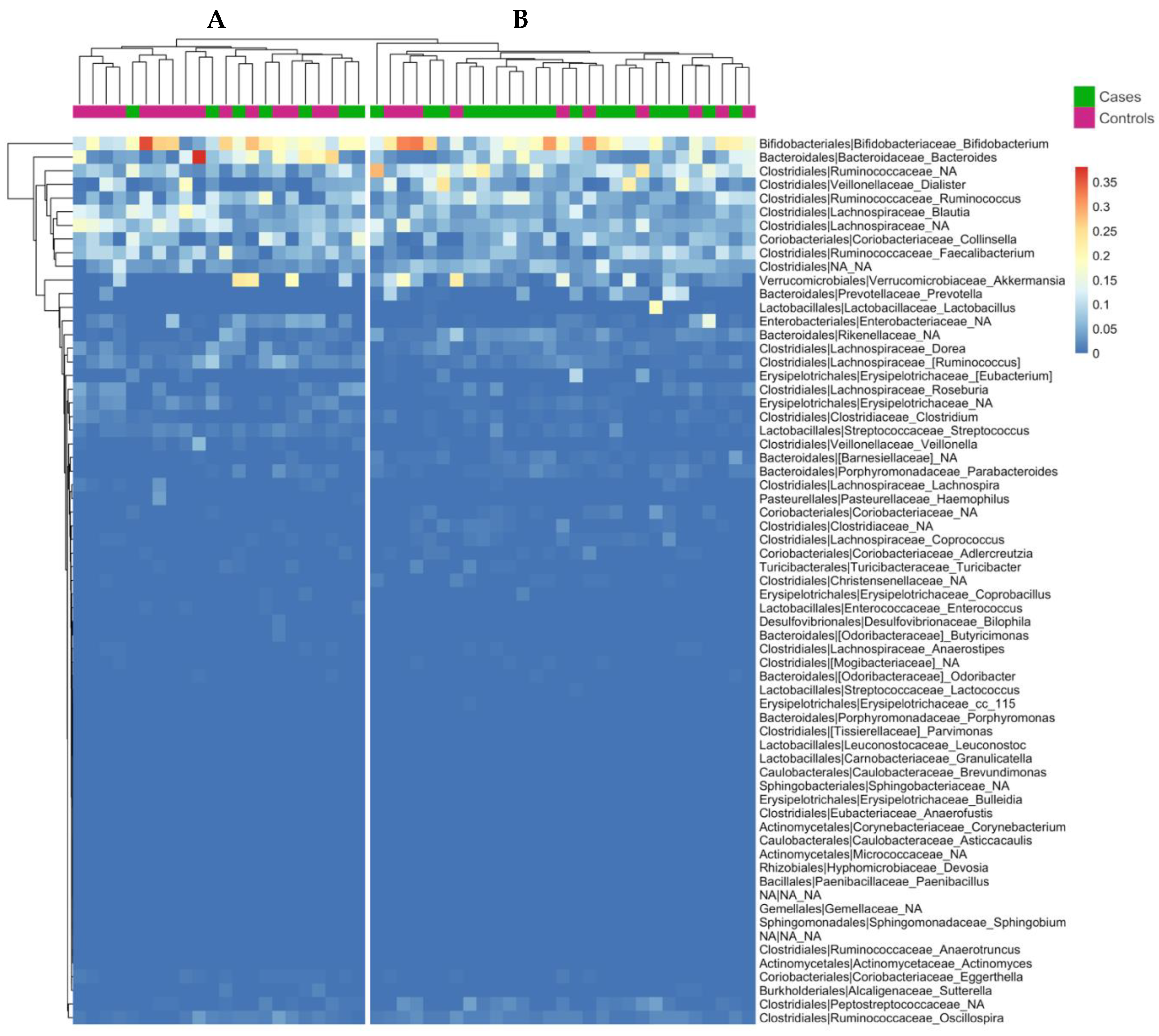
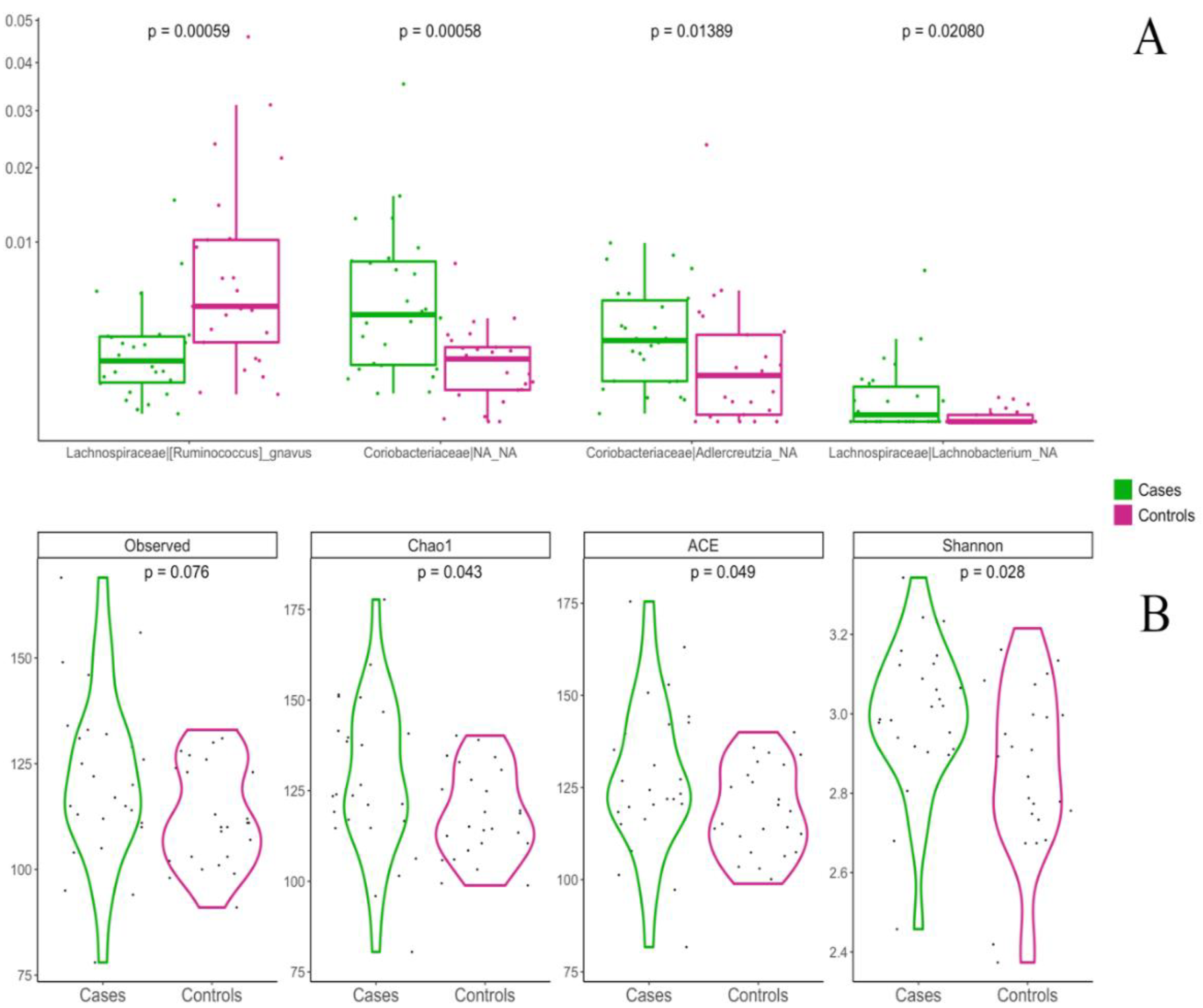
3.5. Influence of Weaning Type on Gut Microbiota Composition
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mozaffarian, D. Dietary and policy priorities for cardiovascular disease, diabetes and obesity: A comprehensive review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy Derek, W.; Fasano, A.; Miller Gary, W.; et al. Chronic inflammation in the aeiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Food, immunity, and the microbiome. Gastroenterology 2015, 148, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Chang, J.; Lartigue, L.; Bolen, C.R.; Haddad, F.; Gaudilliere, B.; Ganio, E.A.; Fragiadakis, G.K.; Spitzer, M.H.; Douchet, I.; et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat. Med. 2017, 23, 174–184. [Google Scholar] [CrossRef]
- Moszak, M.; Szulińska, M.; Bogdański, P. You are what you eat-the relationship between diet, microbiota, and metabolic disorders-a review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprockett, D.; Fukami, T.; Relman, D.A. Role of priority effects in the early-life assembly of the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Jagnow, C.P.; Beauchamp, G.K. Prenatal and postnatal flavor learning by human infants. Pediatrics 2001, 107, E88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forestell, C.A. Flavor perception and preference development in human infants. Ann. Nutr. Metab. 2017, 70, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Townsend, E.; Pitchford, N.J. Baby knows best? The impact of weaning style on food preferences and body mass index in early childood in a case-controlled sample. BMJ Open 2012, 2, e000298. [Google Scholar] [CrossRef] [Green Version]
- Barroso, M.; Sytske, A.B.; Voortman, T.; Jaddoe, V.W.V.; van Zelm, M.C.; Moll, H.A.; Kiefte-de Jong, J.C. Dietary patterns after the weaning and lactation period are associated with celiac disease autoimmunity in children. Gastroenterology 2018, 154, 2087–2096. [Google Scholar] [CrossRef] [Green Version]
- Vrdoljak, J.; Vilović, M.; Zivcović, P.M.; Hadjina, I.T.; Rušić, D.; Bukić, J.; Borovac, J.A.; Božić, J. Mediterranean diet adherence and dietary attitudes in patients with inflammatory bowel disease. Nutrients 2020, 8, 3429. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Håkansson, N.; Chan, S.S.; Chen, Y.; Lochhead, P.; Ludvigsson, J.F.; Chan, A.T.; Hart, A.R.; Olén, O.; Wolk, A. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: Results from two large prospective cohort studies. BMJ 2020, 69, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Buja, A.; Grotto, G.; Brocadello, F.; Sperotto, M.; Baldo, V. Primary school children and nutrition: Lifestyles and behavioral traits associated with a poor-to-moderate adherence to the Mediterranean diet. A cross-sectional study. Eur. J. Pediatr. 2020, 179, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, G.; Furlan, F.; Scocuzza, M.; Lorini, C. Adherence to Mediterranean Diet among students from primary and middle school in the province of Taranto, 2016–2018. Int. J. Environ. Res. Public Health 2020, 28, 5437. [Google Scholar] [CrossRef]
- Grassi, T.; Bagordo, F.; Panico, A.; De Giorgi, M.; Idolo, A.; Serio, F.; Tumolo, M.R.; De Donno, A. Adherence to Mediterranean diet of children living in small Southern Italian villages. Int. J. Food Sci. Nutr. 2020, 71, 490. [Google Scholar] [CrossRef]
- Rosi, A.; Biasini, B.; Donati, M.; Ricci, C.; Scazzina, F. Adherence to the Mediterranean Diet and environmental impact of the diet on primary school children living in Parma (Italy). Int. J. Environ. Res. Public Health 2020, 21, 6105. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; a Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Rogol, A.D.; Hyden, G.F. Etiologies and early diagnosis of short stature and growth failure in children and adolescent. J. Pediatr. 2014, 164, S1–S14. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.O.; García, A.; Pérez-Rodrigo, C.; Aranceta, J. Food, youth and the Mediterranean diet in Spain. Development of KIDMED, Mediterranean Diet Quality Index in children and adolescents. Public Health Nutr. 2004, 7, 931–935. [Google Scholar] [CrossRef]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salvadó, J.S.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Italian Society of Human Nutrition. Reference Levels of Nutrient and Energy Intake for the Italian Population (LARN); IV revision, II reprint; Italian Society of Human Nutrition: Milano, Italy, 2016. [Google Scholar]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Investig. 2006, 29, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; De Filippis, F.; Nocerino, R.; Laiola, M.; Paparo, L.; Calignano, A.; De Caro, C.; Coretti, L.; Chiariotti, L.; Gilbert, J.A.; et al. Specific signatures of the gut microbiota and increased levels of butyrate in children treated with fermented cow’s milk containing heat-killed lactobacillus paracasei CBA L74. Appl. Environ. Microbiol. 2017, 83, e01206-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verduci, E.; Banderali, G.; Montanari, G.; Berni Canani, R.; Cimmino Caserta, L.; Corsello, G.; Mosca, F.; Piazzolla, R.; Rescigno, M.; Terracciano, L.; et al. Childhood Dietary Intake in Italy: The Epidemiological ”MY FOOD DIARY” Survey. Nutrients 2019, 21, 1129. [Google Scholar] [CrossRef] [Green Version]
- De Filippis, F.; Vitaglione, P.; Cuomo, R.; Berni Canani, R.; Ercolini, D. Dietary interventions to modulate the gut microbiome - how far away are from precision medicine. Inflamm. Bowel Dis. 2018, 24, 2142–2154. [Google Scholar] [CrossRef]
- Romo-Vaquero, M.; Cortés-Martín, A.; Loria-Kohen, V.; Ramìrez-de-Molina, A.; Garcìa-Mantrana, I.; Collado, M.C.; Espín, J.C.; Selma, M.V. Deciphering the human gut microbiome of urolithin metabotypes: Association with enterotypes and potential cardiometabolic health implications. Mol. Nutr. Food Res. 2019, 63, 1800958. [Google Scholar] [CrossRef]
- Selma, M.V.; Beltrán, D.; Luna, M.C.; Romo-Vaquero, M.; Garcìa-Villalba, R.; Mira, A.; Espín, J.C.; Tomàs-Barberán, F.A. Isolation of human intestinal bacteria capable of producing the bioactive metabolite isourolithin A from ellagic acid. Front. Microbiol. 2017, 8, 1521. [Google Scholar] [CrossRef]
- Garcìa-Mantrana, I.; Calatayud, M.; Romo-Vaquero, M.; Espín, J.C.; Selma, M.V.; Collado, M.C. Urolithin metabotypes can determine the modulation of gut microbiota in healthy individuals by tracking walnuts consumption over three days. Nutrients 2019, 11, 2483. [Google Scholar] [CrossRef] [Green Version]
- Abdelazeem, K.N.M.; Kalo, M.Z.; Beer-Hammer, S.; Lang, F. The gut microbiota metabolite urolithin A inhibits NF-κB activation in LPS stimulated BMDMs. Sci. Rep. 2021, 11, 7117. [Google Scholar] [CrossRef]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef] [Green Version]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Joossens, M.; Huys, G.; Cnockaert, M.; De Preter, V.; Verbeke, K.; Rutgeerts, P.; Vandamme, P.; Vermeire, S. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut 2011, 60, 631–637. [Google Scholar] [CrossRef] [Green Version]
- Hall, A.B.; Yassour, M.; Sauk, J.; Garner, A.; Jiang, X.; Arthur, T.; Lagoudas, G.K.; Vatanen, T.; Fornelos, N.; Wilson, R.; et al. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017, 9, 103. [Google Scholar] [CrossRef]
- De Filippis, F.; Paparo, L.; Nocerino, R.; Della Gatta, G.; Carucci, L.; Russo, R.; Pasolli, E.; Ercolini, D.; Berni Canani, R. Specific gut microbiome signatures and the associated pro-inflamatory funcions are linked to pediatric allergy and acquisition of immune tolerance. Nat. Commun. 2021, 12, 5958. [Google Scholar]
- Segata, N. Gut microbiome: Westernization and the disappearance of intestinal diversity. Curr. Biol. 2015, 25, R611–R613. [Google Scholar] [CrossRef] [Green Version]
- Hold, G.L. Western lifestyle: A ‘master’ manipulator of the intestinal microbiota? Gut 2014, 63, 5–6. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.D.; Nguyen, L.H.; Li, Y.; Yan, Y.; Ma, W.; Rinott, E.; Ivey, K.L.; Shai, I.; Willett, W.C.; Hu, F.B.; et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat. Med. 2021, 27, 333–343. [Google Scholar] [CrossRef]
- Laursen, M.F.; Bahal, M.I.; Michaelsen, K.F.; Licht, T.R. First foods and gut microbes. Front. Microbiol. 2017, 8, 356. [Google Scholar] [CrossRef] [Green Version]
- Dizzell, S.; Stearns, J.C.; Li, J.; van Best, N.; Bervoets, L.; Mommers, M.; Penders, J.; Morrison, K.M.; Hutton, E.K.; GI-MDH Consortium Partners. Investigating colonization patterns of the infant gut microbiome during the introduction of solid food and weaning from breastmilk: A cohort study protocol. PLoS ONE 2021, 16, e0248924. [Google Scholar]
- Al Nabhani, Z.; Dulauroy, S.; Marques, R.; Cousu, C.; Al Bounny, S.; Déjardin, F.; Sparwasser, T.; Bérard, M.; Cerf-Bensussan, N.; Eberl, G. A Weaning reaction to microbiota is required for resistance to immunopathologies in the adult. Immunity 2019, 50, 1276–1288. [Google Scholar] [CrossRef]
- Spinelli, A.; Nardone, P.; Buoncristiano, M.; Lauria, L.; Pierannunzio, D.; Centro Nazionale per la Prevenzione Delle Malattie e la Promozione Della Salute, Cnapps-Iss. OKKIO Alla SALUTE: I dati Nazionali 2016. Available online: http://www.epicentro.iss.it/okkioallasalute/dati2016.asp (accessed on 21 March 2022).
- Hummel, S.; Weiß, A.; Bonifacio, E.; Agardh, D.; Akolkar, B.; Aronsson, C.A.; Hagopian, W.A.; Koletzko, S.; Krischer, J.P.; Lernmark, A.; et al. Associations of breastfeeding with childhood autoimmunity, allergies, and overweight: The Environmental Determinants of Diabetes in the Young (TEDDY) study. Am. J. Clin. Nutr. 2021, 114, 134–142. [Google Scholar] [CrossRef]
- Platt, R.; Simon, G.E.; Hernandez, A.F. Is learning worth the trouble?—Improving Health Care System participation in embedded research. N. Engl. J. Med. 2021, 385, 5–7. [Google Scholar] [CrossRef]
- Corley, D.A.; Kilbourne, A. The Learning Health System: The tools are here-why aren’t we moving forward? Gastroenterology 2021, 161, 1747–1750. [Google Scholar] [CrossRef]

| Control Arm (n = 200; 50.8%) | Med Diet Arm (n = 194; 49.2%) | |
|---|---|---|
| Child characteristics | ||
| Age at enrollment (days) | 145.6 + −19.8 (47 to 189) | 145.3 + −18.8 (92 to 197) |
| Gender; female | 102 (51) | 103 (53.1) |
| Birth weight (kg) | 3.2 + −0.4 (1.8 to 4.4) | 3.2 + −0.5 (1.2 to 4.5) |
| Weight at enrollment (kg) | 7.2 + −0.9 (3.8 to 10) | 7.2 + −0.9 (4.9 to 9.9) |
| Only child | 86 (43) | 98 (50.5) |
| Feeding | ||
| Breastfeeding | 48 (24) | 64 (33.2) |
| Formula type | 118 (59) | 100 (51.8) |
| Mixed | 34 (17) | 29 (15) |
| Parents characteristics | ||
| Mothers’ degree | ||
| Primary school | 59 (29.5) | 50 (25.9) |
| Secondary school | 90 (45) | 80 (41.5) |
| Degree or higher | 31 (30.5) | 63 (32.6) |
| Mother BMI (kg/m2) | 25.8 + −4.4 (17.7 to 43.1) | 25 + −4.4 (17.6 to 46.9) |
| Father BMI (kg/m2) | 26.3 + −2.9 (18.7 to 34.8) | 25.9 + −3.1 (17.3 to 36.7) |
| Mothers’ adherence to Med Diet | 62.2 + −13.9 (28.6 to 92.9) | 62.7 + −13.5 (28.6 to 85.7) |
| Control Arm (n = 200; 50.8%) | Med Diet Arm (n = 194; 49.2%) | Treatment Effect (95% CI) | p Value | |
|---|---|---|---|---|
| Children | ||||
| KidMed score ≥ 8; n (%) | 62 (34.3%) | 105 (59.3%) | 2.8 (1.82 to 4.3) ° | <0.001 |
| KidMed score | 6.0 ± 2.9 | 7.5 ± 3 | 1.5 (0.9 to 2.1) § | <0.001 |
| BMI (kg/m2) | 16.4 ± 2 | 16.2 ± 1.5 | −0.16 (−0.54 to 0.22) § | 0.413 |
| Overweight; n (%) | 29 (17.7) | 18 (11) | 0.58 (0.31 to 1.1) ° | 0.094 |
| Obese; n (%) | 2 (1.2) | 0 (0) | NA | NA |
| Parents | ||||
| Mothers’ adherence to Med Diet | 61.5 ± 13.8 (28.6 to 92.2) | 70.3 ± 18.2 (28.6 to 92.2) | 8.6 (4.9 to 12.3) § | <0.001 |
| Control Arm (n = 25; 49%) | Med Diet Arm (n = 26; 51%) | |
|---|---|---|
| Gender; female | 14 (56%) | 17 (65.4%) |
| C-Section | 9 (36%) | 9 (34.6%) |
| Weight at T36 (kg) | 14.7 ± 1.6 (12 to 18) | 15.3 ± 1.9 (12.3 to 20.5) |
| Feeding at T36 | ||
| Breastfeeding | 0 (0%) | 1 (3.9%) |
| Formula type | 4 (16.0%) | 3 (11.5%) |
| Cow milk | 21 (84.0%) | 22 (84.6%) |
| KidMed score at T36 | 6.6 ± 2.1 (3 to 11) | 8 ± 3 (0 to 12) |
| Age at microbiota evaluation | 4.0 ± 0.6 (3.3 to 5.5) | 4.3 ± 0.6 (3.4 to 5.6) |
| Number of cumulative antibiotics prescriptions at microbiota evaluation | 4 (1 to 9) | 5 (2 to 7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Franchis, R.; Bozza, L.; Canale, P.; Chiacchio, M.; Cortese, P.; D’Avino, A.; De Giovanni, M.; Iacovo, M.D.; D’Onofrio, A.; Federico, A.; et al. The Effect of Weaning with Adult Food Typical of the Mediterranean Diet on Taste Development and Eating Habits of Children: A Randomized Trial. Nutrients 2022, 14, 2486. https://doi.org/10.3390/nu14122486
de Franchis R, Bozza L, Canale P, Chiacchio M, Cortese P, D’Avino A, De Giovanni M, Iacovo MD, D’Onofrio A, Federico A, et al. The Effect of Weaning with Adult Food Typical of the Mediterranean Diet on Taste Development and Eating Habits of Children: A Randomized Trial. Nutrients. 2022; 14(12):2486. https://doi.org/10.3390/nu14122486
Chicago/Turabian Stylede Franchis, Raffaella, Luigi Bozza, Pasquale Canale, Maria Chiacchio, Paolo Cortese, Antonio D’Avino, Maria De Giovanni, Mirella Dello Iacovo, Antonietta D’Onofrio, Aniello Federico, and et al. 2022. "The Effect of Weaning with Adult Food Typical of the Mediterranean Diet on Taste Development and Eating Habits of Children: A Randomized Trial" Nutrients 14, no. 12: 2486. https://doi.org/10.3390/nu14122486
APA Stylede Franchis, R., Bozza, L., Canale, P., Chiacchio, M., Cortese, P., D’Avino, A., De Giovanni, M., Iacovo, M. D., D’Onofrio, A., Federico, A., Gasparini, N., Iaccarino, F., Romano, G., Spadaro, R., Tedesco, M., Vitiello, G., Antignani, A., Auricchio, S., Valentino, V., ... Bruzzese, D. (2022). The Effect of Weaning with Adult Food Typical of the Mediterranean Diet on Taste Development and Eating Habits of Children: A Randomized Trial. Nutrients, 14(12), 2486. https://doi.org/10.3390/nu14122486









