Effect of a Dietary Supplement Combining Bioactive Peptides and Magnesium on Adjustment Disorder with Anxiety: A Clinical Trial in General Practice
Abstract
:1. Introduction
- -
- Generalized anxiety disorder: a state of permanent anxiety and excessive worry lasting at least 6 months, unrelated to a specific object or situation. In Western countries, it affects 4% of the population, especially women. It is accompanied by motor tension and hypervigilance (concentration difficulties, sleep disorders, irritability). It has a strong impact on the person’s life and is often associated with depression;
- -
- Panic attack: sudden onset of intense fear, a feeling of imminent disaster and loss of control, unrelated to an objective vital risk;
- -
- Panic disorder: repetition of panic attacks, accompanied by the fear of being afraid;
- -
- Phobic disorder: unreasonable, intense, object- or situation-specific fear, considered pathological when it affects the person’s life;
- -
- Obsessive-compulsive disorder: fears that invade the mind permanently and become obsessive fears;
- -
- Post-traumatic stress disorder: development of a pattern of symptoms following exposure to a traumatic event. These symptom types include re-experiencing the traumatic event, avoidance of stimuli associated with the trauma, persistent negative alterations in cognition, a numbing of general responsiveness, and increased symptoms of arousal.
2. Materials and Methods
2.1. Population
2.2. Objectives of the Study
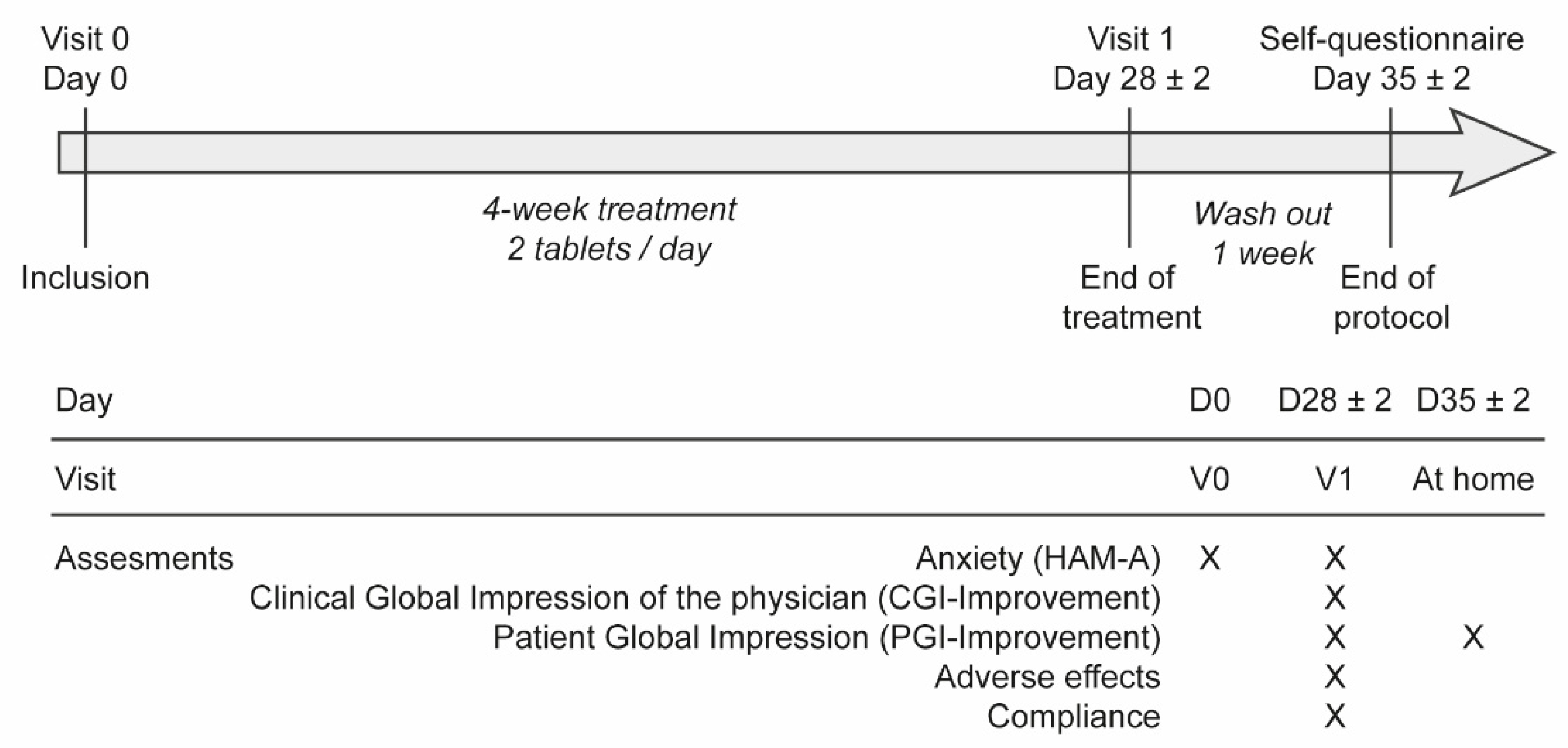
2.3. Study Design and Tested Dietary Supplement
2.4. Inclusion/Exclusion Criteria
2.5. Evaluation Criteria
2.6. Statistical Methods
2.6.1. General
2.6.2. Rationale for the Number of Subjects
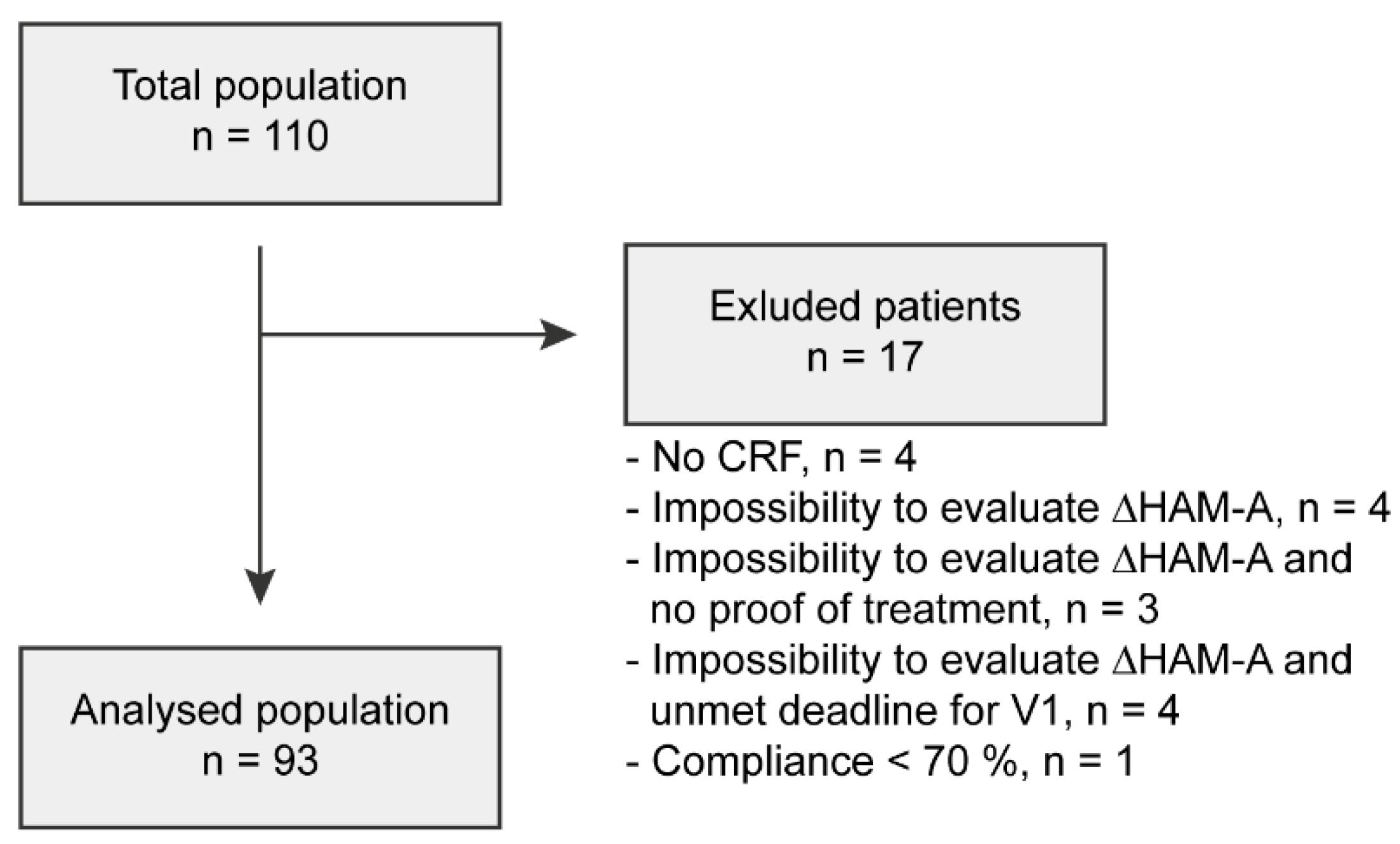
2.6.3. Analysed Population
2.6.4. Imputation of MD
- -
- If more than one box was ticked to answer an item in the questionnaires used in the study, the most severe answer (worst choice) was retained for all analyses
- -
- No estimates of missing questionnaires were made. If the follow-up questionnaire was missing, the data was considered missing. If the patient exits the study due to lack of efficacy, then the treatment will be considered as failed. Concerning the primary endpoint (Ham-A score):
- -
- If more than 20% of the items on the Ham-A scale were missing, the total score was considered missing
- -
- If less than 20% of the items were missing, a correction was applied based on the proportionality rule:
- -
- Missing Ham-A scores were imputed according to the baseline-observation-carried-forward (BOCF) model, which consists of replacing the missing value with the baseline value for the sensitivity analysis of the primary endpoint.
- -
- Incomplete dates: no imputation on missing dates was performed, except for the date of initial diagnosis for which, if the day was missing, it was estimated as the 1st of the month and if the month was missing, it was estimated as January.
2.6.5. Sensitivity Analyses of the Primary Objective
3. Results
3.1. Study Population
- -
- Not returned at V1 (lost to follow-up): three patients;
- -
- Premature discontinuation due to AEs: four patients;
- -
- Premature discontinuation of treatment due to the need to introduce an antidepressant: two patients.
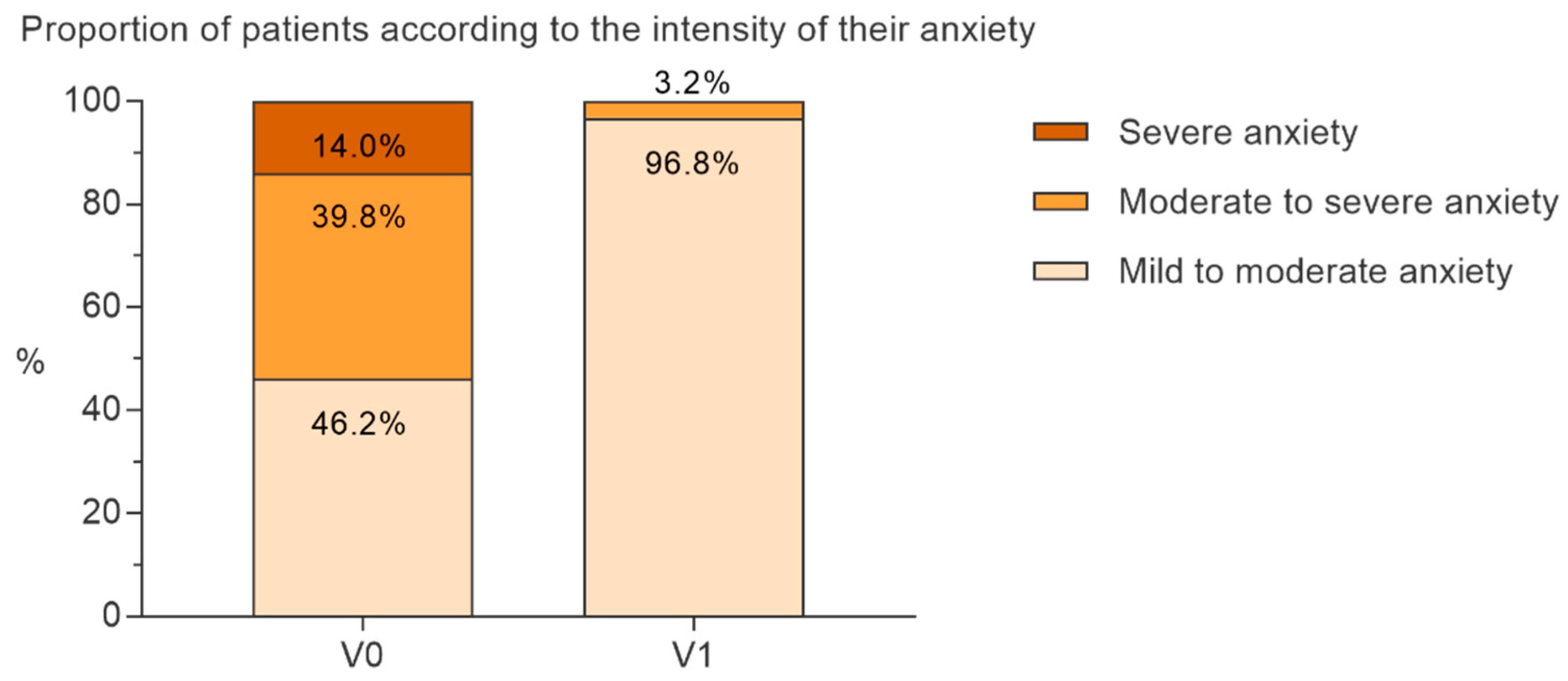
3.2. Primary Objective Analyses
3.3. Secondary Analyses
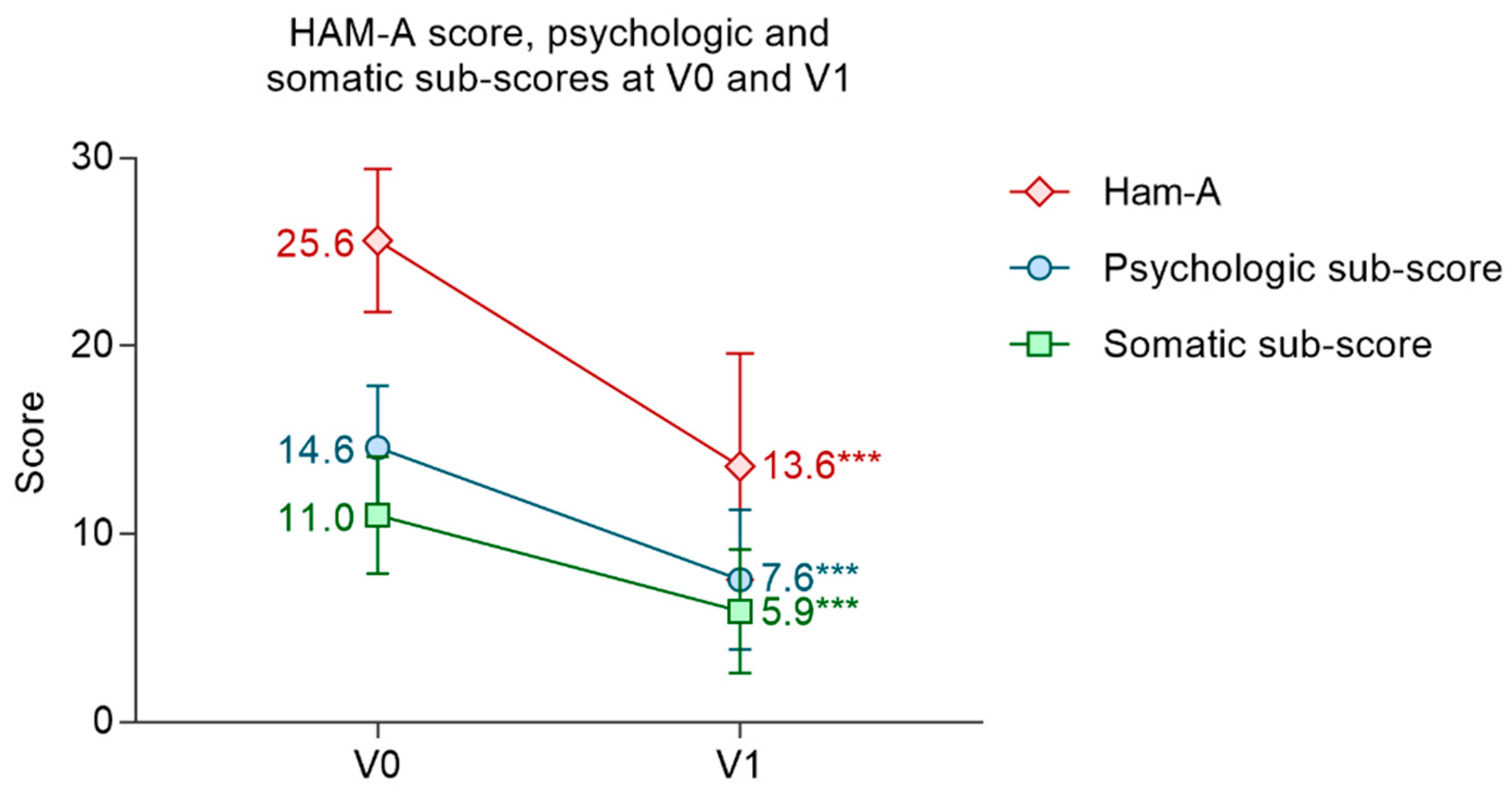
3.3.1. Evolution of the Ham-A Score and Subscores
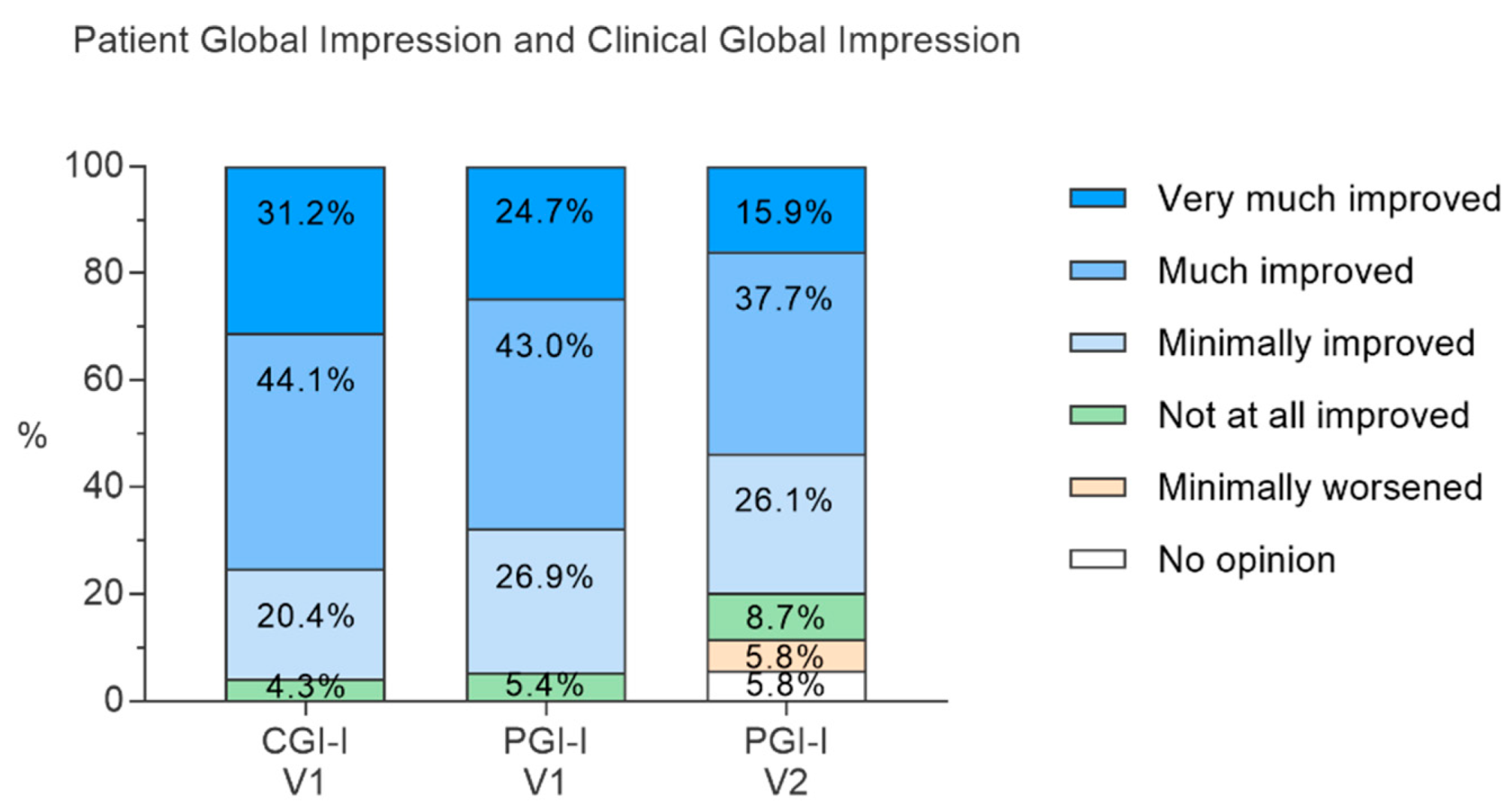
3.3.2. Clinical Improvement According to Physicians (CGI-I Score) and to Patients (PGI-I Score)
3.3.3. Analysis of Compliance
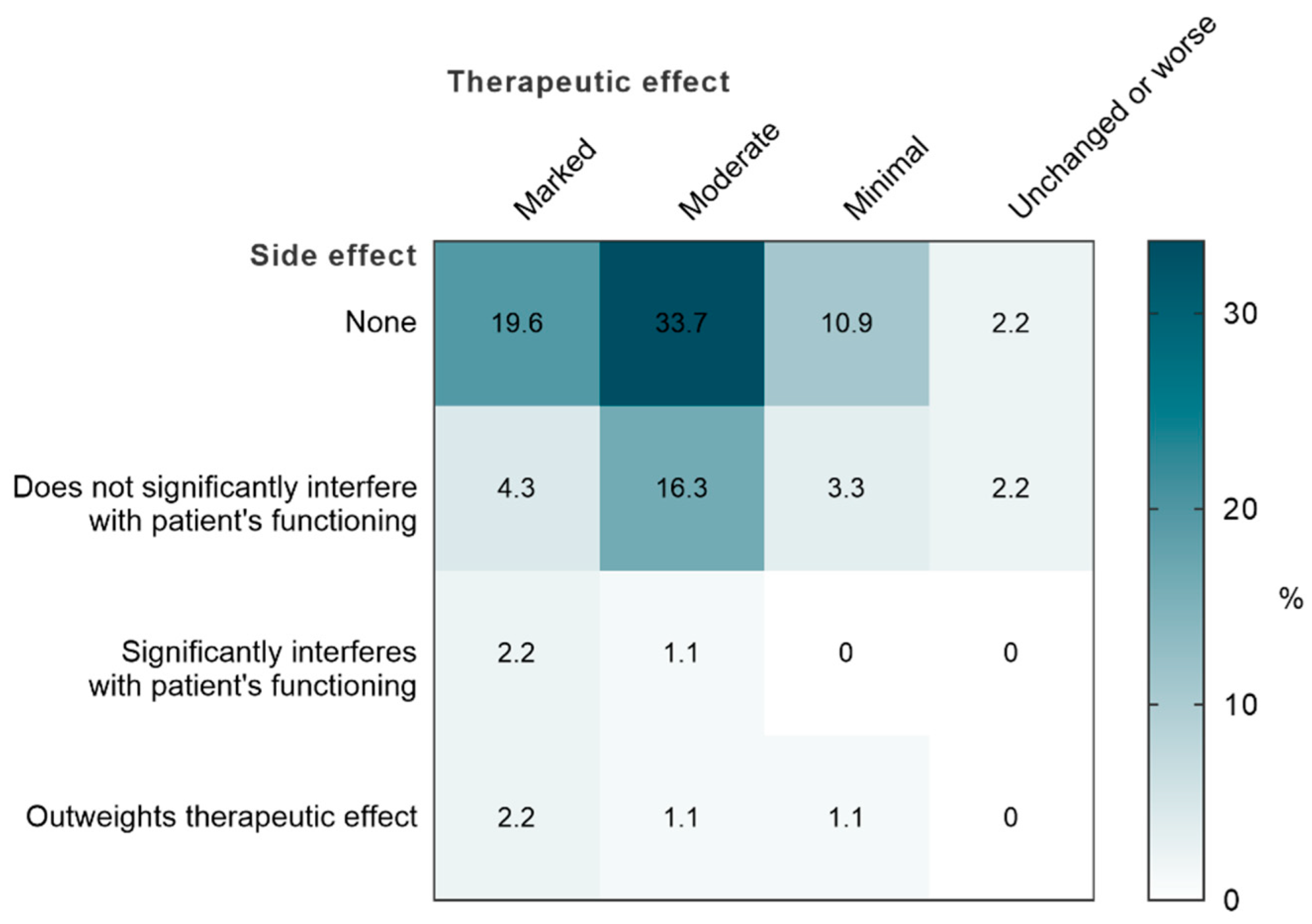
3.3.4. Analysis of the Therapeutic Effect with Regard to Tolerance
4. Discussion
- -
- The responder rate (decline of ≥50% in Ham-A score) after 28 days of treatment was 72% in the etifoxin group and 56% in the lorazepam group. The responder rate in the Stress 2 study was 41.9%.
- -
- The mean Ham-A score at Day 28 was 11.4 ± 5.9 in the etifoxin group (versus 25.2 ± 3.5 at Day 0) and 12.2 ± 6.4 in the lorazepam group (versus 25.6 ± 4.2 at Day 0), a reduction of 54.6 ± 23.5% and 52.3 ± 24.2%, respectively. In the Stress 2 study, the reduction in Ham-A score was 47.3%.
- -
- Clinical improvement according to the CGI score (much or very much improved) was 73.3% in the etifoxin group and 57.1% in the lorazepam group. This improvement was seen in three quarters of the patients in the Stress 2 study.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013; ISBN 978-0-89042-555-8. [Google Scholar]
- Carta, M.; Balestrieri, M.; Murru, A.; Hardoy, M. Adjustment Disorder: Epidemiology, Diagnosis and Treatment. Clin. Pract. Epidemiol. Ment. Health 2009, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Arends, I.; Bruinvels, D.J.; Rebergen, D.S.; Nieuwenhuijsen, K.; Madan, I.; Neumeyer-Gromen, A.; Bültmann, U.; Verbeek, J.H. Interventions to Facilitate Return to Work in Adults with Adjustment Disorders. Cochrane Database Syst. Rev. 2012, 12, CD006389. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.J. Etifoxine Versus Alprazolam for the Treatment of Adjustment Disorder with Anxiety: A Randomized Controlled Trial. Adv. Ther. 2015, 32, 57–68. [Google Scholar] [CrossRef]
- Bandelow, B.; Sher, L.; Bunevicius, R.; Hollander, E.; Kasper, S.; Zohar, J.; Möller, H.-J. WFSBP Task Force on Mental Disorders in Primary Care; WFSBP Task Force on Anxiety Disorders, OCD and PTSD Guidelines for the Pharmacological Treatment of Anxiety Disorders, Obsessive–Compulsive Disorder and Posttraumatic Stress Disorder in Primary Care. Int. J. Psychiatry Clin. Pract. 2012, 16, 77–84. [Google Scholar] [CrossRef]
- Baldwin, D.S.; Aitchison, K.; Bateson, A.; Curran, H.V.; Davies, S.; Leonard, B.; Nutt, D.J.; Stephens, D.N.; Wilson, S. Benzodiazepines: Risks and Benefits. A Reconsideration. J. Psychopharmacol. 2013, 27, 967–971. [Google Scholar] [CrossRef]
- Nasri, M. Protein Hydrolysates and Biopeptides. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 81, pp. 109–159. ISBN 978-0-12-811916-7. [Google Scholar]
- Saadi, S.; Saari, N.; Anwar, F.; Abdul Hamid, A.; Ghazali, H.M. Recent Advances in Food Biopeptides: Production, Biological Functionalities and Therapeutic Applications. Biotechnol. Adv. 2015, 33, 80–116. [Google Scholar] [CrossRef]
- Bernet, F.; Montel, V.; Noël, B.; Dupouy, J.P. Diazepam-like Effects of a Fish Protein Hydrolysate (Gabolysat PC60) on Stress Responsiveness of the Rat Pituitary-Adrenal System and Sympathoadrenal Activity. Psychopharmacology 2000, 149, 34–40. [Google Scholar] [CrossRef]
- Messaoudi, M.; Nejdi, A.; Bisson, J.-F.; Rozan, P.; Javelot, H.; Lalonde, R. Anxiolytic And Antidepressant-Like Effects Of Garum Armoricum® (GA), A Blue Ling Fish Protein Autolysate In Male Wistar Rats. Curr. Top. Nutraceutical Res. 2008, 6, 115–123. [Google Scholar]
- Messaoudi, M.; Lalonde, R.; Nejdi, A.; Bisson, J.-F.; Rozan, P.; Javelot, H.; Schroeder, H. The Effects Of Garum Armoricum® (GA) On Elevated-Plus Maze And Conditioned Light Extinction Tests In Rats. Curr. Top. Nutraceutical Res. 2008, 6, 41–46. [Google Scholar]
- Landsberg, G.M.; Mougeot, I.; Kelly, S.; Milgram, N.W. Assessment of Noise-Induced Fear and Anxiety in Dogs: Modification by a Novel Fish Hydrolysate Supplemented Diet. J. Vet. Behav. 2015, 10, 391–398. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Dorman, T.; Glaze, P.; Hogan, J.; Skinner, R.; Nelson, D.; Bowker, L.; Head, D. The Effectiveness of Garum Armoricum (Stabilium) in Reducing Anxiety in College Students. J. Adv. Med. 1995, 8, 193–200. [Google Scholar]
- Arrêté du 3 Décembre 1993 Portant Application du Décret N° 93-1130 du 27 Septembre 1993 Concernant L’étiquetage Relatif Aux Qualités Nutritionnelles des Denrées Alimentaires; French Decree. Available online: https://www.legifrance.gouv.fr/loda/id/JORFTEXT000000484070/ (accessed on 9 May 2022).
- Zhu, Y.; Auerbach, A. Na+ Occupancy and Mg2+ Block of the N-Methyl-d-Aspartate Receptor Channel. J. Gen. Physiol. 2001, 117, 275–286. [Google Scholar] [CrossRef]
- Schwartz, R.D.; Wagner, J.P.; Yu, X.; Martin, D. Bidirectional Modulation of GABA-Gated Chloride Channels by Divalent Cations: Inhibition by Ca2+ and Enhancement by Mg2+. J. Neurochem. 1994, 62, 916–922. [Google Scholar] [CrossRef]
- Poleszak, E.; Szewczyk, B.; Kędzierska, E.; Wlaź, P.; Pilc, A.; Nowak, G. Antidepressant- and Anxiolytic-like Activity of Magnesium in Mice. Pharmacol. Biochem. Behav. 2004, 78, 7–12. [Google Scholar] [CrossRef]
- Poleszak, E.; Wlaź, P.; Wróbel, A.; Fidecka, S.; Nowak, G. NMDA/Glutamate Mechanism of Magnesium-Induced Anxiolytic-like Behavior in Mice. Pharmacol. Rep. 2008, 60, 655–663. [Google Scholar]
- De Souza, M.C.; Walker, A.F.; Robinson, P.A.; Bolland, K. A Synergistic Effect of a Daily Supplement for 1 Month of 200 Mg Magnesium plus 50 Mg Vitamin B6 for the Relief of Anxiety-Related Premenstrual Symptoms: A Randomized, Double-Blind, Crossover Study. J. Womens Health Gend. Based Med. 2000, 9, 131–139. [Google Scholar] [CrossRef]
- Hanus, M.; Lafon, J.; Mathieu, M. Double-Blind, Randomised, Placebo-Controlled Study to Evaluate the Efficacy and Safety of a Fixed Combination Containing Two Plant Extracts (Crataegus Oxyacantha and Eschscholtzia Californica) and Magnesium in Mild-to-Moderate Anxiety Disorders. Curr. Med. Res. Opin. 2004, 20, 63–71. [Google Scholar] [CrossRef]
- Tarleton, E.K.; Littenberg, B.; MacLean, C.D.; Kennedy, A.G.; Daley, C. Role of Magnesium Supplementation in the Treatment of Depression: A Randomized Clinical Trial. PLoS ONE 2017, 12, e0180067. [Google Scholar] [CrossRef]
- Nightingale, J.M.D. (Ed.) Intestinal Failure; Greenwich Medical Media: London, UK, 2001; ISBN 978-1-900151-93-1. [Google Scholar]
- Bangratz, M.; Guinobert, I.; Dubourdeaux, M.; Guilbot, A. Higher Absorption and Lower Urinary Elimination of a New Magnesium Rice Complex Compared to Two Other Organic Forms of Magnesium: A Pilot Study in Rats. Gavin J. Food Nutrit. Sci. 2016, 1, 23–27. [Google Scholar] [CrossRef]
- Schuette, S.A.; Lashner, B.A.; Janghorbani, M. Bioavailability of Magnesium Diglycinate vs Magnesium Oxide in Patients with Ileal Resection. J. Parenter. Enter. Nutr. 1994, 18, 430–435. [Google Scholar] [CrossRef]
- Siebrecht, S. Magnesium Bisglycinate as Safe Form for MIneral Supplementation in Human Nutrition. Int. J. Orthomol. Relat. Med. 2013, 144, 1–16. [Google Scholar]
- Dakshinamurti, K.; Paulose, C.S.; Viswanathan, M.; Siow, Y.L.; Sharma, S.K.; Bolster, B. Neurobiology of Pyridoxine. Ann. N. Y. Acad. Sci. 1990, 585, 128–144. [Google Scholar] [CrossRef]
- Abraham, G.E.; Schwartz, U.D.; Lubran, M.M. Effect of Vitamin B-6 on Plasma and Red Blood Cell Magnesium Levels in Premenopausal Women. Ann. Clin. Lab. Sci. 1981, 11, 333–336. [Google Scholar]
- Kafeshani, M.; Feizi, A.; Esmaillzadeh, A.; Keshteli, A.H.; Afshar, H.; Roohafza, H.; Adibi, P. Higher Vitamin B6 Intake Is Associated with Lower Depression and Anxiety Risk in Women but Not in Men: A Large Cross-Sectional Study. Int. J. Vitam. Nutr. Res. 2020, 90, 484–492. [Google Scholar] [CrossRef]
- Pouteau, E.; Kabir-Ahmadi, M.; Noah, L.; Mazur, A.; Dye, L.; Hellhammer, J.; Pickering, G.; Dubray, C. Superiority of Magnesium and Vitamin B6 over Magnesium Alone on Severe Stress in Healthy Adults with Low Magnesemia: A Randomized, Single-Blind Clinical Trial. PLoS ONE 2018, 13, e0208454. [Google Scholar] [CrossRef]
- Boyle, N.; Lawton, C.; Dye, L. The Effects of Magnesium Supplementation on Subjective Anxiety and Stress—A Systematic Review. Nutrients 2017, 9, 429. [Google Scholar] [CrossRef]
- Rouillon, F.; Lejoyeux, M.; Martineau, C. A Double-Blind Controlled Study of PCR 7060 vs. Buspirone in the Treatment of Generalised Anxiety Disorder. Eur. Neuropsychopharmacol. 1995, 5, 367. [Google Scholar] [CrossRef]
- Hamilton, M. The Assessment of Anxiety States by Rating. Br. J. Med. Psychol. 1959, 32, 50–55. [Google Scholar] [CrossRef]
- Lépine, J.-P.; Gasquet, I.; Kovess, V.; Arbabzadeh-Bouchez, S.; Nègre-Pagès, L.; Nachbaur, G.; Gaudin, A.-F. Prévalence et comorbidité des troubles psychiatriques dans la population générale française: Résultats de l’étude épidémiologique ESEMeD/MHEDEA 2000/(ESEMeD). L’Encéphale 2005, 31, 182–194. [Google Scholar] [CrossRef]
- Nguyen, N.; Fakra, E.; Pradel, V.; Jouve, E.; Alquier, C.; Le Guern, M.-E.; Micallef, J.; Blin, O. Efficacy of Etifoxine Compared to Lorazepam Monotherapy in the Treatment of Patients with Adjustment Disorders with Anxiety: A Double-Blind Controlled Study in General Practice. Hum. Psychopharmacol. Clin. Exp. 2006, 21, 139–149. [Google Scholar] [CrossRef]
- Freret, T.; Largilliere, S.; Nee, G.; Coolzaet, M.; Corvaisier, S.; Boulouard, M. Fast Anxiolytic-Like Effect Observed in the Rat Conditioned Defensive Burying Test, after a Single Oral Dose of Natural Protein Extract Products. Nutrients 2021, 13, 2445. [Google Scholar] [CrossRef]
- Papadopol, V.; Nechifor, M. Magnesium in Neuroses and Neuroticism. In Magnesium in the Central Nervous System; Vink, R., Nechifor, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011; ISBN 978-0-9870730-5-1. [Google Scholar]
- Baxter, C.F.; Roberts, E. The Gamma-Aminobutyric Acid-Alpha-Ketoglutaric Acid Transaminase of Beef Brain. J. Biol. Chem. 1958, 233, 1135–1139. [Google Scholar] [CrossRef]
- Bénetier, C.; Bertin, M.; Calamassi-Tran, G.; Dubuisson, C.; Dufour, A.; Gauchard, F.; Lafay, L.; Lioret, S.; Touvier, M. Étude Individuelle Nationale des Consommations Alimentaires 2 (INCA 2) 2006–2007; AFSSA: Maisons-Alfort, France, 2009; pp. 1–228. [Google Scholar]
- Avis de l’Agence Nationale de Sécurité Sanitaire de L’alimentation, de L’environnement et du Travail Relatif à L’évaluation des Apports en Vitamines et Minéraux Issus de L’alimentation Non Enrichie, de L’alimentation Enrichie et des Compléments Alimentaires Dans la Population Française: Estimation des Apports Usuels, des Prévalences D’inadéquation et des Risques de Dépassement des Limites de Sécurité; Scientific Opinion; Saisine n°2012-SA-0142; ANSES: Maisons-Alfort, France, 2015; pp. 1–46.
- Nelson, J.; Chouinard, G. Guidelines for the Clinical Use of Benzodiazepines: Pharmacokinetics, Dependency, Rebound and Withdrawal. Canadian Society for Clinical Pharmacology. Can. J. Clin. Pharmacol. J. Can. Pharmacol. Clin. 1999, 6, 69–83. [Google Scholar]
| Parameters | Characteristics | Proportion |
|---|---|---|
| Sex | Male | 16 (17.2%) |
| Female | 77 (82.8%) | |
| Age (years) | Mean ± SD | 49.6 ± 13.1 |
| Min; Max | 21; 73 * | |
| (18–29) years | 6 (6.6%) | |
| (30–45) years | 27 (29.7%) | |
| ≥45 years | 58 (63.7%) | |
| MD | 2 | |
| Weight (kg) | Mean ± SD | 69.4 ± 13.0 |
| Min; Max | 42; 100 | |
| MD | 3 | |
| Size (cm) | Mean ± SD | 165.4 ± 6.8 |
| Min; Max | 150; 182 | |
| MD | 2 | |
| BMI (kg/m2) | Mean ± SD | 25.35 ± 4.41 |
| Min; Max | 16.8; 35.9 | |
| Underweight (BMI < 18.5) | 2 (2.2%) | |
| Normal (18.5 ≤ BMI < 25) | 41 (45.6%) | |
| Overweight (25 ≤ BMI < 30) | 30 (33.3%) | |
| Obese (BMI ≥ 30) | 17 (18.9%) | |
| MD | 3 | |
| Marital status | Single | 30 (32.6%) |
| Couple | 62 (67.4%) | |
| MD | 1 | |
| Children | Mean ± SD | 1.5 ± 1.2 |
| Min; Max | 0; 5 | |
| 0 child | 21 (23.9%) | |
| 1 child | 22 (25.0%) | |
| 2 children | 30 (34.1%) | |
| ≥3 children | 15 (17.0%) | |
| MD | 5 | |
| Professional status | Stable employment | 50 (57.5%) |
| Precarious employment | 5 (5.7%) | |
| In search of employment | 2 (2.3%) | |
| On sick leave | 3 (3.4%) | |
| Unemployed | 27 (31.0%) | |
| MD | 6 | |
| Socio-professional status | Craftsmen, traders and company managers | 4 (4.3%) |
| Executives and higher intellectual professions | 11 (12.0%) | |
| Intermediate occupations | 14 (15.2%) | |
| Employees | 39 (39.1%) | |
| Workers | 4 (4.3%) | |
| Not in the labour force (retired, etc.) | 14 (15.2%) | |
| Unemployed | 9 (9.8%) | |
| MD | 1 | |
| Alcohol consumption | Never | 27 (29.3%) |
| Sometimes | 61 (66.3%) | |
| ≤2 glasses/day | 4 (4.3%) | |
| MD | 1 | |
| Caffein consumption | Never | 21 (22.8%) |
| <3 cups/day | 63 (68.5%) | |
| <6 cups/day | 8 (8.7%) | |
| MD | 1 | |
| Smoking habit | Never | 62 (70,5%) |
| Former smoker | 15 (17.0%) | |
| Smoker | 11 (12.5%) | |
| MD | 5 | |
| Physical activity | Never | 26 (31.3%) |
| <2 times/week | 23 (27.7%) | |
| 2 times/week | 13 (15.7%) | |
| >2 times/week | 21 (25.3%) | |
| MD | 10 |
| Parameters | Characteristics | Proportion |
|---|---|---|
| Age of anxiety disorder (months) | Mean ± SD | 1.30 ± 0.73 |
| Min; Max | 0.1; 3.1 | |
| MD | 5 | |
| At least one identified stressor(s) | No | 9 (9.8%) |
| Yes | 83 (90.2%) | |
| MD | 1 | |
| Identified stressors | Professional difficulties | 41 (49.4%) |
| Family difficulties | 45 (54.2%) | |
| Health issues | 10 (12.0%) | |
| Financial difficulties | 7 (8.4%) | |
| Social conflict | 2 (2.4%) | |
| Other | 10 (12.0%) | |
| Former anxiety episodes requiring treatment or psychotherapy | No | 69 (74.2%) |
| Yes | 24 (25.8%) | |
| Ham-A V0 | Mean ± SD | 25.6 ± 3.8 |
| Min; max | 20; 37 | |
| Psychologic subscore V0 | Mean ± SD | 14.6 ± 3.3 |
| Min; max | 9; 23 | |
| Somatic subscore V0 | Mean ± SD | 11.0 ± 3.1 |
| Min; max | 4; 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oddoux, S.; Violette, P.; Cornet, J.; Akkoyun-Farinez, J.; Besnier, M.; Noël, A.; Rouillon, F. Effect of a Dietary Supplement Combining Bioactive Peptides and Magnesium on Adjustment Disorder with Anxiety: A Clinical Trial in General Practice. Nutrients 2022, 14, 2425. https://doi.org/10.3390/nu14122425
Oddoux S, Violette P, Cornet J, Akkoyun-Farinez J, Besnier M, Noël A, Rouillon F. Effect of a Dietary Supplement Combining Bioactive Peptides and Magnesium on Adjustment Disorder with Anxiety: A Clinical Trial in General Practice. Nutrients. 2022; 14(12):2425. https://doi.org/10.3390/nu14122425
Chicago/Turabian StyleOddoux, Sarah, Paul Violette, Jeanne Cornet, Julie Akkoyun-Farinez, Michel Besnier, Antoine Noël, and Frédéric Rouillon. 2022. "Effect of a Dietary Supplement Combining Bioactive Peptides and Magnesium on Adjustment Disorder with Anxiety: A Clinical Trial in General Practice" Nutrients 14, no. 12: 2425. https://doi.org/10.3390/nu14122425
APA StyleOddoux, S., Violette, P., Cornet, J., Akkoyun-Farinez, J., Besnier, M., Noël, A., & Rouillon, F. (2022). Effect of a Dietary Supplement Combining Bioactive Peptides and Magnesium on Adjustment Disorder with Anxiety: A Clinical Trial in General Practice. Nutrients, 14(12), 2425. https://doi.org/10.3390/nu14122425






