Correlation between Serum 25-Hydroxyvitamin D Level and Peripheral Arterial Stiffness in Chronic Kidney Disease Stage 3–5 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Anthropometric and Biochemical Analysis
2.3. Measurement of Brachial-Ankle Pulse Wave Velocity
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stevens, P.E.; Levin, A.; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Karras, A.; Haymann, J.P.; Bozec, E.; Metzger, M.; Jacquot, C.; Maruani, G.; Houillier, P.; Froissart, M.; Stengel, B.; Guardiola, P.; et al. Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension 2012, 60, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Song, T.J.; Song, D.; Lee, K.J.; Kim, E.H.; Lee, H.S.; Nam, C.M.; Nam, H.S.; Kim, Y.D.; Heo, J.H. Brachial-ankle pulse wave velocity is a strong predictor for mortality in patients with acute stroke. Hypertension 2014, 64, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.S.; Li, Y.; Li, L.H.; Huang, Q.F.; Zeng, W.F.; Kang, Y.Y.; Zhang, L.; Liu, M.; Wei, F.F.; Li, G.L.; et al. Brachial-ankle pulse wave velocity as a predictor of mortality in elderly Chinese. Hypertension 2014, 64, 1124–1130. [Google Scholar] [CrossRef]
- Tomiyama, H.; Koji, Y.; Yambe, M.; Shiina, K.; Motobe, K.; Yamada, J.; Shido, N.; Tanaka, N.; Chikamori, T.; Yamashina, A. Brachial—Ankle pulse wave velocity is a simple and independent predictor of prognosis in patients with acute coronary syndrome. Circ. J. 2005, 69, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Luo, Q.; Zhu, B.X.; Zhou, F.F. Brachial-ankle pulse wave velocity could be a predictor of mortality in patients on peritoneal dialysis. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2018, 38, 215–219. [Google Scholar] [CrossRef]
- Chen, S.C.; Chang, J.M.; Liu, W.C.; Tsai, Y.C.; Tsai, J.C.; Hsu, P.C.; Lin, T.H.; Lin, M.Y.; Su, H.M.; Hwang, S.J.; et al. Brachial-ankle pulse wave velocity and rate of renal function decline and mortality in chronic kidney disease. Clin. J. Am. Soc. Nephrol. CJASN 2011, 6, 724–732. [Google Scholar] [CrossRef]
- Xie, S.; Huang, L.; Cao, W.; Hu, Y.; Sun, H.; Cao, L.; Liu, K.; Liu, C. Association between serum 25-hydroxyvitamin D and diabetic kidney disease in Chinese patients with type 2 diabetes. PLoS ONE 2019, 14, e0214728. [Google Scholar] [CrossRef]
- Damasiewicz, M.J.; Magliano, D.J.; Daly, R.M.; Gagnon, C.; Lu, Z.X.; Ebeling, P.R.; Chadban, S.J.; Atkins, R.C.; Kerr, P.G.; Shaw, J.E.; et al. 25-Hydroxyvitamin D levels and chronic kidney disease in the AusDiab (Australian Diabetes, Obesity and Lifestyle) study. BMC Nephrol. 2012, 13, 55. [Google Scholar] [CrossRef]
- Choi, S.W.; Kweon, S.S.; Lee, Y.H.; Ryu, S.Y.; Nam, H.S.; Park, K.S.; Kim, S.A.; Shin, M.H. 25-Hydroxyvitamin D is associated with kidney function: The Dong-gu study. J. Nutr. Sci. Vitaminol. 2018, 64, 385–390. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, D.; Xu, B.; Huang, C.; Yang, S.; Wang, W.; Liu, W.; Deng, Y.; Li, K.; Liu, D.; et al. Association of serum 25-hydroxyvitamin D with cardiovascular outcomes and all-cause mortality in individuals with prediabetes and diabetes: Results from the UK biobank prospective cohort study. Diabetes Care 2022, 45, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, J.; Cheung, A.K.; Kaufman, J.S.; Greene, T.; Roberts, W.L.; Smits, G.; Chonchol, M.; HOST (Homocysteinemia in Kidney and End Stage Renal Disease) study investigators. Associations of plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D concentrations with death and progression to maintenance dialysis in patients with advanced kidney disease. Am. J. Kidney Dis. 2012, 60, 567–575. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am. J. Kidney Dis. 2003, 42 (4 Suppl. S3), S1–S201. [Google Scholar]
- Carthy, E.P.; Yamashita, W.; Hsu, A.; Ooi, B.S. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension 1989, 13, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Molinari, C.; Uberti, F.; Grossini, E.; Vacca, G.; Carda, S.; Invernizzi, M.; Cisari, C. 1α,25-dihydroxycholecalciferol induces nitric oxide production in cultured endothelial cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2011, 27, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Andrukhova, O.; Slavic, S.; Zeitz, U.; Riesen, S.C.; Heppelmann, M.S.; Ambrisko, T.D.; Markovic, M.; Kuebler, W.M.; Erben, R.G. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol. Endocrinol. 2014, 28, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Mayer, O., Jr.; Filipovský, J.; Seidlerová, J.; Vaněk, J.; Dolejšová, M.; Vrzalová, J.; Cífková, R. The association between low 25-hydroxyvitamin D and increased aortic stiffness. J. Hum. Hypertens. 2012, 26, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Kuloğlu, O.; Gür, M.; Şeker, T.; Kalkan, G.Y.; Şahin, D.Y.; Tanboğa, I.H.; Koyunsever, N.Y.; Harbaloğlu, H.; Türkoğlu, C.; Akyol, S.; et al. Serum 25-hydroxyvitamin D level is associated with arterial stiffness, left ventricle hypertrophy, and inflammation in newly diagnosed hypertension. J. Investig. Med. 2013, 61, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Giallauria, F.; Milaneschi, Y.; Tanaka, T.; Maggio, M.; Canepa, M.; Elango, P.; Vigorito, C.; Lakatta, E.G.; Ferrucci, L.; Strait, J. Arterial stiffness and vitamin D levels: The Baltimore longitudinal study of aging. J. Clin. Endocrinol. Metab. 2012, 97, 3717–3723. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, G.Y.; Gür, M.; Koyunsever, N.Y.; Şeker, T.; Gözükara, M.Y.; Uçar, H.; Kaypaklı, O.; Baykan, A.O.; Akyol, S.; Türkoğlu, C.; et al. Serum 25-hydroxyvitamin d level and aortic intima-media thickness in patients without clinical manifestation of atherosclerotic cardiovascular disease. J. Clin. Lab. Anal. 2015, 29, 305–311. [Google Scholar] [CrossRef]
- Akdam, H.; Alp, A. Arterial stiffness and 25-hydroxyvitamin D levels in chronic kidney disease patients. Rev. Assoc. Med. Bras. 2017, 63, 910–916. [Google Scholar] [CrossRef]
- García-Canton, C.; Bosch, E.; Ramírez, A.; Gonzalez, Y.; Auyanet, I.; Guerra, R.; Perez, M.A.; Fernández, E.; Toledo, A.; Lago, M.; et al. Vascular calcification and 25-hydroxyvitamin D levels in non-dialysis patients with chronic kidney disease stages 4 and 5. Nephrol. Dial. Transplant. 2011, 26, 2250–2256. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2019. Diabetes Care 2019, 42 (Suppl. S1), S13–S28. [Google Scholar]
- Tomiyama, H.; Shiina, K. State of the art review: Brachial-ankle PWV. J. Atheroscler. Thromb. 2020, 27, 621–636. [Google Scholar] [CrossRef]
- Tanaka, A.; Tomiyama, H.; Maruhashi, T.; Matsuzawa, Y.; Miyoshi, T.; Kabutoya, T.; Kario, K.; Sugiyama, S.; Munakata, M.; Ito, H.; et al. Physiological diagnostic criteria for vascular failure. Hypertension 2018, 72, 1060–1071. [Google Scholar] [CrossRef]
- London, G.M.; Safar, M.E.; Pannier, B. Aortic aging in ESRD: Structural, hemodynamic, and mortality implications. J. Am. Soc. Nephrol. 2016, 27, 1837–1846. [Google Scholar] [CrossRef]
- Kim, E.K.; Chang, S.A.; Jang, S.Y.; Choi, K.H.; Huh, E.H.; Kim, J.H.; Kim, S.M.; Choe, Y.H.; Kim, D.K. Brachial-ankle pulse wave velocity as a screen for arterial stiffness: A comparison with cardiac magnetic resonance. Yonsei Med. J. 2015, 56, 617–624. [Google Scholar] [CrossRef][Green Version]
- Kim, H.L.; Lim, W.H.; Seo, J.B.; Kim, S.H.; Zo, Z.H.; Kim, M.A. Prediction of cardiovascular events using brachial-ankle pulse wave velocity in hypertensive patients. J. Clin. Hypertens. 2020, 22, 1659–1665. [Google Scholar] [CrossRef]
- Ohkuma, T.; Ninomiya, T.; Tomiyama, H.; Kario, K.; Hoshide, S.; Kita, Y.; Inoguchi, T.; Maeda, Y.; Kohara, K.; Tabara, Y.; et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: An Individual participant data meta-analysis. Hypertension 2017, 69, 1045–1052. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Terentes-Printzios, D.; Ioakeimidis, N.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension 2012, 60, 556–562. [Google Scholar] [CrossRef]
- Tanaka, H.; Munakata, M.; Kawano, Y.; Ohishi, M.; Shoji, T.; Sugawara, J.; Tomiyama, H.; Yamashina, A.; Yasuda, H.; Sawayama, T.; et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J. Hypertens. 2009, 27, 2022–2027. [Google Scholar] [CrossRef]
- Tsuchikura, S.; Shoji, T.; Kimoto, E.; Shinohara, K.; Hatsuda, S.; Koyama, H.; Emoto, M.; Nishizawa, Y. Brachial-ankle pulse wave velocity as an index of central arterial stiffness. J. Atheroscler. Thromb. 2010, 17, 658–665. [Google Scholar] [CrossRef]
- Yu, W.C.; Chuang, S.Y.; Lin, Y.P.; Chen, C.H. Brachial-ankle vs. carotid-femoral pulse wave velocity as a determinant of cardiovascular structure and function. J. Hum. Hypertens. 2008, 22, 24–31. [Google Scholar] [CrossRef]
- Cheng, Y.B.; Li, Y.; Sheng, C.S.; Huang, Q.F.; Wang, J.G. Quantification of the interrelationship between brachial-ankle and carotid-femoral pulse wave velocity in a workplace population. Pulse 2016, 3, 253–262. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Fahrleitner, A.; Dobnig, H.; Obernosterer, A.; Pilger, E.; Leb, G.; Weber, K.; Kudlacek, S.; Obermayer-Pietsch, B.M. Vitamin D deficiency and secondary hyperparathyroidism are common complications in patients with peripheral arterial disease. J. Gen. Intern. Med. 2002, 17, 663–669. [Google Scholar] [CrossRef][Green Version]
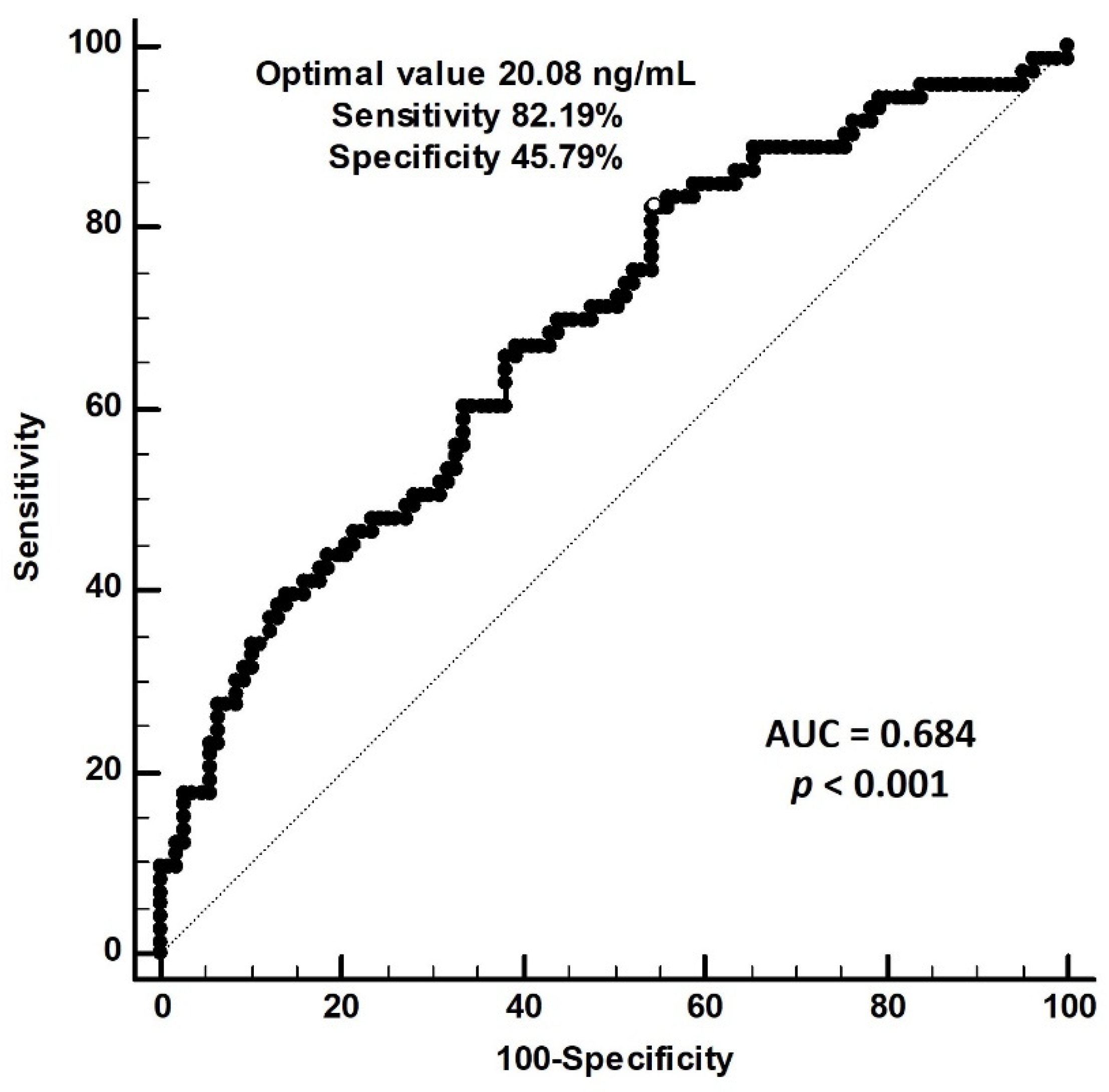
| Characteristics | All Patients (n = 180) | Control Group (n = 107) | PAS Group (n = 73) | p Value |
|---|---|---|---|---|
| Age (years) | 68.39 ± 14.30 | 63.11 ± 13.89 | 76.12 ± 11.05 | <0.001 * |
| Height (cm) | 159.12 ± 8.82 | 159.99 ± 7.96 | 157.84 ± 9.86 | 0.110 |
| Female, n (%) | 81 (45.0) | 43 (40.2) | 38 (52.1) | 0.116 |
| Blood urea nitrogen (mg/dL) | 40.44 (25.25–48.75) | 40.96 (25.00–49.00) | 39.68 (26.00–48.00) | 0.742 |
| Creatinine (mg/dL) | 2.56 (1.50–2.80) | 2.47 (1.40–2.70) | 2.70 (1.60–3.25) | 0.409 |
| eGFR (mL/min) | 30.45 ± 15.25 | 32.83 ± 15.37 | 26.96 ± 14.47 | 0.011 * |
| CKD stage 3, n (%) | 82 (45.6) | 55 (51.4) | 27 (37.0) | 0.129 |
| CKD stage 4, n (%) | 63 (35.0) | 35 (32.7) | 28 (38.4) | |
| CKD stage 5, n (%) | 35 (19.4) | 17 (15.9) | 18 (24.6) | |
| Diabetes mellitus, n (%) | 65 (36.1) | 32 (29.9) | 33 (45.2) | 0.036 * |
| Hypertension, n (%) | 148 (82.2) | 87 (81.3) | 61 (83.6) | 0.698 |
| Body weight (kg) | 66.01 ± 14.41 | 67.23 ± 14.26 | 64.22 ± 14.53 | 0.169 |
| Body mass index (kg/m2) | 25.94 ± 4.52 | 26.16 ± 4.68 | 25.62 ± 4.29 | 0.435 |
| Left baPWV (m/s) | 17.39 ± 3.83 | 14.89 ± 1.86 | 21.06 ± 2.91 | <0.001 * |
| Right baPWV (m/s) | 17.29 ± 3.77 | 14.89 ± 1.91 | 20.79 ± 3.01 | <0.001 * |
| SBP (mmHg) | 149.91 ± 25.52 | 141.55 ± 23.57 | 160.68 ± 24.55 | <0.001 * |
| DBP (mmHg) | 84.49 ± 14.47 | 82.34 ± 13.49 | 87.66 ± 15.34 | 0.015 * |
| Total cholesterol (mg/dL) | 159.87 ± 43.59 | 158.83 ± 45.76 | 161.38 ± 40.46 | 0.701 |
| Triglyceride (mg/dL) | 137.47 (83.25–166.25) | 138.35 (82.00–172.00) | 136.19 (83.50–157.50) | 0.973 |
| LDL-C (mg/dL) | 90.24 ± 36.87 | 90.71 ± 38.47 | 89.55 ± 34.64 | 0.836 |
| Fasting glucose (mg/dL) | 117.40 (93.00–127.75) | 113.03 (92.00–120.00) | 123.81 (94.50–139.00) | 0.015 * |
| Total calcium (mg/dL) | 8.97 ± 0.57 | 8.95 ± 0.54 | 9.01 ± 0.62 | 0.495 |
| Phosphorus (mg/dL) | 3.68 ± 0.72 | 3.63 ± 0.62 | 3.76 ± 0.85 | 0.216 |
| 25-hydroxyvitamin D (ng/mL) | 18.15 ± 5.87 | 19.65 ± 5.41 | 15.96 ± 5.87 | <0.001 * |
| iPTH (pg/mL) | 47.14 (30.58–54.80) | 43.02 (27.00–53.30) | 53.18 (36.65–60.00) | 0.009 * |
| CRP (mg/dL) | 0.81 (0.08–0.98) | 0.51 (0.06–0.79) | 1.25 (0.17–1.25) | <0.001 * |
| Current smoking, n (%) | 20 (11.1) | 12 (11.2) | 8 (11.0) | 0.957 |
| ARB use, n (%) | 112 (62.2) | 68 (63.6) | 44 (60.3) | 0.656 |
| β-blocker use, n (%) | 54 (30.0) | 29 (27.1) | 25 (34.2) | 0.304 |
| CCB use, n (%) | 80 (44.4) | 46 (43.0) | 34 (46.6) | 0.635 |
| α-adrenergic blocker use, n (%) | 26 (14.4) | 15 (14.0) | 11 (15.1) | 0.844 |
| Statin use, n (%) | 80 (44.4) | 45 (42.1) | 35 (47.9) | 0.435 |
| Fibrate use, n (%) | 31 (17.2) | 21 (19.6) | 10 (13.7) | 0.301 |
| Variables | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| 25-hydroxyvitamin D, 1 ng/mL | 0.895 | 0.828–0.968 | 0.005 * |
| Age, 1 year | 1.140 | 1.088–1.194 | <0.001 * |
| Diabetes mellitus, present | 2.445 | 0.886–6.752 | 0.084 |
| Systolic blood pressure, 1 mmHg | 1.017 | 0.989–1.046 | 0.231 |
| Diastolic blood pressure, 1 mmHg | 1.048 | 0.994–1.104 | 0.080 |
| Fasting glucose, 1 mg/dL | 1.001 | 0.991–1.011 | 0.832 |
| Estimated glomerular filtration rate, 1 mL/min | 0.987 | 0.954–1.020 | 0.434 |
| Intact parathyroid hormone, 1 pg/mL | 1.009 | 0.984–1.034 | 0.479 |
| C-reactive protein, 1 mg/dL | 1.444 | 0.885–2.356 | 0.142 |
| Correlation p Value | 25-Hydroxyvitamin D | Age | BMI | eGFR | Calcium | Phosphorus | iPTH # |
|---|---|---|---|---|---|---|---|
| 25-hydroxyvitamin D | −0.011 | −0.177 | 0.138 | 0.052 | 0.020 | −0.203 | |
| 0.880 | 0.018 * | 0.065 | 0.485 | 0.786 | 0.006 * | ||
| Age | −0.011 | −0.035 | −0.040 | −0.014 | −0.022 | 0.044 | |
| 0.880 | 0.644 | 0.592 | 0.850 | 0.774 | 0.554 | ||
| BMI | −0.177 | −0.035 | 0.042 | 0.017 | −0.089 | −0.029 | |
| 0.018 * | 0.644 | 0.580 | 0.817 | 0.237 | 0.701 | ||
| eGFR | 0.138 | −0.040 | 0.042 | 0.142 | −0.490 | −0.593 | |
| 0.065 | 0.592 | 0.580 | 0.058 | <0.001 * | <0.001 * | ||
| Calcium | 0.052 | −0.014 | 0.017 | 0.142 | 0.008 | −0.115 | |
| 0.485 | 0.850 | 0.817 | 0.058 | 0.916 | 0.124 | ||
| Phosphorus | 0.020 | −0.022 | −0.089 | −0.490 | 0.008 | 0.318 | |
| 0.786 | 0.774 | 0.237 | <0.001 * | 0.916 | <0.001 * | ||
| iPTH # | −0.203 | 0.044 | −0.029 | −0.593 | −0.115 | 0.318 | |
| 0.006 * | 0.554 | 0.701 | <0.001 * | 0.124 | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-J.; Hsieh, Y.-J.; Lin, Y.-L.; Wang, C.-H.; Hsu, B.-G.; Tsai, J.-P. Correlation between Serum 25-Hydroxyvitamin D Level and Peripheral Arterial Stiffness in Chronic Kidney Disease Stage 3–5 Patients. Nutrients 2022, 14, 2429. https://doi.org/10.3390/nu14122429
Lee C-J, Hsieh Y-J, Lin Y-L, Wang C-H, Hsu B-G, Tsai J-P. Correlation between Serum 25-Hydroxyvitamin D Level and Peripheral Arterial Stiffness in Chronic Kidney Disease Stage 3–5 Patients. Nutrients. 2022; 14(12):2429. https://doi.org/10.3390/nu14122429
Chicago/Turabian StyleLee, Chung-Jen, Yi-Jen Hsieh, Yu-Li Lin, Chih-Hsien Wang, Bang-Gee Hsu, and Jen-Pi Tsai. 2022. "Correlation between Serum 25-Hydroxyvitamin D Level and Peripheral Arterial Stiffness in Chronic Kidney Disease Stage 3–5 Patients" Nutrients 14, no. 12: 2429. https://doi.org/10.3390/nu14122429
APA StyleLee, C.-J., Hsieh, Y.-J., Lin, Y.-L., Wang, C.-H., Hsu, B.-G., & Tsai, J.-P. (2022). Correlation between Serum 25-Hydroxyvitamin D Level and Peripheral Arterial Stiffness in Chronic Kidney Disease Stage 3–5 Patients. Nutrients, 14(12), 2429. https://doi.org/10.3390/nu14122429









