Camellia (Camellia oleifera Abel.) Seed Oil Regulating of Metabolic Phenotype and Alleviates Dyslipidemia in High Fat-Fed Mice through Serum Branch-Chain Amino Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fatty Acids Profile of CO
2.2. Animal Procedure
2.3. Serum Lipid Biomarkers Parameters
2.4. BCAA Concentration Assay
2.5. RT-PCR and Western Blot Analysis
2.6. Metabolomics Analyses of Serum
2.7. Statistical Analyses
3. Results
3.1. Fatty Acids Profile of CO
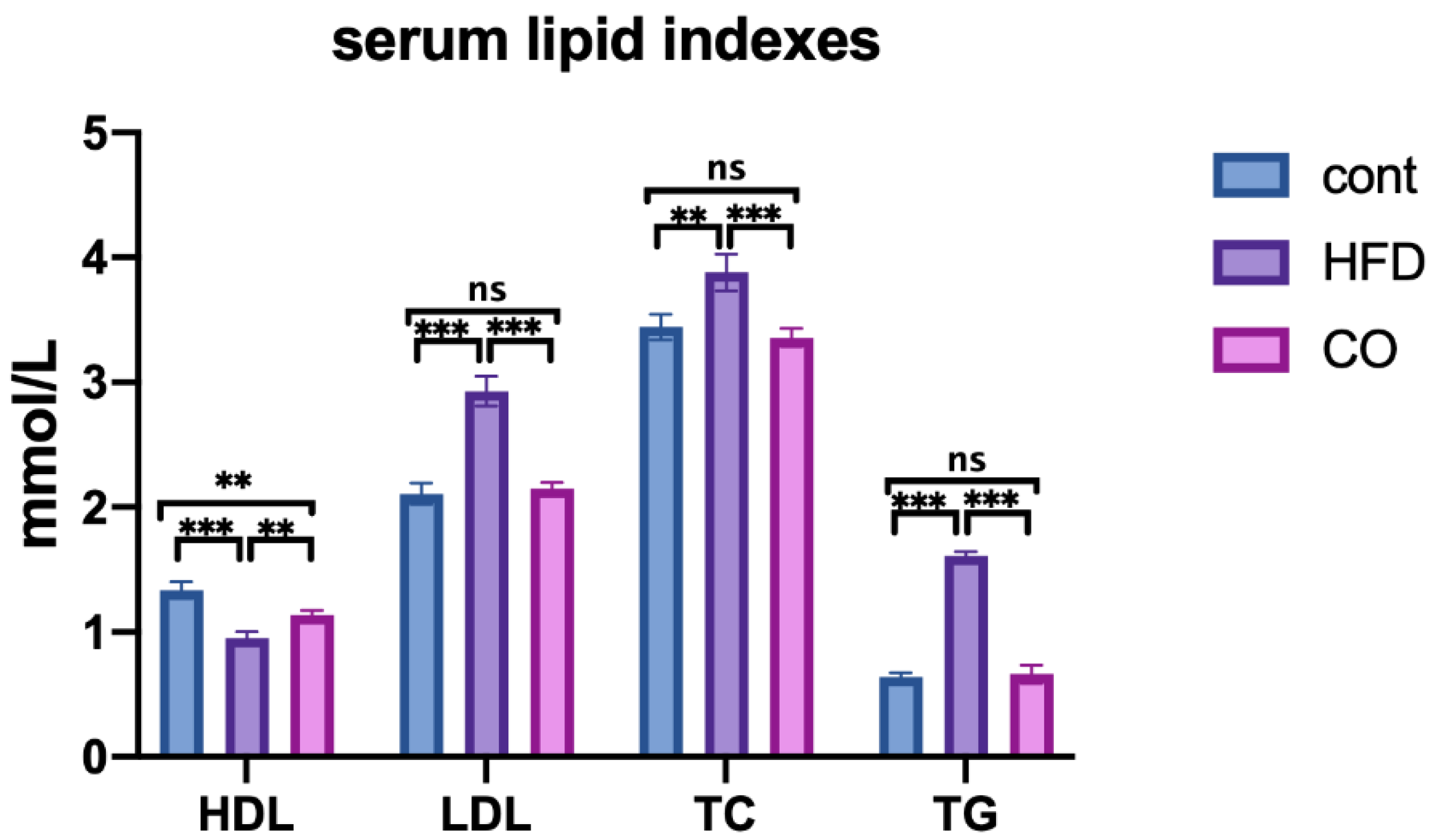
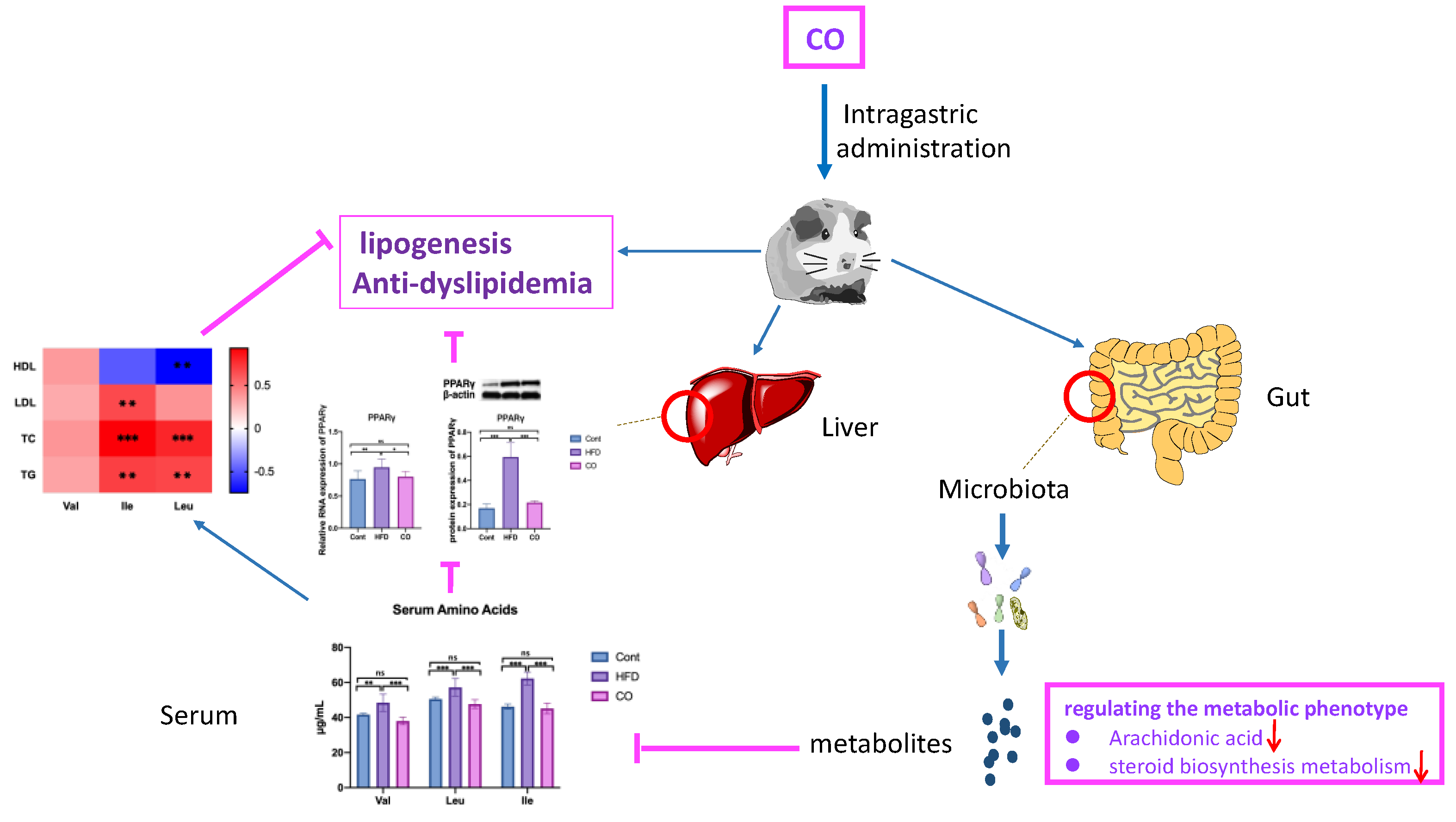
3.2. CO Alleviates Dyslipidemia of HFD-Fed Mice
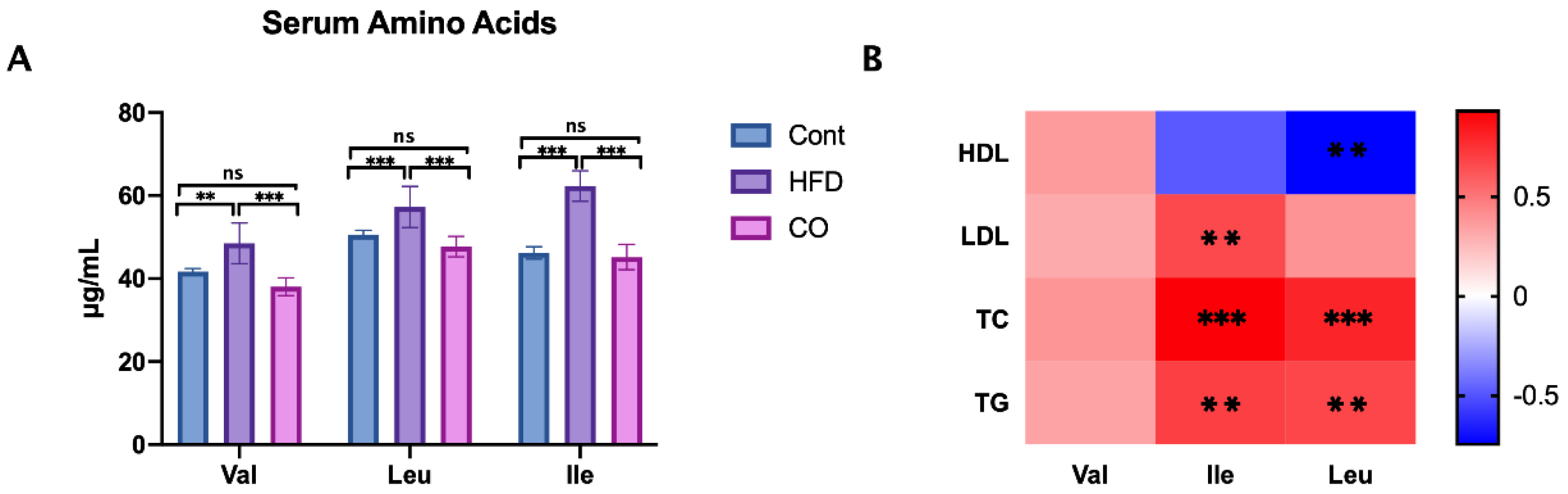
3.3. CO Inhibits the Serum Concentration of BCAA in HFD-Fed Mice
3.4. CO Affected the Expression of PPARγ in HFD-Fed Mice
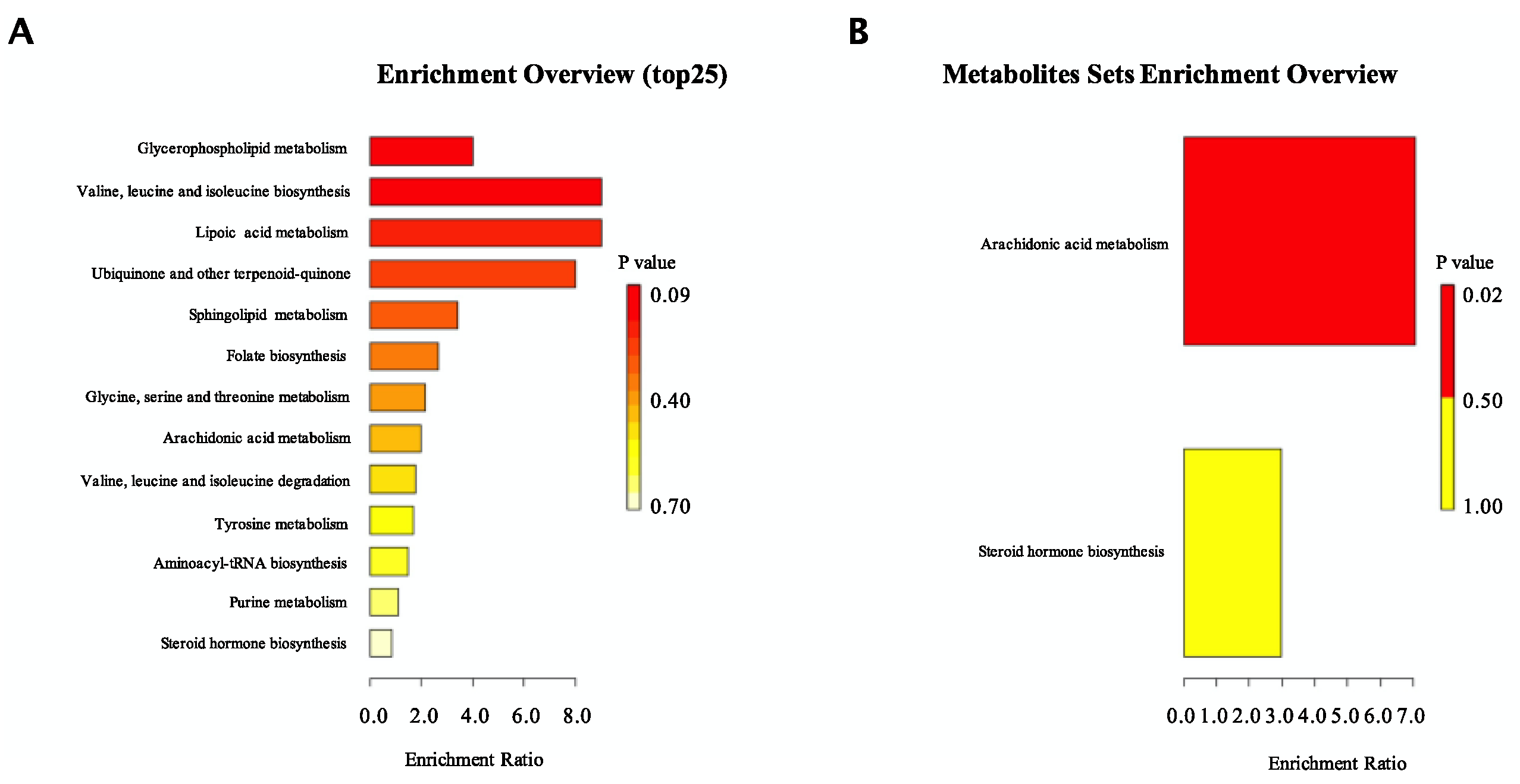
3.5. CO Altered the Metabolic Phenotype of Serum in HFD-Fed Mice
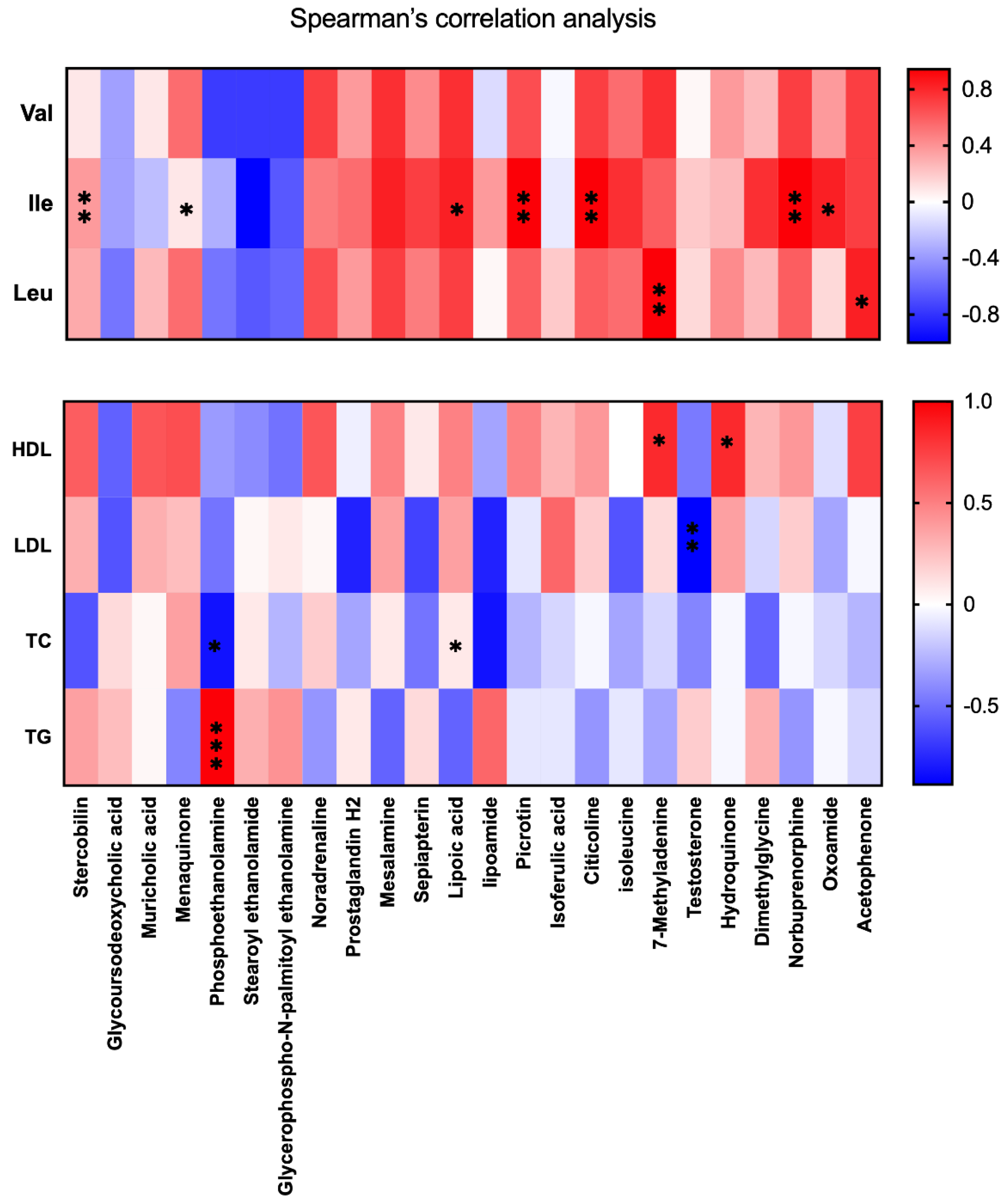
3.6. BCAA Correlated with Metabolic Phenotype and Serum Lipid Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kopin, L.; Lowenstein, C. Dyslipidemia. Ann. Intern. Med. 2017, 167, ITC81–ITC96. [Google Scholar] [CrossRef] [PubMed]
- Lazarte, J.; Hegele, R.A. Dyslipidemia Management in Adults With Diabetes. Can. J. Diabetes 2020, 44, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Whaley-Connell, A.; Sowers, J.R. Diabetic cardiomyopathy: A hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia 2018, 61, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sunil, B.; Ashraf, A.P. Dyslipidemia in Pediatric Type 2 Diabetes Mellitus. Curr. Diab. Rep. 2020, 20, 53. [Google Scholar] [CrossRef]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metabolism 2019, 92, 71–81. [Google Scholar] [CrossRef]
- Trautwein, E.A.; McKay, S. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients 2020, 12, 2671. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Garruti, G.; Fruhbeck, G.; de Angelis, M.; de Bari, O.; Wang, D.Q.; Lammert, F.; Portincasa, P. The Role of Diet in the Pathogenesis of Cholesterol Gallstones. Curr. Med. Chem. 2019, 26, 3620–3638. [Google Scholar] [CrossRef]
- Pastor, R.; Bouzas, C.; Tur, J.A. Beneficial effects of dietary supplementation with olive oil, oleic acid, or hydroxytyrosol in metabolic syndrome: Systematic review and meta-analysis. Free Radic Biol. Med. 2021, 172, 372–385. [Google Scholar] [CrossRef]
- Unhapipatpong, C.; Shantavasinkul, P.C.; Kasemsup, V.; Siriyotha, S.; Warodomwichit, D.; Maneesuwannarat, S.; Vathesatogkit, P.; Sritara, P.; Thakkinstian, A. Tropical Oil Consumption and Cardiovascular Disease: An Umbrella Review of Systematic Reviews and Meta Analyses. Nutrients 2021, 13, 1549. [Google Scholar] [CrossRef]
- Li, C.X.; Shen, L.R. New observations on the effect of camellia oil on fatty liver disease in rats. J. Zhejiang Univ. Sci. B 2020, 21, 657–667. [Google Scholar] [CrossRef]
- Xiao, F.; Du, Y.; Lv, Z.; Chen, S.; Zhu, J.; Sheng, H.; Guo, F. Effects of essential amino acids on lipid metabolism in mice and humans. J. Mol. Endocrinol. 2016, 57, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Wahl, D.; Clark, X.; Bagley, E.; Gregoriou, G.C.; Senior, A.M.; Wang, Q.P.; Brandon, A.E.; et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 2019, 1, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Neinast, M.; Murashige, D.; Arany, Z. Branched Chain Amino Acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Hu, W.; Fu, Z.; Sun, L.; Zhou, Y.; Gong, Y.; Yang, T.; Zhou, H. The positive association of branched-chain amino acids and metabolic dyslipidemia in Chinese Han population. Lipids Health Dis. 2016, 15, 120. [Google Scholar] [CrossRef]
- Yamakado, M.; Nagao, K.; Imaizumi, A.; Tani, M.; Toda, A.; Tanaka, T.; Jinzu, H.; Miyano, H.; Yamamoto, H.; Daimon, T.; et al. Plasma Free Amino Acid Profiles Predict Four-Year Risk of Developing Diabetes, Metabolic Syndrome, Dyslipidemia, and Hypertension in Japanese Population. Sci. Rep. 2015, 5, 11918. [Google Scholar] [CrossRef]
- Blanchard, P.G.; Moreira, R.J.; Castro, E.; Caron, A.; Cote, M.; Andrade, M.L.; Oliveira, T.E.; Ortiz-Silva, M.; Peixoto, A.S.; Dias, F.A.; et al. PPARgamma is a major regulator of branched-chain amino acid blood levels and catabolism in white and brown adipose tissues. Metabolism 2018, 89, 27–38. [Google Scholar] [CrossRef]
- Andrade, M.L.; Gilio, G.R.; Perandini, L.A.; Peixoto, A.S.; Moreno, M.F.; Castro, E.; Oliveira, T.E.; Vieira, T.S.; Ortiz-Silva, M.; Thomazelli, C.A.; et al. PPARgamma-induced upregulation of subcutaneous fat adiponectin secretion, glyceroneogenesis and BCAA oxidation requires mTORC1 activity. Biochim Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158967. [Google Scholar] [CrossRef]
- Artha, I.; Bhargah, A.; Dharmawan, N.K.; Pande, U.W.; Triyana, K.A.; Mahariski, P.A.; Yuwono, J.; Bhargah, V.; Prabawa, I.P.Y.; Manuaba, I.; et al. High level of individual lipid profile and lipid ratio as a predictive marker of poor glycemic control in type-2 diabetes mellitus. Vasc. Health Risk Manag. 2019, 15, 149–157. [Google Scholar] [CrossRef]
- Gao, J.; Ma, L.; Yin, J.; Liu, G.; Ma, J.; Xia, S.; Gong, S.; Han, Q.; Li, T.; Chen, Y.; et al. Camellia (Camellia oleifera bel.) seed oil reprograms gut microbiota and alleviates lipid accumulation in high fat-fed mice through the mTOR pathway. Food Funct. 2022, 13, 4977–4992. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Cummings, N.E.; Apelo, S.I.A.; Neuman, J.C.; Kasza, I.; Schmidt, B.A.; Cava, E.; Spelta, F.; Tosti, V.; Syed, F.A.; et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep. 2016, 16, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ma, J.; Li, Y.; Ma, X.; Chen, J.; Zhang, H.; Wu, X.; Li, F.; Liu, Z.; Li, T.; et al. Branched-chain amino acids, especially of leucine and valine, mediate the protein restricted response in a piglet model. Food Funct. 2020, 11, 1304–1311. [Google Scholar] [CrossRef]
- Duan, Y.H.; Li, F.N.; Wen, C.Y.; Wang, W.L.; Guo, Q.P.; Li, Y.H.; Yin, Y.L. Branched-chain amino acid ratios in low-protein diets regulate the free amino acid profile and the expression of hepatic fatty acid metabolism-related genes in growing pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, e43–e51. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; McGarrah, R.W.; Herman, M.A.; Bain, J.R.; Shah, S.H.; Newgard, C.B. Insulin action, type 2 diabetes, and branched-chain amino acids: A two-way street. Mol. Metab. 2021, 52, 101261. [Google Scholar] [CrossRef]
- Lai, M.; Liu, Y.; Ronnett, G.V.; Wu, A.; Cox, B.J.; Dai, F.F.; Rost, H.L.; Gunderson, E.P.; Wheeler, M.B. Amino acid and lipid metabolism in post-gestational diabetes and progression to type 2 diabetes: A metabolic profiling study. PLoS Med. 2020, 17, e1003112. [Google Scholar] [CrossRef]
- Li, Y.; Wei, H.; Li, F.; Duan, Y.; Guo, Q.; Yin, Y. Effects of Low-Protein Diets Supplemented with Branched-Chain Amino Acid on Lipid Metabolism in White Adipose Tissue of Piglets. J. Agric. Food Chem. 2017, 65, 2839–2848. [Google Scholar] [CrossRef]
- Ma, Q.X.; Zhu, W.Y.; Lu, X.C.; Jiang, D.; Xu, F.; Li, J.T.; Zhang, L.; Wu, Y.L.; Chen, Z.J.; Yin, M.; et al. BCAA-BCKA axis regulates WAT browning through acetylation of PRDM16. Nat. Metab. 2022, 4, 106–122. [Google Scholar] [CrossRef]
- Kaimoto, T.; Shibuya, M.; Nishikawa, K.; Maeda, H. High incidence of lipid deposition in the liver of rats fed a diet supplemented with branched-chain amino acids under vitamin B6 deficiency. J. Nutr. Sci. Vitaminol. 2013, 59, 73–78. [Google Scholar] [CrossRef]
- Saedisomeolia, A.; Ashoori, M. Riboflavin in Human Health: A Review of Current Evidences. Adv. Food Nutr. Res. 2018, 83, 57–81. [Google Scholar]
- Thakur, K.; Tomar, S.K.; Singh, A.K.; Mandal, S.; Arora, S. Riboflavin and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2017, 57, 3650–3660. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Dullaart, R.P.F.; Schreuder, T.; Blokzijl, H.; Faber, K.N. Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2017, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Liu, T.; Xie, P.; Jiang, S.; Yi, W.; Dai, P.; Guo, X. UPLC-MS-based urine nontargeted metabolic profiling identifies dysregulation of pantothenate and CoA biosynthesis pathway in diabetic kidney disease. Life Sci. 2020, 258, 118160. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; Vidimce, J.; Holland, O.J.; Cuffe, J.S.M.; Beck, B.R.; Perkins, A.V.; McAinch, A.J.; Hryciw, D.H. Maternal and Postnatal High Linoleic Acid Diet Impacts Lipid Metabolism in Adult Rat Offspring in a Sex-Specific Manner. Int. J. Mol. Sci. 2021, 22, 2946. [Google Scholar] [CrossRef] [PubMed]
- Marchix, J.; Catheline, D.; Duby, C.; Monthean-Boulier, N.; Boissel, F.; Pedrono, F.; Boudry, G.; Legrand, P. Interactive effects of maternal and weaning high linoleic acid intake on hepatic lipid metabolism, oxylipins profile and hepatic steatosis in offspring. J. Nutr. BioChem. 2020, 75, 108241. [Google Scholar] [CrossRef]
- Bicikova, M.; Hampl, R.; Hill, M.; Stanicka, S.; Tallova, J.; Vondra, K. Steroids, sex hormone-binding globulin, homocysteine, selected hormones and markers of lipid and carbohydrate metabolism in patients with severe hypothyroidism and their changes following thyroid hormone supplementation. Clin. Chem. Lab. Med. 2003, 41, 284–292. [Google Scholar] [CrossRef]
- Nie, Q.; Chen, H.; Hu, J.; Gao, H.; Fan, L.; Long, Z.; Nie, S. Arabinoxylan Attenuates Type 2 Diabetes by Improvement of Carbohydrate, Lipid, and Amino Acid Metabolism. Mol. Nutr. Food Res. 2018, 62, e1800222. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, N.; Wang, Q.; Zhou, W.; Liu, C.; Liu, X.; Chen, S.; Fan, D.; Cao, W. Effects of honey-extracted polyphenols on serum antioxidant capacity and metabolic phenotype in rats. Food Funct. 2019, 10, 2347–2358. [Google Scholar] [CrossRef]
- Sonnweber, T.; Pizzini, A.; Nairz, M.; Weiss, G.; Tancevski, I. Arachidonic Acid Metabolites in Cardiovascular and Metabolic Diseases. Int. J. Mol. Sci. 2018, 19, 3285. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Si, F.; Huang, L.; Gao, A.; Lin, W.; Hoft, D.F.; Shao, Q.; Peng, G. Citrate Promotes Excessive Lipid Biosynthesis and Senescence in Tumor Cells for Tumor Therapy. Adv. Sci. 2022, 9, e2101553. [Google Scholar] [CrossRef]
- Hayakawa, H.; Raij, L. Relationship between hypercholesterolaemia, endothelial dysfunction and hypertension. J. Hypertens 1999, 17, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Szanto, M.; Gupte, R.; Kraus, W.L.; Pacher, P.; Bai, P. PARPs in lipid metabolism and related diseases. Prog. Lipid Res. 2021, 84, 101117. [Google Scholar] [CrossRef] [PubMed]
- Le May, N.; Iltis, I.; Ame, J.C.; Zhovmer, A.; Biard, D.; Egly, J.M.; Schreiber, V.; Coin, F. Poly (ADP-ribose) glycohydrolase regulates retinoic acid receptor-mediated gene expression. Mol. Cell 2012, 48, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.M.; Kennedy, C.C.; Caples, S.M.; Tracz, M.J.; Bolona, E.R.; Sideras, K.; Uraga, M.V.; Erwin, P.J.; Montori, V.M. Testosterone and cardiovascular risk in men: A systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin. Proc. 2007, 82, 29–39. [Google Scholar] [CrossRef]
- He, X.; Li, Z.; Zhang, Q. A UPLC-MRM-MS method for comprehensive profiling of Amadori compound-modified phosphatidylethanolamines in human plasma. Anal. Bioanal. Chem. 2021, 413, 431–443. [Google Scholar] [CrossRef]
- Shen, W.; Hao, J.; Feng, Z.; Tian, C.; Chen, W.; Packer, L.; Shi, X.; Zang, W.; Liu, J. Lipoamide or lipoic acid stimulates mitochondrial biogenesis in 3T3-L1 adipocytes via the endothelial NO synthase-cGMP-protein kinase G signalling pathway. Br. J. Pharmacol. 2011, 162, 1213–1224. [Google Scholar] [CrossRef]
- Erickson, N.; Zafron, M.; Harding, S.V.; Marinangeli, C.P.F.; Rideout, T.C. Evaluating the Lipid-Lowering Effects of alpha-lipoic Acid Supplementation: A Systematic Review. J. Diet Suppl. 2020, 17, 753–767. [Google Scholar] [CrossRef]
- Huang, C.C.; Sun, J.; Ji, H.; Kaneko, G.; Xie, X.D.; Chang, Z.G.; Deng, W. Systemic effect of dietary lipid levels and alpha-lipoic acid supplementation on nutritional metabolism in zebrafish (Danio rerio): Focusing on the transcriptional level. Fish Physiol. BioChem. 2020, 46, 1631–1644. [Google Scholar] [CrossRef]
- Li, P.; Slaughter, M. Glycine receptor subunit composition alters the action of GABA antagonists. Vis. Neurosci. 2007, 24, 513–521. [Google Scholar] [CrossRef]


| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| PPARγ | CCATTCTGGCCCACCAAC | AATGCGAGTGGTCTTCCATCA |
| β-actin | GTCCACCTTCCAGCAGATGT | GAAAGGGTGTAAAACGCAGC |
| Fatty Acid | Relative Content |
|---|---|
| Myristic Acid (C14:0) | 0.09% |
| Palmitic Acid (C16:0) | 0.09% |
| Palmitoleic Acid (C16:1n7) | 81.30% |
| heptadecanoic acid (C17:0) | 0.59% |
| 10-Heptadecenoicacid (C17:1n7) | 0.35% |
| Stearic Acid (C18:0) | 6.27% |
| oleic acid (C18:1n9c) | 0.06% |
| linoleic acid (C18:2n6c) | 0.04% |
| Linolenic Acid (C18:3n3) | 8.01% |
| Arachidic Acid (C20:0) | 0.09% |
| 11-Eicosenoic Acid (C20:1) | 3.12% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Ma, L.; Ma, J.; Xia, S.; Gong, S.; Yin, Y.; Chen, Y. Camellia (Camellia oleifera Abel.) Seed Oil Regulating of Metabolic Phenotype and Alleviates Dyslipidemia in High Fat-Fed Mice through Serum Branch-Chain Amino Acids. Nutrients 2022, 14, 2424. https://doi.org/10.3390/nu14122424
Gao J, Ma L, Ma J, Xia S, Gong S, Yin Y, Chen Y. Camellia (Camellia oleifera Abel.) Seed Oil Regulating of Metabolic Phenotype and Alleviates Dyslipidemia in High Fat-Fed Mice through Serum Branch-Chain Amino Acids. Nutrients. 2022; 14(12):2424. https://doi.org/10.3390/nu14122424
Chicago/Turabian StyleGao, Jing, Li Ma, Jie Ma, Siting Xia, Saiming Gong, Yulong Yin, and Yongzhong Chen. 2022. "Camellia (Camellia oleifera Abel.) Seed Oil Regulating of Metabolic Phenotype and Alleviates Dyslipidemia in High Fat-Fed Mice through Serum Branch-Chain Amino Acids" Nutrients 14, no. 12: 2424. https://doi.org/10.3390/nu14122424
APA StyleGao, J., Ma, L., Ma, J., Xia, S., Gong, S., Yin, Y., & Chen, Y. (2022). Camellia (Camellia oleifera Abel.) Seed Oil Regulating of Metabolic Phenotype and Alleviates Dyslipidemia in High Fat-Fed Mice through Serum Branch-Chain Amino Acids. Nutrients, 14(12), 2424. https://doi.org/10.3390/nu14122424






