Risk of Chronic Disease after an Episode of Marasmus, Kwashiorkor or Mixed–Type Severe Acute Malnutrition in the Democratic Republic of Congo: The Lwiro Follow-Up Study
Abstract
:1. Introduction
2. Methodology
2.1. Study Area
2.2. Study Design and Population
2.3. Data Collection
2.4. Outcomes
Potential Adult Confounder Variables
2.5. Statistical Analysis
3. Results
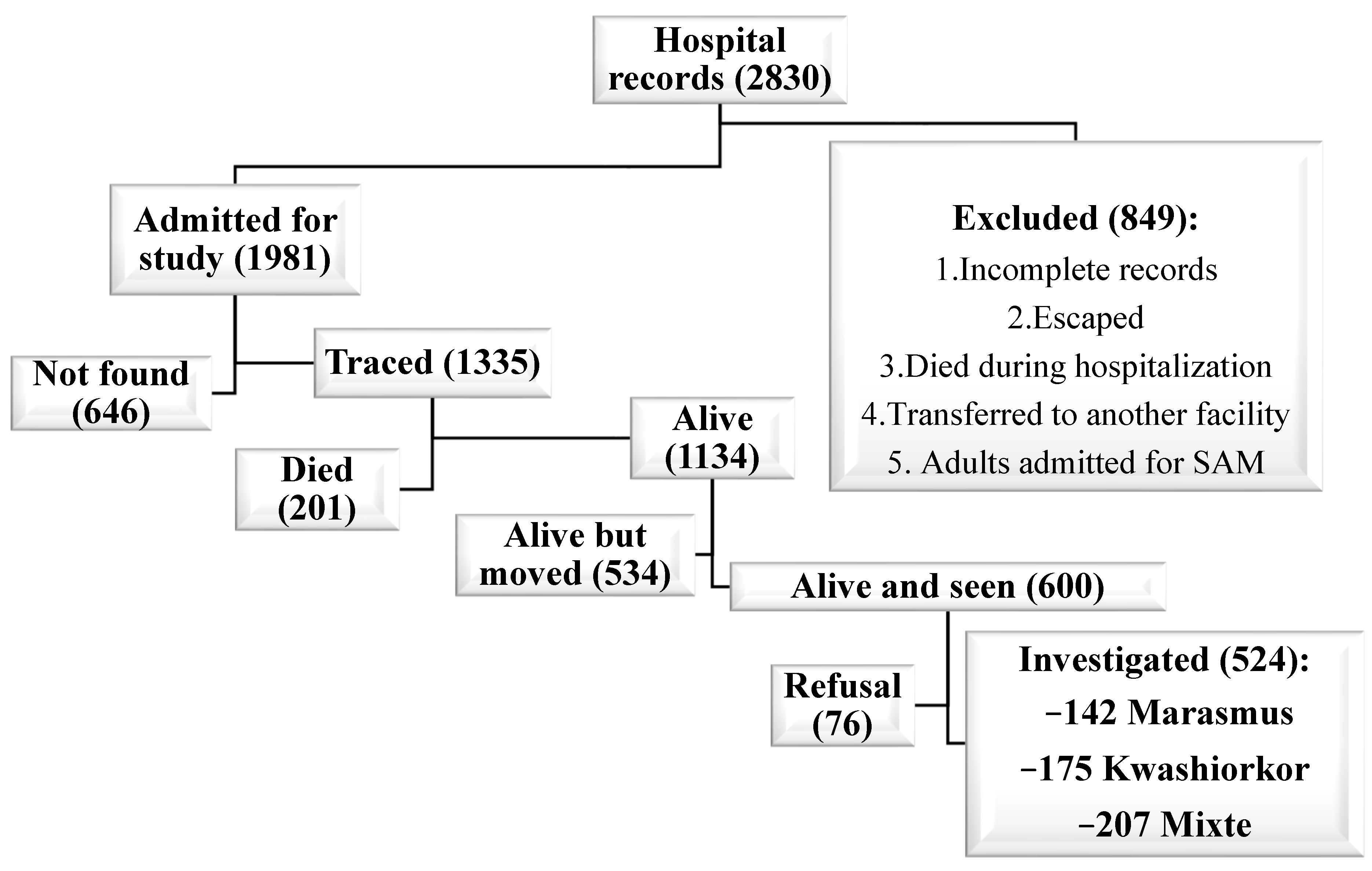
3.1. Recruitment of Exposed Group
3.1.1. Sociodemographic and Economic Characteristics of the Different Subgroups
3.1.2. Mean Differences in Clinical and Biological Markers for NCDs between Subgroups
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abrreviations
| BMI | Body Mass Index |
| BP | Blood Pressure |
| CVRFs | Cardiovascular Risk Factors |
| DRC | Democratic Republic of the Congo |
| DBP | Diastolic Blood Pressure |
| HDL-C | High-Density Lipoprotein Cholesterol |
| HMIC | High- and Middle-Income Countries |
| HBP | High Blood Pressure or Hypertension |
| HC | Hip circumference |
| LDL-C | Low-Density Lipoprotein cholesterol |
| LIC | Low-Income Countries |
| MAM | Moderate Acute Malnutrition |
| MBP | Mean Blood Pressure |
| MUAC | Mid-Upper Arm Circumference |
| NCDs | Noncommunicable Diseases |
| SAM | Severe Acute Malnutrition |
| SBP | Systolic Blood Pressure |
| SES | Socio-Economic Status |
| TG | Triglycerides |
| WC | Waist Circumference |
| WHO | World Health Organization |
| WHR | Waist-to-Hip Ratio |
| WHtR | Waist-to-Height Ratio |
References
- Goday, P.S. Malnutrition in Children in Ressource-Limited Countries: Clinical Assessment; Motil, K.J., Hoppin, A.G., Eds.; Uptodate: Waltham, MA, USA, 2020. [Google Scholar]
- Barker, D.J. The developmental origins of adult disease. J. Am. Coll. Nutr. 2004, 23 (Suppl. 6), 5885–5995. [Google Scholar] [CrossRef]
- Roseboom, T.J.; van der Meulen, J.H.P.; Ravelli, A.C.J.; Osmond, C.; Barker, D.J.P.; Bleker, O.P. Effects of prenatal exposure to the Dutch famine on adult disease in later life: An overview. Mol. Cell. Endocrinol. 2001, 185, 93–98. [Google Scholar] [CrossRef]
- Bleker, L.S.; de Rooij, S.R.; Painter, R.C.; Ravelli, A.C.; Roseboom, T.J. Cohort profile: The Dutch famine birth cohort (DFBC)—A prospective birth cohort study in the Netherlands. BMJ Open 2021, 11, e042078. [Google Scholar] [CrossRef]
- Sawaya, A.L.; Sesso, R.; Florencio, T.M.; Fernandes, M.T.; Martins, P.A. Association between chronic undernutrition and hypertension. Matern. Child Nutr. 2005, 1, 155–163. [Google Scholar] [CrossRef]
- Victora, C.G.; Adair, L.; Fall, C.; Hallal, P.C.; Martorell, R.; Richter, L.; Sachdev, H.S. Maternal and child undernutrition: Consequences for adult health and human capital. Lancet N. Am. Ed. 2008, 371, 340–357. [Google Scholar] [CrossRef] [Green Version]
- Grey, K.; Gonzales, G.B.; Abera, M.; Lelijveld, N.; Thompson, D.; Berhane, M.; Abdissa, A.; Girma, T.; Kerac, M. Severe malnutrition or famine exposure in childhood and cardiometabolic non-communicable disease later in life: A systematic review. BMJ Glob. Health 2021, 6, e003161. [Google Scholar] [CrossRef]
- Asiki, G.; Newton, R.; Marions, L.; Kamali, A.; Smedman, L. The effect of childhood stunting and wasting on adolescent cardiovascular diseases risk and educational achievement in rural Uganda: A retrospective cohort study. Glob. Health Action 2019, 12, 1626184. [Google Scholar] [CrossRef]
- Lelijveld, N.; Seal, A.; Wells, J.C.; Kirkby, J.; Opondo, C.; Chimwezi, E.; Bunn, J.; Bandsma, R.; Heyderman, R.S.; Nyirenda, M.J.; et al. Chronic disease outcomes after severe acute malnutrition in Malawian children (ChroSAM): A cohort study. Lancet Glob. Health 2016, 4, e654–e662. [Google Scholar] [CrossRef] [Green Version]
- Mwene-Batu, P.; Bisimwa, G.; Ngaboyeka, G.; Dramaix, M.; Macq, J.; Hermans, M.P.; Lemogoum, D.; Donnen, P. Severe acute malnutrition in childhood, chronic diseases, and human capital in adulthood in the Democratic Republic of Congo: The Lwiro cohort study. Am. J. Clin. Nutr. 2021, 114, 70–79. [Google Scholar] [CrossRef]
- Francis-Emmanuel, P.M.; Thompson, D.S.; Barnett, A.T.; Osmond, C.; Byrne, C.D.; Hanson, M.A.; Gluckman, P.D.; Forrester, T.E.; Boyne, M.S. Glucose metabolism in adult survivors of severe acute malnutrition. J. Clin. Endocrinol. Metab. 2014, 99, 2233–2240. [Google Scholar] [CrossRef] [Green Version]
- Action Contre la Faim. Enquête Nutritionnelle Anthropométrique dans la Zone de Santé de MitiMurhesa, Province du Sud-Kivu, République Démocratique du Congo; Action contre la Faim: Bukavu, Congo, 2011. [Google Scholar]
- Mwene-Batu, P.; Bisimwa, G.; Ngaboyeka, G.; Dramaix, M.; Macq, J.; Lemogoum, D.; Donnen, P. Follow-up of a historic cohort of children treated for severe acute malnutrition between 1988 and 2007 in eastern Democratic Republic of Congo. PLoS ONE 2020, 15, e0229675. [Google Scholar] [CrossRef] [PubMed]
- Lemonnier, D.; Ingenbleek, Y. les carences nutritionnelles dans les pays en voie de développement. In Les Malnutritions Dans les Pays du Tiersmonde, Les Éditions; Institut National de la Santé et de la Recherche Médicale (INSERM): Paris, France, 1989. [Google Scholar]
- Paluku, B. Contribution à L’amelioration et à L’évaluation de la Prise en Charge Globale de L’enfant Hospitalisé en Afrique Centrale (Sud-Kivu); Université Libre de Bruxelles: Bruxelles, Belgium, 2002. [Google Scholar]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006; Available online: https://apps.who.int/iris/handle/10665/44129 (accessed on 26 September 2020).
- Mwene-Batu, P.; Lemogoum, D.; de le Hoye, L.; Bisimwa, G.; Hermans, M.P.; Minani, J.; Amani, G.; Mateso, G.-Q.; Cikomola, J.C.; Dramaix, M.; et al. Association between severe acute malnutrition during childhood and blood pressure during adulthood in the eastern Democratic Republic of the Congo: The Lwiro cohort study. BMC Public Health 2021, 21, 847. [Google Scholar] [CrossRef] [PubMed]
- Ministère du Plan et Suivi de la Mise en œuvre de la Révolution de la Modernité, Ministère de la Santé Publique, ICF International. Enquête Démographique et de Santé en République Démocratique du Congo 2013–2014; MEASURE DHS, ICF International: Rockville, MD, USA, 2014; Available online: http://dhsprogram.com/pubs/pdf/FR300/FR300.pdf (accessed on 15 October 2020).
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association (ADA). Standards of medical care in diabetes—2010. Diabetes Care 2010, 33, 11–61. [Google Scholar] [CrossRef] [Green Version]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redón, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension. The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2013, 31, 1281–1357. [Google Scholar] [CrossRef] [Green Version]
- Katchunga, P.B.; Cikomola, J.; Tshongo, C.; Baleke, A.; Kaishusha, D.; Mirindi, P.; Tamburhe, T.; Kluyskens, Y.; Sadiki, A.; Bwanamudogo, S.; et al. Obesity and diabetes mellitus association in rural community of Katana, South Kivu, in Eastern Democratic Republic of Congo: Bukavu Observ Cohort Study Results. BMC Endocr. Disord. 2016, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Longo-mbenza, B.; Lasi, J.B.K.; Okwe, A.N.; Kabangu, N.K. The metabolic syndrome in a Congolese population and its implications for metabolic syndrome definitions. Diabetes Metab. Syndr. Clin. Res. Rev. 2011, 5, 17–24. [Google Scholar] [CrossRef]
- Ashwell, M.; Gibson, S. Waist to Height Ratio Is a Simple and Effective Obesity Screening Tool for Cardiovascular Risk Factors: Analysis of Data from the British National Diet and Nutrition Survey of Adults Aged 19–64 Years. Eur. J. Obes. 2009, 2, 97–103. [Google Scholar] [CrossRef]
- Boursier, V. Le syndrome métabolique. J. Mal. Vasc. 2006, 31, 190–201. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation and T of HBC in A (Adult TPI). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection; National Cholesterol Education Program: Bethesda, MD, USA, 2002. [Google Scholar]
- Celis-morales, C.A.; Welsh, P.; Lyall, D.M.; Steell, L.; Petermann, F.; Anderson, J.; Iliodromiti, S.; Sillars, A.; Graham, N.; Mackey, D.F.; et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: Prospective cohort study of half a million UK Biobank participants. BMJ 2018, 361, k1651. [Google Scholar]
- World Food Programme. Food Consumption Analysis: Calculation and Use of the Food Consumption Score in Food Security Analysis; World Health Organization (WHO): Geneva, Switzerland, 2008. [Google Scholar]
- International Food Policy Research Institute (IFPRI). Validation du Score de Consommation Alimentaire du PAM par IFPRI. 2014. Available online: http://www.ifpri.org/sites/default/files/publications/ifpridp00870.pdf (accessed on 20 May 2022).
- Convention Cadre de l’OMS Pour la Lutte Antitabac; Recueil des Indicateurs: Genève, Switzerland, 2015.
- Belgherbi, S.; Mutatayi, C.; Palle, C. Les Repères de Consommation D’alcool: Les Standards mis en Question; Observatoire Français des Nouvelles Routes de la Soie: Paris, France, 2015; pp. 1–160. [Google Scholar]
- Lee, C.M.; Huxley, R.R.; Wildman, R.P.; Woodward, M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: A meta-analysis. J. Clin. Epidemiol. 2008, 61, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Bosomworth, N.J. Obésité centrale malgré un poids normal. Can. Fam. Physician 2019, 65, 251–260. [Google Scholar]
- Seidell, J.C.; Pérusse, L.; Després, J.P.; Bouchard, C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: The Quebec Family Study. Am. J. Clin. Nutr. 2001, 74, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Kajubi, S.K. The endocrine pancreas after kwashiorkor. Am. J. Clin. Nutr. 1972, 25, 1140–1142. [Google Scholar] [CrossRef]
- Becker, D.J.; Pimstone, B.L.; Hansen, J.D.; Hendricks, S. Insulin secretion in protein-calorie malnutrition. I. Quantitative abnormalities and response to treatment. Diabetes 1971, 20, 542–551. [Google Scholar] [CrossRef]
- Forrester, T.E.; Badaloo, A.V.; Boyne, M.S.; Osmond, C.; Thompson, D.; Green, C.; Taylor-Bryan, C.; Barnett, A.; Soares-Wynter, S.; Hanson, M.A.; et al. Prenatal factors contribute to the emergence of kwashiorkor or marasmus in severe undernutrition: Evidence for the predictive adaptation model. PLoS ONE 2012, 7, e35907. [Google Scholar] [CrossRef] [Green Version]
- McDonald, C.M.; Olofin, I.; Flaxman, S.; Fawzi, W.W.; Spiegelman, D.; Caulfield, L.E.; Black, R.E.; Ezzati, M.; Danaei, G.; Nutrition Impact Model Study. The effect of multiple anthropometric deficits on child mortality: Meta-analysis of individual data in 10 prospective studies from developing countries. Am. J. Clin. Nutr. 2013, 97, 896–901. [Google Scholar] [CrossRef] [Green Version]
| Marasmus | Kwashiorkor | Mixed-Type | Unexposed | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | ||
| Age (years) Mean (SD) | 142 | 22.68 (4.63) | 175 | 22.32 (4.03) | 207 | 21.98 (4.63) | 407 | 22.14 (4.62) | |
| Male gender | 53.5 | 49.7 | 53.1 | 50.6 | 0.848 | ||||
| Food consumption score | 142 | 175 | 207 | 407 | |||||
| Insufficient | 13.4 | 8.6 | 11.6 | 6.9 | |||||
| Bordeline | 37.3 | 42.3 | 38.2 | 31.7 | 0.011 | ||||
| Satisfactory | 49.3 | 49.1 1 | 50.2 1 | 61.4 | |||||
| Socioeconomic status | 124 | 164 | 184 | 357 | |||||
| Low | 64.5 | 60.4 | 66.8 | 55.5 | |||||
| Average | 32.3 | 36.0 | 32.0 | 37.8 | 0.069 | ||||
| High | 3.2 | 3.7 | 2.2 | 6.7 | |||||
| Marasmus | Kwashiorkor | Mixed-Type | Unexposed | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Dyslipidemia | 118 | 134 | 172 | 331 | ||||
| High LDL-C | 2.6 | 3.1 | 1.8 | 1.6 | ||||
| Low HDL-C | 39.8 | 26.92 | 46.5 1,2 | 34.1 | ||||
| High TG | 7.8 | 6.1 | 4.2 | 4.7 | ||||
| Diabetes mellitus (DM) | 107 | 8.4 | 129 | 7.8 | 162 | 11.7 | 319 | 7.5 |
| Hypertension | 104 | 7.7 | 125 | 5.8 | 156 | 5.7 | 301 | 6.6 |
| Metabolic syndrome | 92 | 12.0 | 108 | 8.3 | 132 | 12.1 1 | 265 | 4.9 |
| Overweight/Obesity | 141 | 12.8 | 167 | 18.0 | 201 | 9.0 | 396 | 13.1 |
| Visceral obesity | 136 | 52.9 | 161 | 54.0 | 195 | 50.8 | 372 | 43.8 |
| Android obesity | 142 | 61.3 | 171 | 64.9 | 203 | 72.4 1 | 405 | 54.6 |
| Cardio-Vascular Risk factor | 142 | 175 | 207 | 407 | ||||
| Alcohol (yes) | 35.2 | 32.6 | 39.1 | 40.3 | ||||
| Tobacco (yes) | 3.5 | 1.7 | 3.9 | 1.5 | ||||
| First-degree relative with HBP and/or DM (yes) | 34.5 | 34.9 | 28.5 | 32.9 | ||||
| Variable | Marasmus vs. Unexp | Kwash vs. Unexp | Mixed vs. Unexp | Kwash vs. Marasmus | Mixed-Form vs. Marasmus | Mixed-Type vs. Kawsh |
|---|---|---|---|---|---|---|
| Overweight/Obesity | ||||||
| aOR (95% CI) | 1.10 (0.50; 2.44) | 1.39 (0.70; 2.76) | 0.76 (0.35; 1.65) | 1.27 (0.53; 3.03) | 0.69 (0.27; 1.77) | 0.54 (0.23–1; 0.28) |
| Metabolic syndrome | ||||||
| aOR (95% CI) | 2.38 (0.68; 8.24) | 1.60 (0.45; 5.73) | 2.68 (1.18; 8.07) 1 | 0.67 (0.17; 2.61) | 1.13 (0.34; 3.71) | 1.67 (0.49; 5.67) |
| Hypertension | ||||||
| aOR (95% CI) | 1.32 (0.40; 4.25) | 0.74 (0.20; 2.67) | 0.83 (0.26; 2.65) | 0.56 (0.12; 2.49) | 0.63 (0.15; 2.50) | 1.12 (0.25; 4.90) |
| Diabetes | ||||||
| aOR (95% CI) | 0.84 (0.25; 2.77) | 0.93 (0.32; 2.66) | 1.34 (0.54; 3.29) | 1.1 (0.28; 4.30) | 1.59 (0.45; 5.50) | 1.43 (0.47; 4.37) |
| High TG | ||||||
| aOR (95% CI) | 1.78 (0.55; 5.73) | 1.25 (0.37; 4.14) | 0.92 (0.26; 3.23) | 0.69 (0.18; 2.66) | 0.51 (0.13; 2.05) | 0.74 (0.18; 3.03) |
| Low HDL-C | ||||||
| aOR (95% CI) | 1.31 (0.70; 2.44) | 0.64 (0.34; 1.21) | 1.52 (1.08; 2.62) 1 | 0.49 (0.23; 1.04) | 1.16 (0.58; 2.29) | 2.34 (1.18; 4.65) 2 |
| High LDL-C | ||||||
| aOR (95% CI) | 2.05 (0.28; 15.08) | 2.1 (0.34; 12.94) | 0.92 (0.09; 8.84) | 1.02 (0.12; 8.05) | 0.45 (0.03; 5.18) | 0.44 (0.04; 4.49) |
| Visceral Obesity | ||||||
| aOR (95% CI) | 1.59 (0.89; 2.85) | 1.42 (0.84; 2.42) | 1.28 (0.77; 2.13) | 0.89 (0.46; 1.73) | 0.8 (0.42; 1.52) | 0.89 (0.49; 1.63) |
| Android obesity | ||||||
| aOR (95% CI) | 1.43 (0.80; 2.55) | 1.35 (0.81; 2.28) | 1.89 (1.11; 3.21) 2 | 0.94 (0.48; 1.84) | 1.32 (0.68; 2.58) | 1.39 (0.76; 2.61) |
| Marasmus | Kwashiorkor | Mixed-Type | Unexposed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (Total) | % | Mean (SD) | N (Total) | % | Mean (SD) | N (Total) | % | Mean (SD) | N (Total) | % | Mean (SD) | |
| Anthropometry | ||||||||||||
| Weight (kg) | 141 | 53.5 (8.2) | 167 | 55.7 (7.4)2 | 201 | 51.4 (7.5)1,2 | 396 | 55.1 (7.2) | ||||
| Height (cm) | 142 | 156.1 (8.8) | 173 | 156.7 (9.1) | 205 | 154.9 (9.1)1 | 406 | 157.6 (8.8) | ||||
| Waist circumference (cm) | 142 | 79.4 (9.1) | 172 | 80.1 (9.2)1 | 205 | 78.0 (9.1) | 406 | 77.9 (8.2) | ||||
| Hip circumference (cm) | 142 | 84.7 (8.9) | 172 | 85.6 (8.4)2 | 203 | 83.3 (8.4) 1,2 | 405 | 86.0 (7.6) | ||||
| Waist-to-Hip ratio (WHR) | 142 | 0.94 (0.14) 1 | 171 | 0.93 (0.12)1 | 203 | 0.94 (0.11) 1 | 405 | 0.91 (0.11) | ||||
| Waist-to-Height ratio WHtR | 142 | 0.51 (0.06) | 172 | 0.51 (0.06)1 | 205 | 0.50 (0.06) | 406 | 0.49 (0.05) | ||||
| Muscle strength (Kg) | 106 | 30.7 (9.7) | 122 | 30.1 (8.4)1 | 157 | 29.3 (8.0) 1 | 303 | 32.8 (8.8) | ||||
| Body Mass Index (Kg/m2) | 141 | 21.9 (2.9) | 167 | 22.7 (2.8) 2 | 201 | 21.4 (2.7) 1,2 | 396 | 22.2 (2.5) | ||||
| Blood pressure (BP) mmHg | 105 | 125 | 156 | 301 | ||||||||
| Systolic BP | 119.2 (12.9) | 120.1 (13.6) | 117.0 (13.1) | 119.6 (13.2) | ||||||||
| Diastolic BP | 70.4 (10.6) | 71.6 (11.5) | 70.6 (10.1) | 71.6 (10.1) | ||||||||
| Mean BP | 86.7 (9.9) | 87.7 (10.4) | 86.1 (9.8) | 87.5 (9.5) | ||||||||
| Pulse pressure | 48.8 (11.9) | 48.5 (13.6) | 46.4 (10.9) | 47.9 (12.7) | ||||||||
| Glucose homeostasis | ||||||||||||
| Fasting glycemia (mg/dL) | 107 | 103.7 (17.1) | 129 | 103.2 (14.5) | 162 | 107.5 (17.3) 1 | 319 | 103.7 (14.5) | ||||
| HbA1c (%) | 30 | 4.6 (0.4) 1 | 30 | 4.7 (0.5) 1 | 30 | 4.6 (0.5) 1 | 52 | 4.1 (0.2) | ||||
| Lipids (mg/dL) | 118 | 134 | 172 | 331 | ||||||||
| Total cholesterol | 155.9 (35.8) | 159.5 (34.6) | 148.7 (35.5) 1 | 159.1 (36.6) | ||||||||
| Non-HDL-C | 112.5 (30.9) | 113.4 (29.5) | 106.3 (30.5) 1 | 114.6 (32.0) | ||||||||
| HDL-C | 43.4 (7.9) | 46.1 (9.0) 2 | 42.3 (8.1) 2 | 44.4 (8.4) | ||||||||
| LDL-C | 92.0 (30.6) | 93.6 (29.8) | 86.2 (30.7) 1 | 94.2 (31.2) | ||||||||
| TG 3 | 97.8 (74.6,128.3) 3 | 97.6 (74.5,127.6) 3 | 97.9 (75.1,128.9) 3 | 96.9 (74.7,126.4) 3 | ||||||||
| Creatinine (mg/dL) | 117 | 0.88 (0.18) | 133 | 0.86 (0.15) | 171 | 0.87 (0.16) | 331 | 0.88 (0.19) | ||||
| Albumin (mg/dL) | 118 | 4.4 (0.3) | 134 | 4.4 (0.3) | 172 | 4.3 (0.3) 1 | 328 | 4.4 (0.3) | ||||
| Thinness (BMI < 18.5) | 141 | 6.4 | 167 | 3.6 | 201 | 11.9 1 | 396 | 3.8 | ||||
| Variable | Marasmus vs. Unexp | Kwash vs. Unexp | Mixed vs. Unexp | Kwash vs. Marasmus | Mixed-type vs. Marasmus | Mixed-type vs. Kawsh |
|---|---|---|---|---|---|---|
| BMI (kg/m2) | −0.04 (−0.79; 0.72) | 0.50 (−0.19; 1.19) | −0.56 (−1.23; 0.10) | 0.54 (−0.2; 1.40) | −0.53 (−1.36; 0.31) | −1.07 (−1.85; −0.28) 2 |
| Weight (kg) | −1.25 (−3.2; 0.77) | 0.91 (−0.94; 2.77) | −3.05 (−4.83; −1.26) 3 | 2.16 (−0.15; 4.48) | −1.79 (−4.05; 0.45) | −3.96 (−6.07; −1.85) 3 |
| Height (cm) | −1.78 (−4.16; 0.59) | −0.52 (−2.68; 1.63) | −2.48 (−4.57; −0.4) 1 | 1.26 (−1.44; 3.97) | −0.7 (−3.34; 1.94) | −1.96 (−4.41; 0.48) |
| Waist circumference (cm) | 1.85 (−0.56; 4.27) | 1.99 (−0.19; 4.19) | 0.15 (−1.96; 2.27) | 0.14 (−2.61; 2.9) | −1.7 (−4.39; 0.98) | −1.84 (−4.34; 0.65) |
| Hip circumference (cm) | −1.38 (−3.62; 0.85) | −0.21 (−2.25; 1.81) | −2.27 (−4.24; −0.31) 1 | 1.16 (−1.39; 3.72) | −0.89 (−3.38; 1.59) | −2.05 (−4.37; 0.25) |
| Muscle strength (Kg) | −2.16 (−4.88; 0.56) | −2.35 (−4.88; 0.16) | −3.47 (−5.82; −1.11) 2 | −0.19 (−3.34; 2.95) | −1.30 (−4.31; 1.7) | −1.11 (−3.94; 1.72) |
| Glycemia (mg/dL) | −0.53 (−5.56; 4.5) | −0.38 (−4.96; 4.19) | 3.38 (0.92; 7.7) 1 | 0.14 (−5.63; 5.93) | 3.92 (−1.64; 9.48) | 3.77 (−1.38; 8.93) |
| HbA1c (%) | 0.49 (0.18; 0.8) 3 | 0.59 (0.29; 0.88) 3 | 0.46 (0.17; 0.76) 3 | 0.09 (−0.25; 0.44) | −0.03 (−0.38; 0.32) | −0.12 (−0.45; 0.2) |
| total Cholesterol (mg/dl) | −2.62 (−13.56; 8.30) | 0.28 (−9.84; 10.41) | −7.96 (−17.54; 1.61) | 2.91 (−9.68; 15.5) | −5.33 (−17.48; 6.8) | −8.25 (−19.6; 3.19) |
| HDL-C (mg/dL) | −1.29 (−3.88; 1.3) | 1.65 (−0.75; 4.05) | −1.86 (−4.14; 0.4) | 2.94 (−0.05; 5.93) | −0.57 (−3.45; 2.3) | −3.51 (−6.23; −0.8) 2 |
| Albumin (mg/dL) | −0.00 (−0.10; 0.09) | −0.02 (−0.11; 0.06) | −0.07 (−0.16; 0.01) | −0.02 (−0.13; 0.09) | −0.06 (−0.18; 0.04) | −0.04 (−0.15; 0.05) |
| Systolic pressure | −0.87 (−5.09; 3.34) | 0.03 (−3.83; 3.9) | −2.07 (−5.72; 1.58) | 0.91 (−3.93; 5.76) | −1.19 (−5.86; 3.47) | −2.10 (−6.46; 2.25) |
| Diastolic pressure | −1.27 (−4.54; 1.99) | −1.08 (−4.08; 1.91) | −0.77 (−3.6; 2.06) | 0.19 (−3.56; 3.95) | 0.49 (−3.12; 4.11) | 0.3 (−3.07; 3.68) |
| Pulse Pressure | 0.39 (−3.55; 4.34) | 1.11 (−2.50; 4.73) | −1.29 (−4.71; 2.12) | 0.72 (−3.81; 5.25) | −1.68 (−6.06; 2.68) | −2.41 (−6.49; 1.66) |
| Mean Pressure | −1.14 (−4.24; 1.95) | −0.71 (−3.55; 2.13) | −1.20 (−3.89; 1.47) | 0.43 (−3.13; 3.99) | −0.06 (−3.49; 3.36) | −0.49 (−3.69; 2.7) |
| Non-HDL-C | −1.14 (−4.24; 1.95) | −0.71 (−3.55; 2.13) | −1.2 (−3.89; 1.47) | 0.43 (−3.13; 3.99) | −0.06 (−3.49; 3.36) | −0.49 (−3.69; 2.7) |
| Creatinine | 0.00 (−0.04; 0.05) | −0.01 (−0.06; 0.03) | 0.00 (−0.04; 0.04) | −0.01 (−0.07; 0.04) | −0.00 (−0.06; 0.05) | 0.01 (−0.04; 0.07) |
| TG (mg/dL) | 1.01 (0.97; 1.04) | 1.00 (0.96, 1.05) | 1.01 (0.96, 1.05) | −0.21 (−0.71; 2.32) | 0.01 (−0.04; 1.92) | −0.97 (−2.24; 3.05) |
| LDL-C (mg/dL) | −1.48 (−10.92; 7.96) | −0.55 (−9.31; 8.21) | −5.58 (−13.92; 2.75) | 0.92 (−9.93; 11.79) | −4.10 (−14.60; 6.39) | −5.03 (−14.95; 4.89) |
| WHR | 0.04 (0.00; 0.07) 2 | 0.02 (−0.00; 0.05) | 0.02 (−0.00; 0.05) | −0.01 (−0.05; 0.02) | −0.01 (−0.05; 0.02) | 0.00 (−0.03; 0.03) |
| WHtR | 0.01 (0.00; 0.03) 1 | 0.01 (−0.00; 0.02) | 0.00 (−0.00; 0.02) | −0.00 (−0.02; 0.01) | −0.00 (−0.02; 0.01) | −0.00 (−0.02; 0.01) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwene-Batu, P.; Bisimwa, G.; Donnen, P.; Bisimwa, J.; Tshongo, C.; Dramaix, M.; Hermans, M.P.; Briend, A. Risk of Chronic Disease after an Episode of Marasmus, Kwashiorkor or Mixed–Type Severe Acute Malnutrition in the Democratic Republic of Congo: The Lwiro Follow-Up Study. Nutrients 2022, 14, 2465. https://doi.org/10.3390/nu14122465
Mwene-Batu P, Bisimwa G, Donnen P, Bisimwa J, Tshongo C, Dramaix M, Hermans MP, Briend A. Risk of Chronic Disease after an Episode of Marasmus, Kwashiorkor or Mixed–Type Severe Acute Malnutrition in the Democratic Republic of Congo: The Lwiro Follow-Up Study. Nutrients. 2022; 14(12):2465. https://doi.org/10.3390/nu14122465
Chicago/Turabian StyleMwene-Batu, Pacifique, Ghislain Bisimwa, Philippe Donnen, Jocelyne Bisimwa, Christian Tshongo, Michelle Dramaix, Michel P. Hermans, and André Briend. 2022. "Risk of Chronic Disease after an Episode of Marasmus, Kwashiorkor or Mixed–Type Severe Acute Malnutrition in the Democratic Republic of Congo: The Lwiro Follow-Up Study" Nutrients 14, no. 12: 2465. https://doi.org/10.3390/nu14122465
APA StyleMwene-Batu, P., Bisimwa, G., Donnen, P., Bisimwa, J., Tshongo, C., Dramaix, M., Hermans, M. P., & Briend, A. (2022). Risk of Chronic Disease after an Episode of Marasmus, Kwashiorkor or Mixed–Type Severe Acute Malnutrition in the Democratic Republic of Congo: The Lwiro Follow-Up Study. Nutrients, 14(12), 2465. https://doi.org/10.3390/nu14122465






