Abstract
Previous studies have highlighted links between a high-glycemic-load (GL) diet and Alzheimer’s disease in apolipoprotein E ε4 (APOE4) carriers. However, the impact of high-GL diet on plasma amyloid-β (Aβ), an Alzheimer’s disease hallmark that can be detected decades before clinical symptomatology, is unknown. This study examined the association between plasma Aβ peptides (Aβ40, Aβ42 concentration and Aβ42/Aβ40 ratio) and GL. The influence of the GL of four meal types (breakfast, lunch, afternoon snack, and dinner) was also determined. From the prospective Three-City study, 377 participants with plasma Aβ measurements, and who completed the Food Frequency Questionnaire, were selected. The association between plasma Aβ and GL was tested using an adjusted linear regression model. Lunch GL was associated with a lower plasma Aβ42 concentration (β = −2.2 [CI = −4.27, −0.12], p = 0.038) and lower Aβ42/Aβ40 ratio (β = −0.009 [CI = −0.0172, −0.0007], p = 0.034) in the model adjusted for center, age, sex, education level, APOE4 status, energy intake, serum creatinine, total cholesterol, and Mediterranean-like diet. No significant association was found with the GL of the other meal types. These results suggest that dietary GL may independently modulate the plasma Aβ of the APOE4 status. The mechanism underlying diet, metabolic response, and Aβ peptide regulation must be elucidated.
1. Introduction
Alzheimer’s disease (AD), the most common form of dementia, is a neurodegenerative disease characterized by amyloid-β (Aβ) deposition and neurofibrillary tangles. Dementia affects 50 million people worldwide and represents the main cause of dependency and disability in older adults [1]. To date, no curative pharmacological treatment is available for dementia. Therefore, it is essential to develop effective and widely applicable strategies to prevent or delay AD onset. Accumulating evidence indicates that diet interventions could be one of these strategies [2,3]. Indeed, a refined carbohydrate-rich diet is known to increase Aβ in mice brains [4,5]. Recently, a meal intervention study suggested that the Western diet, rich in saturated fat and refined carbohydrates, could exacerbate Aβ peptide concentration in plasma [6]. Importantly, the plasma concentration of Aβ42 and Aβ40 peptides may reflect Aβ burden in the brain [7]. Moreover, a high plasma Aβ40 concentration, low plasma Aβ42 concentration, and low Aβ42/Aβ40 ratio have been linked to a higher dementia risk [8,9]. However, no study in the general population has specifically examined the association between a refined carbohydrate diet and plasma Aβ biomarkers. Thus, the investigation of this association may provide evidence that a refined carbohydrate diet is an environmental factor that promotes AD pathogenesis via plasma Aβ. Additionally, this would open the possibility of acting on this modifiable dietary factor to reduce the prevalence of dementia in the general population. In this context, our previous work emphasized that a high-glycemic-load (GL) diet increases the risk of dementia in apolipoprotein E ε4 (APOE4) carriers, the main genetic risk factor of AD [10]. In addition, an interventional study demonstrated that diet may change the plasma concentration of Aβ peptides according to the APOE4 carrier status [6,11], suggesting that APOE4 status could be an effect modifier. Moreover, our work highlighted the stronger impact of the GL of an afternoon snack (the meal with the highest content in refined carbohydrates) on the risk of dementia [10], suggesting a risk variability according to the meal type. Indeed, snacks and breakfast tend to be richer in refined carbohydrates and therefore have higher GL values than the main meals (i.e., lunch and dinner) [12].
Based on these findings, we hypothesized that a high-GL diet might be associated with plasma Aβ peptide levels. Therefore, we used data from the prospective Three-City (3C) cohort study to examine the association between GL and plasma Aβ40 and Aβ42 concentration, as well as Aβ42/Aβ40 ratio. We also evaluated the potential interactive effect of APOE4 carrier status and the effect of the daily, breakfast, lunch, afternoon snack, and dinner GL values on this association.
2. Materials and Methods
2.1. Study Sample
The 3C study is a population-based, prospective study on the relationship between vascular factors and dementia. The study started in 1999–2000 and included participants, aged 65 years or older, from three French cities: Bordeaux, Dijon, and Montpellier [13]. The Consultative Committee for the Protection of Persons Participating in Biomedical Research at Kremlin-Bicêtre University Hospital (Paris, France) approved the 3C Study protocol, and all participants gave their written informed consent. Baseline data collection included sociodemographic, clinical, and lifestyle characteristics, medication use, neuropsychological testing, and clinical examination with blood sampling. At baseline, 1254 participants were randomly selected from the three centers, stratified by center, sex, and age, for plasma Aβ concentration measurement [9]. From this subsample, participants at the Bordeaux and Montpellier centers who completed a 148-item Food Frequency Questionnaire (FFQ) at 2-year and 4-year follow-up visits, respectively, (Figure 1) were selected.
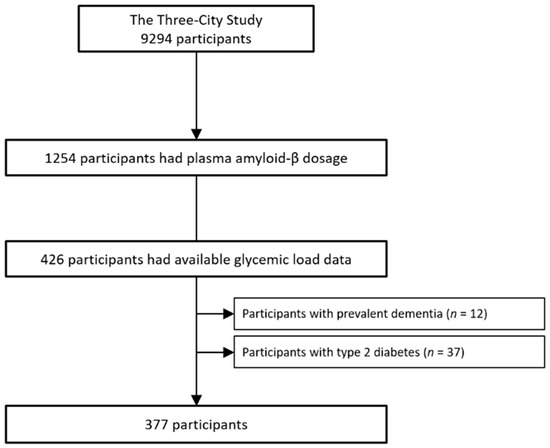
Figure 1.
Flow chart.
2.2. Dietary Data
The 148-item semi-quantitative FFQ was divided into breakfast, lunch, dinner, and snacks between meals. The GL is an indicator of the cumulative exposure to postprandial glycemia and reflects insulin demand, induced by the carbohydrate intake [14,15]. GL values were obtained from the International Table of Glycemic Index [16] and internet updates (www.glycemicindex.com (accessed on 11 June 2019)). GL items are defined as “low” when the GL value is below 10 and as “high” when the value is above 20 [17]. As previously described, GL was calculated for breakfast, lunch, afternoon snack (“goûter” in French, corresponding to a snack between lunch and dinner), dinner, and the entire day, and validated with the GL values from the 24 h dietary recall [10]. GL values were within the same range of variation as those from another study on a French population (Table S1) [18]. In the Bordeaux 3C study, carbohydrate intake did not seem to change during the follow-up [19]. Thus, it was hypothesized that the GL did not change from baseline until the visit when the FFQ was completed. Energy intake and adherence to a Mediterranean-like diet were also determined [10]. Diet quality, for example, from a Mediterranean diet, may influence the plasma concentration of Aβ peptides [20] as well as the GL values [21,22]. The Mediterranean-like diet score was categorized as low (0–3), moderate (4–5), and high (6–9). Missing values of the Mediterranean-like diet score (8%) were added in a fourth category.
2.3. Plasma Amyloid-β Peptide Assessment
The plasma Aβ peptide assay is described elsewhere [9]. Briefly, blood samples were collected in tubes containing sodium EDTA. After centrifugation, samples were aliquoted and stored at −80 °C. Plasma Aβ peptides were measured using the INNO-BIA Kit (Innogenetics, Ghent, Belgium) based on the multiplex xMAP technique (Luminex, Austin, TX, USA). Aβ40 and Aβ42 concentrations were expressed in pg/mL, and the Aβ42/Aβ40 ratio was calculated.
2.4. Incident Dementia Diagnosis
At the 2-, 4-, 7-, 10-, 12- and 15-year follow-up visits, a neurologist examined participants with suspected dementia on the basis of their neuropsychological test scores. Then, an independent committee of neurologists evaluated all potential cases of dementia to reach a consensus on the diagnosis and etiology based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [23].
2.5. Other Covariates
Education level was defined as no school, primary school, high school, or graduate level. APOE genotyping was performed as previously described [24]. APOE4 carriers were defined as carrying at least one ε4 allele. Diabetes was defined as treated diabetes, fasting glycemia > 7 mmol/L, and self-reported diabetes. Serum creatinine (mmol/L) was measured with the Jaffe method [25]. Total cholesterol (mmol/L) was quantified using routine enzymatic methods. Serum creatinine and total cholesterol are biological factors that may influence the plasma concentration of Aβ peptides [26,27].
2.6. Statistical Analyses
Linear regression models were used to evaluate the association between GL and Aβ peptide concentration/ratio. The models were first adjusted for energy intake. Then, multicollinearity was tested using the variance inflation factor [28]. As GL showed a high variance inflation factor value when energy intake was added to the models, the residual method was used with all GL values [14,29]. The first model was adjusted for center, age, sex, education level, APOE4 status, and energy intake. The second model was additionally adjusted for serum creatinine, total cholesterol, and Mediterranean-like diet, which may influence the plasma concentration of Aβ peptides [20,26,27]. The interaction with APOE4 carrier status was also tested. The model assumptions were checked, and outliers were removed (n = 4 for Aβ40, n = 3 for Aβ42, and n = 2 for Aβ42/Aβ40) by looking at the graphs and using the Bonferroni method with the ‘car’ package in R [30]. For the sensitivity analysis, participants with incident dementia were excluded to avoid potential reverse causation. All statistical analyses were performed using R version 3.6.1(R Core Team, Vienna, Austria). Two-sided Fisher tests were used, and a p value < 0.05 was considered statistically significant.
3. Results
At FFQ completion, the mean age of the 377 participants was 76.1 (±5.2) years and 60.2% were women (Table 1). Participants in the high tertile group of the daily GL included fewer women, and these participants tended to have a higher Mediterranean-like diet score compared with the low tertile group.

Table 1.
Characteristics of the study sample according to the tertile of the total GL residuals.
To evaluate the association between GL and plasma Aβ peptide concentration and ratio according to APOE4 status, the GL × APOE4 interaction was tested. As no significant interaction was detected between GL and APOE4 status, the results are presented for the whole sample.
After removing outliers, no significant association between daily GL and plasma Aβ peptide concentration and ratio was detected in both models (Table 2). In model 1, breakfast GL tended to be associated with higher Aβ40, but not after additional adjustments in model 2. Lunch GL tended to be associated with lower Aβ42 in model 1, and the association between lunch GL and lower Aβ42 and ratio became significant after the adjustment for serum creatinine, total cholesterol, and a Mediterranean-like diet (model 2). The predicted Aβ42 and Aβ42/Aβ40 ratio values as a function of the lunch GL are presented in Figure 2. Finally, in both models, afternoon-snack and dinner GL were not associated with plasma Aβ peptide concentrations and ratio.

Table 2.
Association between glycemic load residuals and amyloid-β peptides.
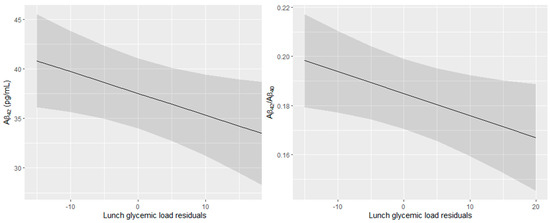
Figure 2.
Predicted Aβ42 concentration and Aβ42/Aβ40 ratio in plasma in function of the lunch glycemic load residuals (95% confidence intervals). All models were adjusted for center, age, sex, education level, APOE4 status, energy intake, serum creatinine, total cholesterol, and Mediterranean-like diet.
Participants who developed dementia during the 15-year follow-up (n = 51) were older than those who remained dementia-free. They were more likely to be women and APOE4 carriers. They also had a lower education level and a lower Mediterranean-like diet score than dementia-free participants (Table S2). In the sensitivity analysis that evaluated the association between GL and Aβ, after exclusion of the participants with incident dementia, the results were similar to those of the main analysis. The associations between lunch GL and plasma Aβ42 concentration and ratio became stronger (Table S3).
These results showed that lunch GL was associated with lower plasma Aβ42 concentration and lower Aβ42/Aβ40 ratio for a 10-point increase in the GL value per day, independently of the APOE4 carrier status.
4. Discussion
This study highlights the links between dietary GL and plasma Aβ biomarkers independently of the APOE4 carrier status. We found that lunch GL was associated with a lower plasma Aβ42 concentration and lower Aβ42/Aβ40 ratio. These associations remained significant after adjustment for center, age, sex, education level, APOE4 status, energy intake, serum creatinine, total cholesterol, and Mediterranean-like diet. Despite the cross-sectional design of our study, these findings suggest that dietary GL, as a proxy of refined carbohydrate consumption, may negatively modulate the plasma Aβ biomarkers. Indeed, lower plasma Aβ42 and lower Aβ42/Aβ40 ratio have been associated with higher risk of dementia in longitudinal studies [8,9]. Nevertheless, since no association was found between other meal types and plasma Aβ biomarkers, this study should be interpreted with caution and replicated in longitudinal studies with larger sample sizes.
Studies on the relationship between refined carbohydrate consumption and Aβ are scarce. Only two interventional studies investigated the impact of a refined carbohydrate-rich diet on Aβ in plasma and cerebrospinal fluid. However, these studies combined a diet rich in refined carbohydrates and in saturated fat, and thus could not highlight the specific effect of refined carbohydrates [6,31]. One neuroimaging study showed that high-GL diet was associated with global and regional Aβ burden in the brain of cognitively normal subjects [32]. Indeed, a high-GL diet was associated with higher Aβ burden in the anterior and posterior cingulate gyrus, the lateral temporal lobe, the precuneus, and the superior parietal lobe.
Previous studies on the relationship between dietary GL and Aβ never considered the different meal types. Our results highlight that lunch GL specifically contributes to worsening plasma Aβ biomarkers. This result is unexpected because lunch is the main meal of the day in France and contains large amounts of vegetables and legumes (i.e., fibers), fat, and protein [10], which contribute to limiting the increase in blood glucose [21]. Thus, for this meal, GL might reflect the postprandial glycemia and insulin demand to a lesser extent [22]. Moreover, 47% of individuals in the high-daily-GL tertile group had a good adherence to the Mediterranean diet (score from 6 to 9), and healthy meals (i.e., composed of vegetables, fruits and some cereals) have been associated with lower Aβ burden [20]. However, two plausible explanations can be suggested. First, as lunch is the main meal of the day in France, with various food types, lunch GL could be a good proxy of GL inter-individual variability, although lunch GL is not the best proxy of postprandial glycemia. In our sample, the GL of the other meals might not have been diverse enough to capture information, and the daily GL may have smoothed out this low variability by summing the different GL values. The second explanation concerns the lunch composition. An increase in lunch GL might be associated with a decrease in the consumption of fat and protein that could be protective factors. Indeed, it has been shown that replacing carbohydrates with fat decreases fasting triglycerides and glycemia and increases HDL cholesterol [33,34]. Particularly, the increased consumption of plant-based proteins and fat instead of carbohydrates has been associated with a significant decrease in all-cause mortality [35]. Moreover, if fewer proteins and fat are consumed, GL could ultimately be a good proxy of postprandial glycemia and insulin demand.
Our results suggest that high-GL diets could negatively affect plasma Aβ biomarkers. A possible biological explanation is that high GL indirectly participates in the mechanisms involving Aβ burden. First, a high-GL diet could promote insulin resistance through inflammation and oxidative stress [36,37]. This increases the blood-brain barrier permeability, and consequently, Aβ transport and clearance are impaired [38]. Second, Aβ degradation by an insulin-degrading enzyme (IDE) could be inhibited by GL-induced hyperinsulinemia. Both insulin and Aβ are IDE ligands, and excess insulin could compete with Aβ and, consequently, decrease its clearance [39]. Moreover, it has been shown that hyperinsulinemia modulates plasma Aβ peptide concentration in people with normal cognition [40,41]. However, moving to a healthy diet rich in anti-oxidants and anti-inflammatory components that improve insulin sensitivity could compensate for the harmful effect of a high-GL diet [42,43].
In this study, we did not find an interactive effect of APOE4 status. Yet, higher amyloid deposits were detected in the brain of APOE4 carriers with AD or with normal cognition [44,45]. Moreover, ApoE4 promotes blood–brain barrier disruption, increases Aβ aggregation, and decreases Aβ clearance [46]. Our study may have lacked the statistical power to show a significant interaction between GL and APOE4 carrier status.
This study presents some limitations. First, Aβ peptide concentration was measured in the early 2000s. Since then, more sensitive and effective methods have been developed [47,48]. Moreover, these results could not be compared with Aβ in cerebrospinal fluid because it was not measured in the 3C study. Second, our results concern a single Aβ measurement in plasma, but possible confounding factors were taken into account in the analysis. Specifically, our results are independent from serum creatinine, total cholesterol, and diet quality, three factors that could influence Aβ concentration in plasma [20,26,27], even though these factors cannot truly take into account the lipid dyshomeostasis that could interfere with Aβ trafficking [49]. Third, we assumed that GL did not change between baseline and the time when FFQ was completed. However, the diets measured with the FFQ might not reflect the participants’ diets at baseline. Although this is a cross-sectional analysis, the prospective population-based design with a long follow-up period allowed us to consider reverse causation concerning dementia in the 15 years of follow-up.
5. Conclusions
Dietary GL is associated with plasma Aβ42 concentration and Aβ42/Aβ40 ratio independently of the APOE4 carrier status, suggesting that AD biomarkers can be modulated by diet. As AD biomarker changes can be detected a long time before cognitive symptom appearance, these findings support the idea that long-term dietary choices constitute a critical environmental factor in the AD causal pathway. These findings pave the way for identifying new therapeutic targets and preventive measures because diet is a potentially modifiable risk factor. Experimental studies are now required to determine the underlying mechanism linking refined carbohydrate consumption and plasma Aβ peptides with dementia development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14122485/s1, Table S1: Glycemic load values of each item of the FFQ and mean glycemic load of the sample according to the meal types; Table S2: Characteristics of the study sample; Table S3: Association between plasma amyloid-β peptides and glycemic load residuals after exclusion of participants with incident dementia (n = 51).
Author Contributions
Conceptualization, M.G., M.R., C.B. and S.A.; methodology, M.G.; software, M.G.; validation, M.G., C.B. and S.A.; formal analysis, M.G.; investigation, M.G.; resources, C.F., C.S., C.B. and S.A.; data curation, S.A.; writing—original draft preparation, M.G.; writing—review and editing, M.G., M.R., C.S., V.C., C.F., C.B. and S.A.; visualization, M.G.; supervision, C.B. and S.A.; project administration, S.A.; funding acquisition, S.A. All authors have read and agreed to the published version of the manuscript.
Funding
The 3C Study is carried out under a partnership agreement between Inserm, the Victor Segalen–Bordeaux II University, and Sanofi-Synthélabo. The Fondation pour la Recherche Médicale funded the preparation and first phase of the study. The 3C Study is also supported by the Caisse Nationale Maladie des Travailleurs Salariés, Direction Générale de la Santé, MGEN, the Institut de la Longévité, Agence Française de Sécurité Sanitaire des Produits de Santé; the Regional Governments of Aquitaine, Bourgogne and Languedoc-Roussillon, Fondation de France; and the Ministry of Research-Inserm Programme ‘Cohorts and collection of biological material’. This research was funded by Union France Alzheimer, grant number RAK17019FFA.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the 3C study was approved by the Consultative Committee for the Protection of Persons Participating in Biomedical Research at Kremlin-Bicêtre University Hospital (Project no. 99-28, June 1999).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request to the 3C scientific committee.
Acknowledgments
We thank Elisabetta Andermarcher for English editing. This is contribution ISEM 2022—139 of the Institute of Evolutionary Science of Montpellier.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- World Health Organization Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 11 June 2019).
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia Prevention, Intervention, and Care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [Green Version]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and Prevention of Cognitive Impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef]
- Carvalho, C.; Cardoso, S.; Correia, S.C.; Santos, R.X.; Santos, M.S.; Baldeiras, I.; Oliveira, C.R.; Moreira, P.I. Metabolic Alterations Induced by Sucrose Intake and Alzheimer’s Disease Promote Similar Brain Mitochondrial Abnormalities. Diabetes 2012, 61, 1234–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, S.H.-H.; Shie, F.-S.; Liu, H.-K.; Yao, H.-H.; Kao, P.-C.; Lee, Y.-H.; Chen, L.-M.; Hsu, S.-M.; Chao, L.-J.; Wu, K.-W.; et al. A High-Sucrose Diet Aggravates Alzheimer’s Disease Pathology, Attenuates Hypothalamic Leptin Signaling, and Impairs Food-Anticipatory Activity in APPswe/PS1dE9 Mice. Neurobiol. Aging 2020, 90, 60–74. [Google Scholar] [CrossRef]
- Hanson, A.J.; Bayer, J.L.; Baker, L.D.; Cholerton, B.; VanFossen, B.; Trittschuh, E.; Rissman, R.A.; Donohue, M.C.; Moghadam, S.H.; Plymate, S.R.; et al. Differential Effects of Meal Challenges on Cognition, Metabolism, and Biomarkers for Apolipoprotein E Ɛ4 Carriers and Adults with Mild Cognitive Impairment. J. Alzheimers Dis. 2015, 48, 205–218. [Google Scholar] [CrossRef]
- DeMattos, R.B.; Bales, K.R.; Cummins, D.J.; Paul, S.M.; Holtzman, D.M. Brain to Plasma Amyloid-Beta Efflux: A Measure of Brain Amyloid Burden in a Mouse Model of Alzheimer’s Disease. Science 2002, 295, 2264–2267. [Google Scholar] [CrossRef]
- van Oijen, M.; Hofman, A.; Soares, H.D.; Koudstaal, P.J.; Breteler, M.M.B. Plasma Aβ1-40 and Aβ1-42 and the Risk of Dementia: A Prospective Case-Cohort Study. Lancet Neurol. 2006, 5, 655–660. [Google Scholar] [CrossRef]
- Lambert, J.-C.; Schraen-Maschke, S.; Richard, F.; Fievet, N.; Rouaud, O.; Berr, C.; Dartigues, J.-F.; Tzourio, C.; Alpérovitch, A.; Buée, L.; et al. Association of Plasma Amyloid Beta with Risk of Dementia: The Prospective Three-City Study. Neurology 2009, 73, 847–853. [Google Scholar] [CrossRef]
- Gentreau, M.; Chuy, V.; Féart, C.; Samieri, C.; Ritchie, K.; Raymond, M.; Berticat, C.; Artero, S. Refined Carbohydrate-Rich Diet Is Associated with Long-Term Risk of Dementia and Alzheimer’s Disease in Apolipoprotein E Ε4 Allele Carriers. Alzheimers Dement. 2020, 16, 1043–1053. [Google Scholar] [CrossRef]
- Hanson, A.J.; Bayer-Carter, J.L.; Green, P.S.; Montine, T.J.; Wilkinson, C.W.; Baker, L.D.; Watson, G.S.; Bonner, L.M.; Callaghan, M.; Leverenz, J.B.; et al. Effect of Apolipoprotein E Genotype and Diet on Apolipoprotein E Lipidation and Amyloid Peptides. JAMA Neurol. 2013, 70, 972–980. [Google Scholar] [CrossRef] [Green Version]
- Bellisle, F.; Dalix, A.M.; Mennen, L.; Galan, P.; Hercberg, S.; de Castro, J.M.; Gausseres, N. Contribution of Snacks and Meals in the Diet of French Adults: A Diet-Diary Study. Physiol. Behav. 2003, 79, 183–189. [Google Scholar] [CrossRef]
- 3C Study Group Vascular Factors and Risk of Dementia: Design of the Three-City Study and Baseline Characteristics of the Study Population. Neuroepidemiology 2003, 22, 316–325. [CrossRef] [PubMed]
- Salmerón, J.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Wing, A.L.; Willett, W.C. Dietary Fiber, Glycemic Load, and Risk of Non-Insulin-Dependent Diabetes Mellitus in Women. JAMA 1997, 277, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Monro, J.A.; Shaw, M. Glycemic Impact, Glycemic Glucose Equivalents, Glycemic Index, and Glycemic Load: Definitions, Distinctions, and Implications. Am. J. Clin. Nutr. 2008, 87, 237S–243S. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, L.; Leloup, C. Mens Sana in Corpore Sano: Does the Glycemic Index Have a Role to Play? Nutrients 2020, 12, 2989. [Google Scholar] [CrossRef]
- Berticat, C.; Durand, V.; Raymond, M. Refined Carbohydrate Consumption and Facial Attractiveness. Evol. Psychol. 2020, 18, 1474704920960440. [Google Scholar] [CrossRef]
- Wagner, M.; Dartigues, J.-F.; Samieri, C.; Proust-Lima, C. Modeling Risk-Factor Trajectories When Measurement Tools Change Sequentially During Follow-up in Cohort Studies: Application to Dietary Habits in Prodromal Dementia. Am. J. Epidemiol. 2018, 187, 845–854. [Google Scholar] [CrossRef]
- Hill, E.; Goodwill, A.M.; Gorelik, A.; Szoeke, C. Diet and Biomarkers of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neurobiol. Aging 2019, 76, 45–52. [Google Scholar] [CrossRef]
- Post, R.E.; Mainous, A.G.; King, D.E.; Simpson, K.N. Dietary Fiber for the Treatment of Type 2 Diabetes Mellitus: A Meta-Analysis. J. Am. Board Fam. Med. 2012, 25, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Hätönen, K.A.; Virtamo, J.; Eriksson, J.G.; Sinkko, H.K.; Sundvall, J.E.; Valsta, L.M. Protein and Fat Modify the Glycaemic and Insulinaemic Responses to a Mashed Potato-Based Meal. Br. J. Nutr. 2011, 106, 248–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; Text Revision (DSM-IV-TR); American Psychiatric Association: Washington, DC, USA, 2000; ISBN 978-0-89042-062-1. [Google Scholar]
- Dufouil, C.; Richard, F.; Fiévet, N.; Dartigues, J.F.; Ritchie, K.; Tzourio, C.; Amouyel, P.; Alpérovitch, A. APOE Genotype, Cholesterol Level, Lipid-Lowering Treatment, and Dementia: The Three-City Study. Neurology 2005, 64, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Stengel, B.; Metzger, M.; Froissart, M.; Rainfray, M.; Berr, C.; Tzourio, C.; Helmer, C. Epidemiology and Prognostic Significance of Chronic Kidney Disease in the Elderly—The Three-City Prospective Cohort Study. Nephrol. Dial. Transplant 2011, 26, 3286–3295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledo, J.B.; Vanderstichele, H.; Figurski, M.; Aisen, P.S.; Petersen, R.C.; Weiner, M.W.; Jack, C.R.; Jagust, W.; Decarli, C.; Toga, A.W.; et al. Factors Affecting Aβ Plasma Levels and Their Utility as Biomarkers in ADNI. Acta Neuropathol. 2011, 122, 401–413. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Shou, Y.; Pan, J.; Du, Y.; Liu, C.; Wang, H. The Relationship between Cholesterol Level and Alzheimer’s Disease-Associated APP Proteolysis/Aβ Metabolism. Nutr. Neurosci. 2019, 22, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.C.; Peck, E.A.; Vining, G.G. Introduction to Linear Regression Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-0-470-54281-1. [Google Scholar]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for Total Energy Intake in Epidemiologic Studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef] [PubMed]
- Fox, J. Applied Regression Analysis and Generalized Linear Models, 3rd ed.; SAGE: Singapore, 2016; ISBN 978-1-4833-1088-6. [Google Scholar]
- Bayer-Carter, J.L.; Green, P.S.; Montine, T.J.; VanFossen, B.; Baker, L.D.; Watson, G.S.; Bonner, L.M.; Callaghan, M.; Leverenz, J.B.; Walter, B.K.; et al. Diet Intervention and Cerebrospinal Fluid Biomarkers in Amnestic Mild Cognitive Impairment. Arch. Neurol. 2011, 68, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.K.; Sullivan, D.K.; Swerdlow, R.H.; Vidoni, E.D.; Morris, J.K.; Mahnken, J.D.; Burns, J.M. A High-Glycemic Diet Is Associated with Cerebral Amyloid Burden in Cognitively Normal Older Adults. Am. J. Clin. Nutr. 2017, 106, 1463–1470. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated Fat, Carbohydrate, and Cardiovascular Disease. Am. J. Clin. Nutr. 2010, 91, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Clifton, P. Metabolic Syndrome—Role of Dietary Fat Type and Quantity. Nutrients 2019, 11, 1438. [Google Scholar] [CrossRef] [Green Version]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary Carbohydrate Intake and Mortality: A Prospective Cohort Study and Meta-Analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Di Domenico, F.; Barone, E. Elevated Risk of Type 2 Diabetes for Development of Alzheimer Disease: A Key Role for Oxidative Stress in Brain. Biochim. Biophys. Acta 2014, 1842, 1693–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, C.J.; Ruderman, N.B.; Kahn, S.E.; Pedersen, O.; Prentki, M. Insulin Resistance as a Physiological Defense against Metabolic Stress: Implications for the Management of Subsets of Type 2 Diabetes. Diabetes 2015, 64, 673–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhea, E.M.; Banks, W.A. Role of the Blood-Brain Barrier in Central Nervous System Insulin Resistance. Front. Neurosci. 2019, 13, 521. [Google Scholar] [CrossRef] [Green Version]
- Farris, W.; Mansourian, S.; Chang, Y.; Lindsley, L.; Eckman, E.A.; Frosch, M.P.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J.; Guenette, S. Insulin-Degrading Enzyme Regulates the Levels of Insulin, Amyloid Beta-Protein, and the Beta-Amyloid Precursor Protein Intracellular Domain in Vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 4162–4167. [Google Scholar] [CrossRef] [Green Version]
- Kulstad, J.J.; Green, P.S.; Cook, D.G.; Watson, G.S.; Reger, M.A.; Baker, L.D.; Plymate, S.R.; Asthana, S.; Rhoads, K.; Mehta, P.D.; et al. Differential Modulation of Plasma β-Amyloid by Insulin in Patients with Alzheimer Disease. Neurology 2006, 66, 1506–1510. [Google Scholar] [CrossRef]
- Karczewska-Kupczewska, M.; Lelental, N.; Adamska, A.; Nikołajuk, A.; Kowalska, I.; Górska, M.; Zimmermann, R.; Kornhuber, J.; Strączkowski, M.; Lewczuk, P. The Influence of Insulin Infusion on the Metabolism of Amyloid β Peptides in Plasma. Alzheimers Dement. 2013, 9, 400–405. [Google Scholar] [CrossRef]
- Williamson, G.; Sheedy, K. Effects of Polyphenols on Insulin Resistance. Nutrients 2020, 12, 3135. [Google Scholar] [CrossRef]
- Schenk, S.; Saberi, M.; Olefsky, J.M. Insulin Sensitivity: Modulation by Nutrients and Inflammation. J. Clin. Investig. 2008, 118, 2992–3002. [Google Scholar] [CrossRef] [Green Version]
- Dorey, E.; Chang, N.; Liu, Q.Y.; Yang, Z.; Zhang, W. Apolipoprotein E, Amyloid-Beta, and Neuroinflammation in Alzheimer’s Disease. Neurosci. Bull. 2014, 30, 317–330. [Google Scholar] [CrossRef] [Green Version]
- Kok, E.; Haikonen, S.; Luoto, T.; Huhtala, H.; Goebeler, S.; Haapasalo, H.; Karhunen, P.J. Apolipoprotein E-Dependent Accumulation of Alzheimer Disease-Related Lesions Begins in Middle Age. Ann. Neurol. 2009, 65, 650–657. [Google Scholar] [CrossRef]
- Montagne, A.; Nation, D.A.; Sagare, A.P.; Barisano, G.; Sweeney, M.D.; Chakhoyan, A.; Pachicano, M.; Joe, E.; Nelson, A.R.; D’Orazio, L.M.; et al. APOE4 Leads to Blood-Brain Barrier Dysfunction Predicting Cognitive Decline. Nature 2020, 581, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Doré, V.; Fowler, C.; Li, Q.-X.; Martins, R.; Rowe, C.; et al. High Performance Plasma Amyloid-β Biomarkers for Alzheimer’s Disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Li, Y.; Gordon, B.A.; Holtzman, D.M.; Morris, J.C.; Benzinger, T.L.S.; Xiong, C.; et al. High-Precision Plasma β-Amyloid 42/40 Predicts Current and Future Brain Amyloidosis. Neurology 2019, 93, e1647–e1659. [Google Scholar] [CrossRef] [PubMed]
- García-Viñuales, S.; Sciacca, M.F.M.; Lanza, V.; Santoro, A.M.; Grasso, G.; Tundo, G.R.; Sbardella, D.; Coletta, M.; Grasso, G.; La Rosa, C.; et al. The Interplay between Lipid and Aβ Amyloid Homeostasis in Alzheimer’s Disease: Risk Factors and Therapeutic Opportunities. Chem. Phys. Lipids 2021, 236, 105072. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).