Cardiovascular Safety and Effectiveness of Bisphosphonates: From Intervention Trials to Real-Life Data
Abstract
1. Epidemiology of Fragility Fractures and Cardiovascular Diseases: Introduction and Brief Overview
2. Cardiovascular Morbidity and Mortality after Fragility Fractures
3. Bisphosphonates in the Prevention of Fragility Fractures
4. Bisphosphonates and Mortality
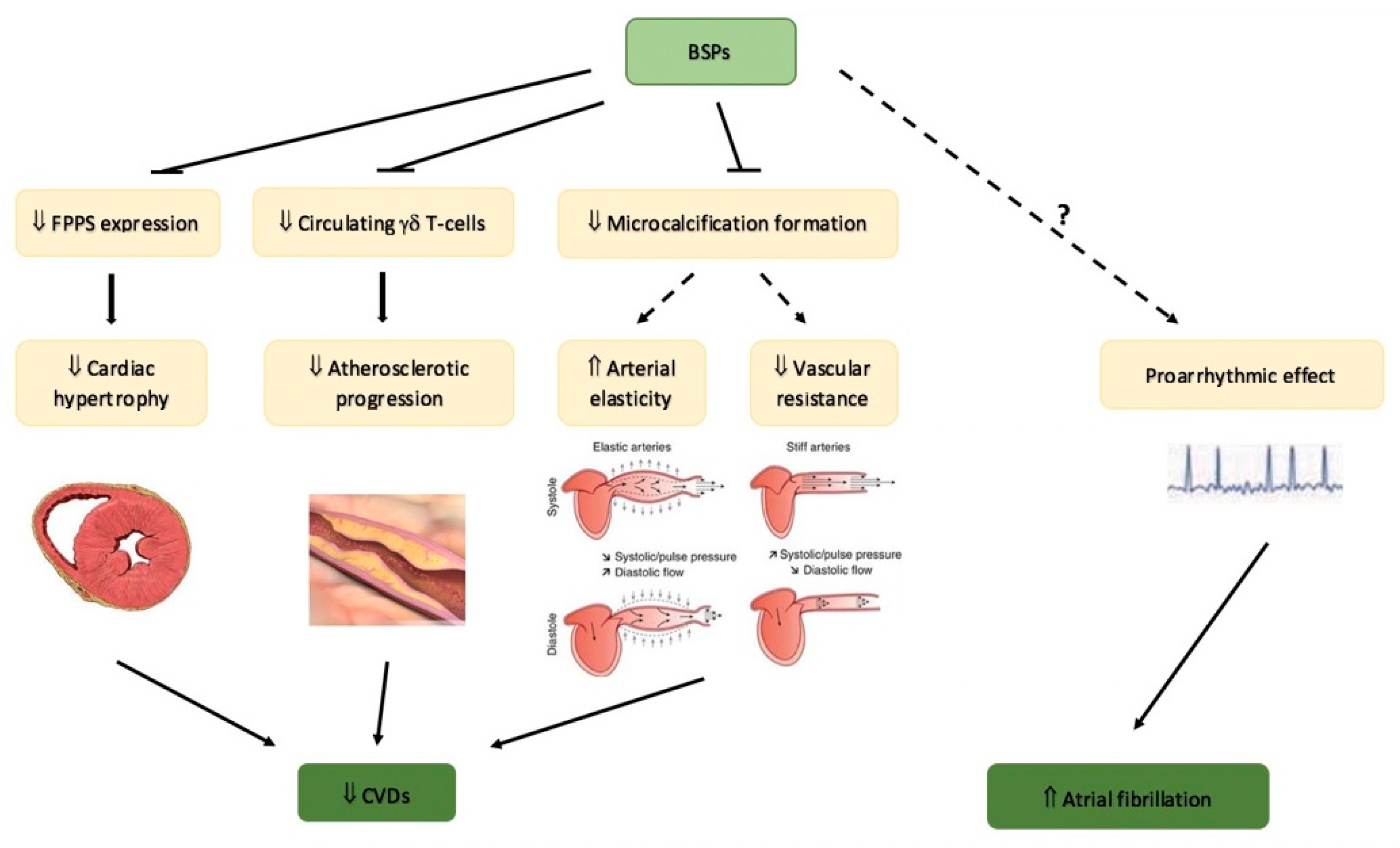
5. Bisphosphonates and Cardiovascular Disease
5.1. Bisphosphonates and Cardiovascular Protection
5.2. Safety of Bisphosphonates on Cardiovascular System: Atrial Fibrillation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis. Report of a WHO Study Group—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/7941614/ (accessed on 4 March 2022).
- Borgström, F.; Karlsson, L.; Ortsäter, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Javaid, M.K.; et al. Fragility Fractures in Europe: Burden, Management and Opportunities. Arch. Osteoporos. 2020, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Willers, C.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; McCloskey, E.V.; Borgström, F.; Kanis, J.A. SCOPE review panel of the IOF Osteoporosis in Europe: A Compendium of Country-Specific Reports. Arch. Osteoporos. 2022, 17, 23. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, A.; Laurberg, P.; Vestergaard, P.; Andersen, S. Clinical Risk Factors for Osteoporosis Are Common among Elderly People in Nuuk, Greenland. Int. J. Circumpolar Health 2013, 72, 19596. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Karagiannis, A.; Kakafika, A.I.; Tziomalos, K.; Athyros, V.G.; Mikhailidis, D.P. Atherosclerosis and Osteoporosis: Age-Dependent Degenerative Processes or Related Entities? Osteoporos. Int. 2009, 20, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johnell, O. The Burden of Osteoporosis. J. Endocrinol. Investig. 1999, 22, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-H.; Liu, C.-J.; Chen, P.-J.; Huang, C.-C.; Hsu, C.-Y.; Chen, Z.-Y.; Chan, W.-L.; Huang, P.-H.; Chen, T.-J.; Chung, C.-M.; et al. Hip Fracture and Risk of Acute Myocardial Infarction: A Nationwide Study. J. Bone Miner. Res. 2013, 28, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Chung, S.-D.; Xirasagar, S.; Jaw, F.-S.; Lin, H.-C. Increased Risk of Stroke in the Year after a Hip Fracture: A Population-Based Follow-up Study. Stroke 2011, 42, 336–341. [Google Scholar] [CrossRef]
- Cameron, I.D.; Chen, J.S.; March, L.M.; Simpson, J.M.; Cumming, R.G.; Seibel, M.J.; Sambrook, P.N. Hip Fracture Causes Excess Mortality Owing to Cardiovascular and Infectious Disease in Institutionalized Older People: A Prospective 5-Year Study. J. Bone Miner. Res. 2010, 25, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Klop, C.; van Staa, T.P.; Cooper, C.; Harvey, N.C.; de Vries, F. The Epidemiology of Mortality after Fracture in England: Variation by Age, Sex, Time, Geographic Location, and Ethnicity. Osteoporos. Int. 2017, 28, 161–168. [Google Scholar] [CrossRef]
- Tankò, L.B.; Bagger, Y.Z.; Christiansen, C. Low Bone Mineral Density in the Hip as a Marker of Advanced Atherosclerosis in Elderly Women. Calcif. Tissue Int. 2003, 73, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.N.; Cho, J.-Y.; Eun, Y.-M.; Song, S.-W.; Moon, K.-W. Associations between Osteoporosis and Coronary Artery Disease in Postmenopausal Women. Climacteric 2016, 19, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Hogan, C.; Lyubomirsky, G.; Sambrook, P.N. Women with Cardiovascular Disease Have Increased Risk of Osteoporotic Fracture. Calcif. Tissue Int. 2011, 88, 9–15. [Google Scholar] [CrossRef]
- Fusaro, M.; Tripepi, G.; Plebani, M.; Politi, C.; Aghi, A.; Taddei, F.; Schileo, E.; Zaninotto, M.; La Manna, G.; Cianciolo, G.; et al. The Vessels-Bone Axis: Iliac Artery Calcifications, Vertebral Fractures and Vitamin K from VIKI Study. Nutrients 2021, 13, 3567. [Google Scholar] [CrossRef]
- Cremers, S.; Ebetino, F.H.; Phipps, R. On the Pharmacological Evaluation of Bisphosphonates in Humans. Bone 2020, 139, 115501. [Google Scholar] [CrossRef]
- Sanderson, J.; Martyn-St James, M.; Stevens, J.; Goka, E.; Wong, R.; Campbell, F.; Selby, P.; Gittoes, N.; Davis, S. Clinical Effectiveness of Bisphosphonates for the Prevention of Fragility Fractures: A Systematic Review and Network Meta-Analysis. Bone 2016, 89, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y. Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF) European Guidance for the Diagnosis and Management of Osteoporosis in Postmenopausal Women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef]
- Black, D.M.; Cummings, S.R.; Karpf, D.B.; Cauley, J.A.; Thompson, D.E.; Nevitt, M.C.; Bauer, D.C.; Genant, H.K.; Haskell, W.L.; Marcus, R.; et al. Randomised Trial of Effect of Alendronate on Risk of Fracture in Women with Existing Vertebral Fractures. Fracture Intervention Trial Research Group. Lancet 1996, 348, 1535–1541. [Google Scholar] [CrossRef]
- Cummings, S.R.; Black, D.M.; Thompson, D.E.; Applegate, W.B.; Barrett-Connor, E.; Musliner, T.A.; Palermo, L.; Prineas, R.; Rubin, S.M.; Scott, J.C.; et al. Effect of Alendronate on Risk of Fracture in Women with Low Bone Density but without Vertebral Fractures: Results from the Fracture Intervention Trial. JAMA 1998, 280, 2077–2082. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, K.F.; Wallander, M.; Johansson, H.; Lundh, D.; Lorentzon, M. Hip Fracture Risk and Safety with Alendronate Treatment in the Oldest-Old. J. Intern. Med. 2017, 282, 546–559. [Google Scholar] [CrossRef]
- Harris, S.T.; Watts, N.B.; Genant, H.K.; McKeever, C.D.; Hangartner, T.; Keller, M.; Chesnut, C.H.; Brown, J.; Eriksen, E.F.; Hoseyni, M.S.; et al. Effects of Risedronate Treatment on Vertebral and Nonvertebral Fractures in Women with Postmenopausal Osteoporosis: A Randomized Controlled Trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA 1999, 282, 1344–1352. [Google Scholar] [CrossRef]
- Reginster, J.; Minne, H.W.; Sorensen, O.H.; Hooper, M.; Roux, C.; Brandi, M.L.; Lund, B.; Ethgen, D.; Pack, S.; Roumagnac, I.; et al. Randomized Trial of the Effects of Risedronate on Vertebral Fractures in Women with Established Postmenopausal Osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos. Int. 2000, 11, 83–91. [Google Scholar] [CrossRef]
- McClung, M.R.; Geusens, P.; Miller, P.D.; Zippel, H.; Bensen, W.G.; Roux, C.; Adami, S.; Fogelman, I.; Diamond, T.; Eastell, R.; et al. Effect of Risedronate on the Risk of Hip Fracture in Elderly Women. Hip Intervention Program Study Group. N. Engl. J. Med. 2001, 344, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.-Y.; Adami, S.; Lakatos, P.; Greenwald, M.; Stepan, J.J.; Silverman, S.L.; Christiansen, C.; Rowell, L.; Mairon, N.; Bonvoisin, B.; et al. Efficacy and Tolerability of Once-Monthly Oral Ibandronate in Postmenopausal Osteoporosis: 2 Year Results from the MOBILE Study. Ann. Rheum. Dis. 2006, 65, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D.; Recker, R.R.; Harris, S.; Silverman, S.; Felsenberg, D.; Reginster, J.; Day, B.-M.; Barr, C.; Masanauskaite, D. Long-Term Fracture Rates Seen with Continued Ibandronate Treatment: Pooled Analysis of DIVA and MOBILE Long-Term Extension Studies. Osteoporos. Int. 2014, 25, 349–357. [Google Scholar] [CrossRef]
- McCloskey, E.; Selby, P.; Davies, M.; Robinson, J.; Francis, R.M.; Adams, J.; Kayan, K.; Beneton, M.; Jalava, T.; Pylkkänen, L.; et al. Clodronate Reduces Vertebral Fracture Risk in Women with Postmenopausal or Secondary Osteoporosis: Results of a Double-Blind, Placebo-Controlled 3-Year Study. J. Bone Miner. Res. 2004, 19, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Delmas, P.D.; Eastell, R.; Reid, I.R.; Boonen, S.; Cauley, J.A.; Cosman, F.; Lakatos, P.; Leung, P.C.; Man, Z.; et al. Once-Yearly Zoledronic Acid for Treatment of Postmenopausal Osteoporosis. N. Engl. J. Med. 2007, 356, 1809–1822. [Google Scholar] [CrossRef]
- Lyles, K.W.; Colón-Emeric, C.S.; Magaziner, J.S.; Adachi, J.D.; Pieper, C.F.; Mautalen, C.; Hyldstrup, L.; Recknor, C.; Nordsletten, L.; Moore, K.A.; et al. Zoledronic Acid and Clinical Fractures and Mortality after Hip Fracture. N. Engl. J. Med. 2007, 357, 1799–1809. [Google Scholar] [CrossRef]
- Bougioukli, S.; Κollia, P.; Koromila, T.; Varitimidis, S.; Hantes, M.; Karachalios, T.; Malizos, Κ.Ν.; Dailiana, Z.H. Failure in Diagnosis and Under-Treatment of Osteoporosis in Elderly Patients with Fragility Fractures. J. Bone Miner. Metab. 2019, 37, 327–335. [Google Scholar] [CrossRef]
- Diez-Perez, A.; Naylor, K.E.; Abrahamsen, B.; Agnusdei, D.; Brandi, M.L.; Cooper, C.; Dennison, E.; Eriksen, E.F.; Gold, D.T.; Guañabens, N.; et al. International Osteoporosis Foundation and European Calcified Tissue Society Working Group. Recommendations for the Screening of Adherence to Oral Bisphosphonates. Osteoporos. Int. 2017, 28, 767–774. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.B.; Gamble, G.D.; Reid, I.R. Effect of Osteoporosis Treatment on Mortality: A Meta-Analysis. J. Clin. Endocrinol. Metab. 2010, 95, 1174–1181. [Google Scholar] [CrossRef]
- Reid, I.R.; Horne, A.M.; Mihov, B.; Stewart, A.; Garratt, E.; Bastin, S.; Gamble, G.D. Effects of Zoledronate on Cancer, Cardiac Events, and Mortality in Osteopenic Older Women. J. Bone Miner. Res. 2020, 35, 20–27. [Google Scholar] [CrossRef]
- Brozek, W.; Reichardt, B.; Zwerina, J.; Dimai, H.P.; Klaushofer, K.; Zwettler, E. Antiresorptive Therapy and Risk of Mortality and Refracture in Osteoporosis-Related Hip Fracture: A Nationwide Study. Osteoporos. Int. 2016, 27, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Beaupre, L.A.; Morrish, D.W.; Hanley, D.A.; Maksymowych, W.P.; Bell, N.R.; Juby, A.G.; Majumdar, S.R. Oral Bisphosphonates Are Associated with Reduced Mortality after Hip Fracture. Osteoporos. Int. 2011, 22, 983–991. [Google Scholar] [CrossRef]
- Sambrook, P.N.; Cameron, I.D.; Chen, J.S.; March, L.M.; Simpson, J.M.; Cumming, R.G.; Seibel, M.J. Oral Bisphosphonates Are Associated with Reduced Mortality in Frail Older People: A Prospective Five-Year Study. Osteoporos. Int. 2011, 22, 2551–2556. [Google Scholar] [CrossRef]
- Goodbrand, J.A.; Hughes, L.D.; Cochrane, L.; Donnan, P.T.; McGilchrist, M.; Frost, H.; McMurdo, M.E.T.; Witham, M.D. Association between Bisphosphonate Therapy and Outcomes from Rehabilitation in Older People. Arch. Gerontol. Geriatr. 2017, 70, 195–200. [Google Scholar] [CrossRef]
- Center, J.R.; Bliuc, D.; Nguyen, N.D.; Nguyen, T.V.; Eisman, J.A. Osteoporosis Medication and Reduced Mortality Risk in Elderly Women and Men. J. Clin. Endocrinol. Metab. 2011, 96, 1006–1014. [Google Scholar] [CrossRef]
- Lee, P.; Ng, C.; Slattery, A.; Nair, P.; Eisman, J.A.; Center, J.R. Preadmission Bisphosphonate and Mortality in Critically Ill Patients. J. Clin. Endocrinol. Metab. 2016, 101, 1945–1953. [Google Scholar] [CrossRef]
- Bergman, J.; Nordström, A.; Hommel, A.; Kivipelto, M.; Nordström, P. Bisphosphonates and Mortality: Confounding in Observational Studies? Osteoporos. Int. 2019, 30, 1973–1982. [Google Scholar] [CrossRef]
- Cummings, S.R.; Lui, L.-Y.; Eastell, R.; Allen, I.E. Association Between Drug Treatments for Patients with Osteoporosis and Overall Mortality Rates: A Meta-Analysis. JAMA Intern. Med. 2019, 179, 1491–1500. [Google Scholar] [CrossRef]
- Fuggle, N.R.; Cooper, C.; Harvey, N.C.; Al-Daghri, N.; Brandi, M.-L.; Bruyere, O.; Cano, A.; Dennison, E.M.; Diez-Perez, A.; Kaufman, J.-M.; et al. Assessment of Cardiovascular Safety of Anti-Osteoporosis Drugs. Drugs 2020, 80, 1537–1552. [Google Scholar] [CrossRef]
- Yang, Y.; Rong, X.; Lv, X.; Jiang, W.; Yang, Y.; Lai, D.; Xu, S.; Fu, G. Inhibition of Mevalonate Pathway Prevents Ischemia-Induced Cardiac Dysfunction in Rats via RhoA-Independent Signaling Pathway. Cardiovasc. Ther. 2017, 35, e12285. [Google Scholar] [CrossRef]
- Giollo, A.; Rossini, M.; Gatti, D.; Adami, G.; Orsolini, G.; Fassio, A.; Caimmi, C.; Idolazzi, L.; Viapiana, O. Amino-Bisphosphonates and Cardiovascular Risk: A New Hypothesis Involving the Effects on Gamma-Delta T Cells. J. Bone Miner. Res. 2019, 34, 570–571. [Google Scholar] [CrossRef]
- Ruiz, J.L.; Hutcheson, J.D.; Cardoso, L.; Bakhshian Nik, A.; Condado de Abreu, A.; Pham, T.; Buffolo, F.; Busatto, S.; Federici, S.; Ridolfi, A.; et al. Nanoanalytical Analysis of Bisphosphonate-Driven Alterations of Microcalcifications Using a 3D Hydrogel System and in Vivo Mouse Model. Proc. Natl. Acad. Sci. USA 2021, 118, e1811725118. [Google Scholar] [CrossRef]
- Cai, G.; Keen, H.I.; Host, L.V.; Aitken, D.; Laslett, L.L.; Winzenberg, T.; Wluka, A.E.; Black, D.; Jones, G. Once-Yearly Zoledronic Acid and Change in Abdominal Aortic Calcification over 3 Years in Postmenopausal Women with Osteoporosis: Results from the HORIZON Pivotal Fracture Trial. Osteoporos. Int. 2020, 31, 1741–1747. [Google Scholar] [CrossRef]
- Kranenburg, G.; Bartstra, J.W.; Weijmans, M.; de Jong, P.A.; Mali, W.P.; Verhaar, H.J.; Visseren, F.L.J.; Spiering, W. Bisphosphonates for Cardiovascular Risk Reduction: A Systematic Review and Meta-Analysis. Atherosclerosis 2016, 252, 106–115. [Google Scholar] [CrossRef]
- Elmariah, S.; Delaney, J.A.C.; O’Brien, K.D.; Budoff, M.J.; Vogel-Claussen, J.; Fuster, V.; Kronmal, R.A.; Halperin, J.L. Bisphosphonate Use and Prevalence of Valvular and Vascular Calcification in Women MESA (The Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2010, 56, 1752–1759. [Google Scholar] [CrossRef]
- Okamoto, M.; Yamanaka, S.; Yoshimoto, W.; Shigematsu, T. Alendronate as an Effective Treatment for Bone Loss and Vascular Calcification in Kidney Transplant Recipients. J. Transplant. 2014, 2014, 269613. [Google Scholar] [CrossRef]
- Sing, C.-W.; Wong, A.Y.; Kiel, D.P.; Cheung, E.Y.; Lam, J.K.; Cheung, T.T.; Chan, E.W.; Kung, A.W.; Wong, I.C.; Cheung, C.-L. Association of Alendronate and Risk of Cardiovascular Events in Patients with Hip Fracture. J. Bone Miner. Res. 2018, 33, 1422–1434. [Google Scholar] [CrossRef]
- Cummings, S.R.; McCulloch, C. Explanations for the Difference in Rates of Cardiovascular Events in a Trial of Alendronate and Romosozumab. Osteoporos. Int. 2020, 31, 1019–1021. [Google Scholar] [CrossRef]
- Rodríguez, A.J.; Ernst, M.T.; Nybo, M.; Prieto-Alhambra, D.; Ebeling, P.R.; Hermann, A.P.; Abrahamsen, B. Oral Bisphosphonate Use Reduces Cardiovascular Events in a Cohort of Danish Patients Referred for Bone Mineral Density. J. Clin. Endocrinol. Metab. 2020, 105, dgaa481. [Google Scholar] [CrossRef] [PubMed]
- Casula, M.; Olmastroni, E.; Galimberti, F.; Tragni, E.; Corrao, G.; Scotti, L.; Catapano, A.L. Association between the Cumulative Exposure to Bisphosphonates and Hospitalization for Atherosclerotic Cardiovascular Events: A Population-Based Study. Atherosclerosis 2020, 301, 1–7. [Google Scholar] [CrossRef]
- Kirchmayer, U.; Sorge, C.; Sultana, J.; Lapi, F.; Onder, G.; Agabiti, N.; Cascini, S.; Roberto, G.; Corrao, G.; Vitale, C.; et al. Bisphosphonates and Cardiovascular Risk in Elderly Patients with Previous Cardiovascular Disease: A Population-Based Nested Case-Control Study in Italy. Ther. Adv. Drug Saf. 2019, 10, 2042098619838138. [Google Scholar] [CrossRef] [PubMed]
- Asghar, Z.B.; Godoy Caballero, A.; Pathirannehelage, S.; Williams, J.; McKay, S.; Grassby, P.; de Lusignan, S.; Niroshan Siriwardena, A. Saving Bones without Risking Brain-Bisphosphonates and Risk of Stroke: Matched Case-Control Study. Osteoporos. Int. 2019, 30, 1845–1854. [Google Scholar] [CrossRef]
- Kang, J.-H.; Keller, J.J.; Lin, H.-C. Bisphosphonates Reduced the Risk of Acute Myocardial Infarction: A 2-Year Follow-up Study. Osteoporos. Int. 2013, 24, 271–277. [Google Scholar] [CrossRef]
- Reid, I.R.; Horne, A.M.; Mihov, B.; Stewart, A.; Garratt, E.; Wong, S.; Wiessing, K.R.; Bolland, M.J.; Bastin, S.; Gamble, G.D. Fracture Prevention with Zoledronate in Older Women with Osteopenia. N. Engl. J. Med. 2018, 379, 2407–2416. [Google Scholar] [CrossRef]
- Cummings, S.R.; Schwartz, A.V.; Black, D.M. Alendronate and Atrial Fibrillation. N. Engl. J. Med. 2007, 356, 1895–1896. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsen, B.; Eiken, P.; Brixen, K. Atrial Fibrillation in Fracture Patients Treated with Oral Bisphosphonates. J. Intern. Med. 2009, 265, 581–592. [Google Scholar] [CrossRef]
- Barrett-Connor, E.; Swern, A.S.; Hustad, C.M.; Bone, H.G.; Liberman, U.A.; Papapoulos, S.; Wang, H.; de Papp, A.; Santora, A.C. Alendronate and Atrial Fibrillation: A Meta-Analysis of Randomized Placebo-Controlled Clinical Trials. Osteoporos. Int. 2012, 23, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Rogers, J.R.; Fulchino, L.A.; Kim, C.A.; Solomon, D.H.; Kim, S.C. Bisphosphonates and Risk of Cardiovascular Events: A Meta-Analysis. PLoS ONE 2015, 10, e0122646. [Google Scholar] [CrossRef] [PubMed]
- Rubin, K.H.; Möller, S.; Choudhury, A.; Zorina, O.; Kalsekar, S.; Eriksen, E.F.; Andersen, M.; Abrahamsen, B. Cardiovascular and Skeletal Safety of Zoledronic Acid in Osteoporosis Observational, Matched Cohort Study Using Danish and Swedish Health Registries. Bone 2020, 134, 115296. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, H.T.; Christensen, S.; Mehnert, F.; Pedersen, L.; Chapurlat, R.D.; Cummings, S.R.; Baron, J.A. Use of Bisphosphonates among Women and Risk of Atrial Fibrillation and Flutter: Population Based Case-Control Study. BMJ 2008, 336, 813–816. [Google Scholar] [CrossRef]
- Fazmin, I.T.; Huang, C.L.-H.; Jeevaratnam, K. Bisphosphonates and Atrial Fibrillation: Revisiting the Controversy. Ann. N. Y. Acad. Sci. 2020, 1474, 15–26. [Google Scholar] [CrossRef]
- Bunch, T.J.; Anderson, J.L.; May, H.T.; Muhlestein, J.B.; Horne, B.D.; Crandall, B.G.; Weiss, J.P.; Lappé, D.L.; Osborn, J.S.; Day, J.D. Relation of Bisphosphonate Therapies and Risk of Developing Atrial Fibrillation. Am. J. Cardiol. 2009, 103, 824–828. [Google Scholar] [CrossRef]
- Grosso, A.; Douglas, I.; Hingorani, A.; MacAllister, R.; Smeeth, L. Oral Bisphosphonates and Risk of Atrial Fibrillation and Flutter in Women: A Self-Controlled Case-Series Safety Analysis. PLoS ONE 2009, 4, e4720. [Google Scholar] [CrossRef]
- Wilkinson, G.S.; Baillargeon, J.; Kuo, Y.-F.; Freeman, J.L.; Goodwin, J.S. Atrial Fibrillation and Stroke Associated with Intravenous Bisphosphonate Therapy in Older Patients with Cancer. J. Clin. Oncol. 2010, 28, 4898–4905. [Google Scholar] [CrossRef]
- Vestergaard, P.; Schwartz, K.; Pinholt, E.M.; Rejnmark, L.; Mosekilde, L. Risk of Atrial Fibrillation Associated with Use of Bisphosphonates and Other Drugs against Osteoporosis: A Cohort Study. Calcif. Tissue Int 2010, 86, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-F.; Tsai, Y.-W.; Wen, Y.-W.; Hsiao, F.-Y.; Kuo, K.N.; Tsai, C.-R. Osteoporosis Treatment and Atrial Fibrillation: Alendronate versus Raloxifene. Menopause 2010, 17, 57–63. [Google Scholar] [CrossRef]
- Erichsen, R.; Christiansen, C.F.; Frøslev, T.; Jacobsen, J.; Sørensen, H.T. Intravenous Bisphosphonate Therapy and Atrial Fibrillation/Flutter Risk in Cancer Patients: A Nationwide Cohort Study. Br. J. Cancer 2011, 105, 881–883. [Google Scholar] [CrossRef][Green Version]
- Lu, P.-Y.; Hsieh, C.-F.; Tsai, Y.-W.; Huang, W.-F. Alendronate and Raloxifene Use Related to Cardiovascular Diseases: Differentiation by Different Dosing Regimens of Alendronate. Clin. Ther. 2011, 33, 1173–1179. [Google Scholar] [CrossRef]
- Pazianas, M.; Cooper, C.; Wang, Y.; Lange, J.L.; Russell, R.G.G. Atrial Fibrillation and the Use of Oral Bisphosphonates. Ther. Clin. Risk Manag. 2011, 7, 131–144. [Google Scholar] [CrossRef]
- Arslan, C.; Aksoy, S.; Dizdar, O.; Dede, D.S.; Harputluoglu, H.; Altundag, K. Zoledronic Acid and Atrial Fibrillation in Cancer Patients. Support. Care Cancer 2011, 19, 425–430. [Google Scholar] [CrossRef]
- Rhee, C.W.; Lee, J.; Oh, S.; Choi, N.K.; Park, B.J. Use of Bisphosphonate and Risk of Atrial Fibrillation in Older Women with Osteoporosis. Osteoporos. Int. 2012, 23, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Herrera, L.; Leal, I.; Lapi, F.; Schuemie, M.; Arcoraci, V.; Cipriani, F.; Sessa, E.; Vaccheri, A.; Piccinni, C.; Staniscia, T.; et al. Risk of Atrial Fibrillation among Bisphosphonate Users: A Multicenter, Population-Based, Italian Study. Osteoporos. Int. 2015, 26, 1499–1506. [Google Scholar] [CrossRef]
- Wang, J.-C.; Chien, W.-C.; Chung, C.-H.; Liao, W.-I.; Tsai, S.-H. Adverse Cardiovascular Effects of Nitrogen-Containing Bisphosphonates in Patients with Osteoporosis: A Nationwide Population-Based Retrospective Study. Int. J. Cardiol. 2016, 215, 232–237. [Google Scholar] [CrossRef]
- Thadani, S.R.; Ristow, B.; Blackwell, T.; Mehra, R.; Stone, K.L.; Marcus, G.M.; Varosy, P.D.; Cummings, S.R.; Cawthon, P.M. Osteoporotic Fractures in Men Study (MrOS) Research Group Relationship of Bisphosphonate Therapy and Atrial Fibrillation/Flutter: Outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study. Chest 2016, 149, 1173–1180. [Google Scholar] [CrossRef][Green Version]
- Tisdale, J.E.; Chung, M.K.; Campbell, K.B.; Hammadah, M.; Joglar, J.A.; Leclerc, J.; Rajagopalan, B.; American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing. Drug-Induced Arrhythmias: A Scientific Statement from the American Heart Association. Circulation 2020, 142, e214–e233. [Google Scholar] [CrossRef] [PubMed]
- Billington, E.O.; Reid, I.R. Benefits of Bisphosphonate Therapy: Beyond the Skeleton. Curr. Osteoporos. Rep. 2020, 18, 587–596. [Google Scholar] [CrossRef] [PubMed]
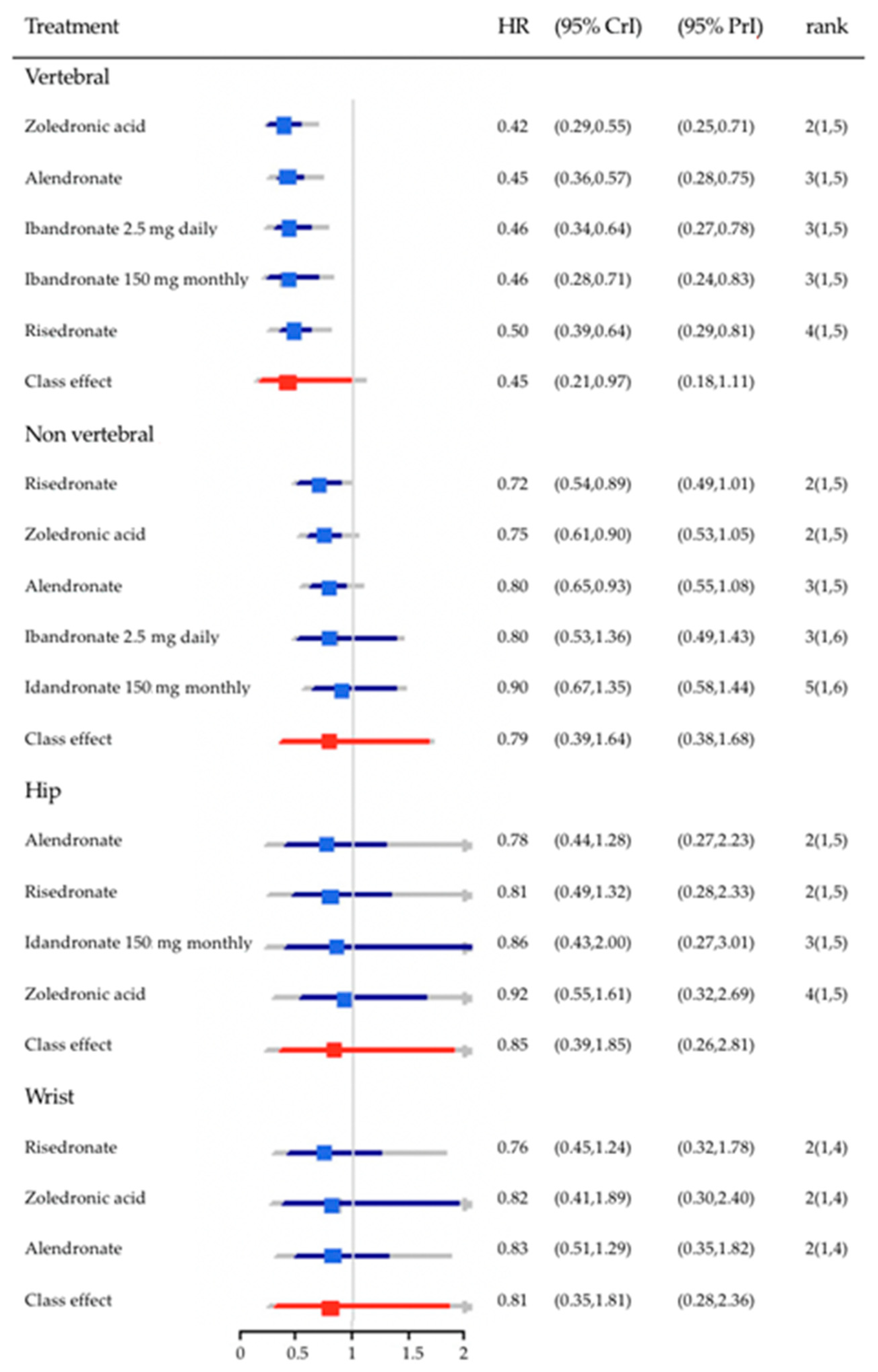
| Type of Study | Study Population | Suggested Relationship of BP in Causing AF | |
|---|---|---|---|
| Black et al. [28] | Double-blind, placebo controlled | Osteoporotic patients | Proarrhythmic |
| Abrahamsen et al. [59] | Retrospective | Fracture patients | Nonarrhythmic |
| Sorensen et al. [63] | Retrospective | Atrial fibrillation/flutter patients | Nonarrhythmic |
| Bunch et al. [65] | Prospective | Coronary angiography patients | Nonarrhythmic |
| Grosso et al. [66] | Retrospective | BP patients | Nonarrhythmic |
| Wilkinson et al. [67] | Retrospective | Cancer patients | Proarrhythmic |
| Vestergaard et al. [68] | Retrospective | Osteoporotic patients | Nonarrhythmic |
| Huang et al. [69] | Retrospective | Osteoporotic patients | Nonarrhythmic |
| Erichsen et al. [70] | Retrospective | Cancer patients | Proarrhythmic |
| Lu et al. [71] | Retrospective | Osteoporotic patients | Proarrhythmic a |
| Pazianas et al. [72] | Retrospective | BP users | Nonarrhythmic |
| Arslan et al. [73] | Cross-sectional | Cancer patients | Nonarrhythmic |
| Rhee et al. [74] | Retrospective | Osteoporotic patients | Antiarrhythmic |
| Herrera et al. [75] | Retrospective | Osteoporotic patients | Proarrhythmic |
| Wang et al. [76] | Retrospective | Osteoporotic patients | Proarrhythmic |
| Thadani et al. [77] | Prospective | Older male patients | Proarrhythmic b |
| Bisphosphonates and Cardiovascular Outcomes |
|---|
|
|
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delli Poggi, C.; Fusaro, M.; Mereu, M.C.; Brandi, M.L.; Cianferotti, L. Cardiovascular Safety and Effectiveness of Bisphosphonates: From Intervention Trials to Real-Life Data. Nutrients 2022, 14, 2369. https://doi.org/10.3390/nu14122369
Delli Poggi C, Fusaro M, Mereu MC, Brandi ML, Cianferotti L. Cardiovascular Safety and Effectiveness of Bisphosphonates: From Intervention Trials to Real-Life Data. Nutrients. 2022; 14(12):2369. https://doi.org/10.3390/nu14122369
Chicago/Turabian StyleDelli Poggi, Chiara, Maria Fusaro, Maria Cristina Mereu, Maria Luisa Brandi, and Luisella Cianferotti. 2022. "Cardiovascular Safety and Effectiveness of Bisphosphonates: From Intervention Trials to Real-Life Data" Nutrients 14, no. 12: 2369. https://doi.org/10.3390/nu14122369
APA StyleDelli Poggi, C., Fusaro, M., Mereu, M. C., Brandi, M. L., & Cianferotti, L. (2022). Cardiovascular Safety and Effectiveness of Bisphosphonates: From Intervention Trials to Real-Life Data. Nutrients, 14(12), 2369. https://doi.org/10.3390/nu14122369








