Randomized Clinical Trial: Effects of β-Hydroxy-β-Methylbutyrate (HMB)-Enriched vs. HMB-Free Oral Nutritional Supplementation in Malnourished Cirrhotic Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Outcome Measures
2.3. Statistical Analyses
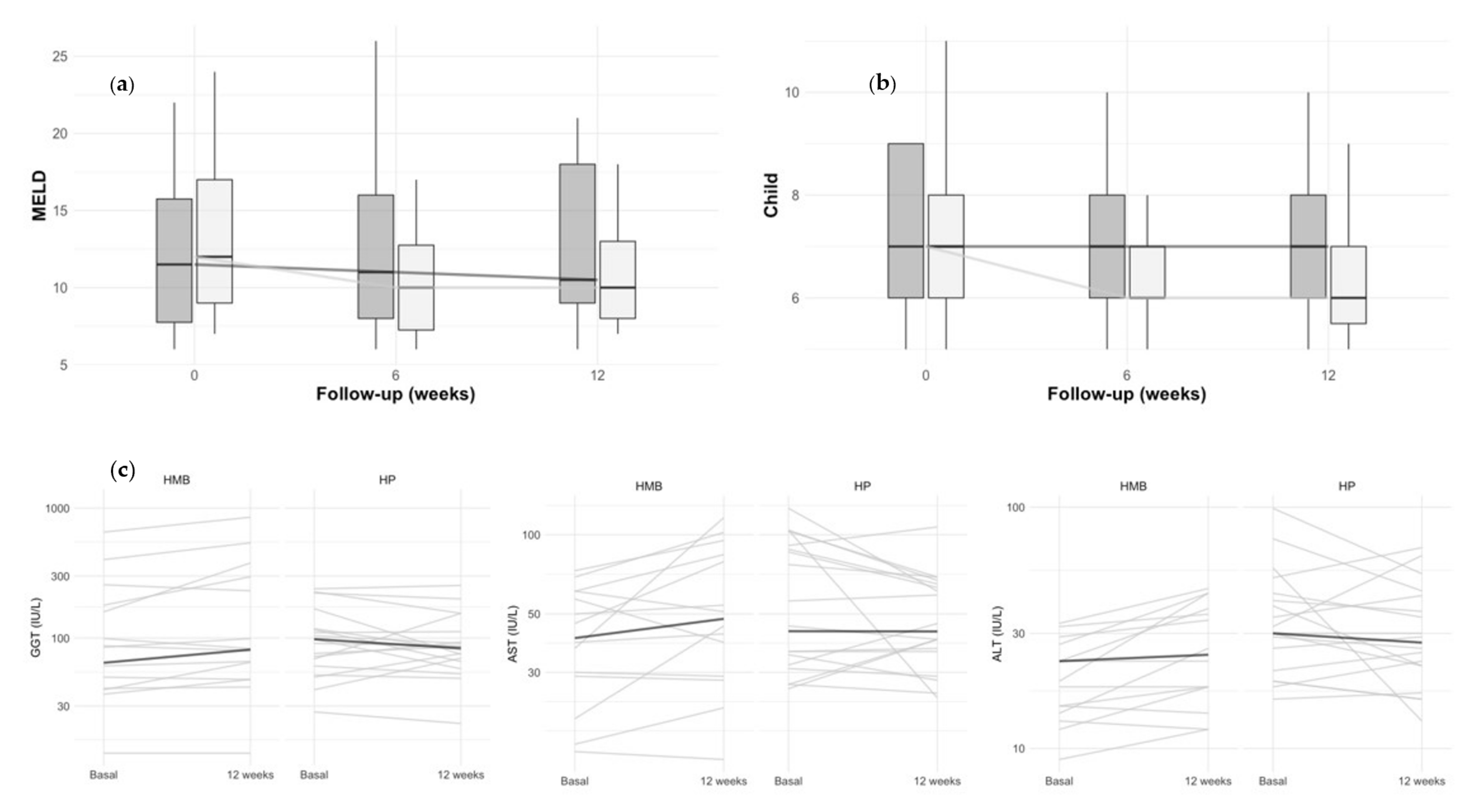
3. Results
3.1. Baseline Characteristics
3.2. Longitudinal Changes in Body Composition, Handgrip Strength, and Liver Status
3.3. Longitudinal Changes in Plasma Biochemistry Analyses and Nutritional Status
3.4. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Periyalwar, P.; Dasarathy, S. Malnutrition in Cirrhosis: Contribution and Consequences of Sarcopenia on Metabolic and Clinical Responses. Clin. Liver Dis. 2012, 16, 95–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moctezuma-Velázquez, C.; García-Juárez, I.; Soto-Solís, R.; Hernández-Cortés, J.; Torre, A. Nutritional assessment and treatment of patients with liver cirrhosis. Nutrition 2013, 29, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Eriksson, L.S.; Hagenfeldt, L.; Wahren, J. Splanchnic and leg exchange of free fatty acids in patients with liver cirrhosis. J. Hepatol. 1986, 3, 348–355. [Google Scholar] [CrossRef]
- Dasarathy, S. Cause and management of muscle wasting in chronic liver disease. Curr. Opin. Gastroenterol. 2016, 32, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Klionsky, D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Tsien, C.; Thapalaya, S.; Narayanan, A.; Weihl, C.C.; Ching, J.K.; Eghtesad, B.; Singh, K.; Fu, X.; Dubyak, G.; et al. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. AJP Endocrinol. Metab. 2012, 303, E983–E993. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Thapaliya, S.; Runkana, A.; Yang, Y.; Tsien, C.; Mohan, M.L.; Narayanan, A.; Eghtesad, B.; Mozdziak, P.E.; McDonald, C.; et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB–mediated mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 18162–18167. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Kang, S.H.; Kim, M.Y.; Baik, S.K. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186990. [Google Scholar] [CrossRef] [Green Version]
- Dasarathy, S.; Merli, M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J. Hepatol. 2016, 65, 1232–1244. [Google Scholar] [CrossRef] [Green Version]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef] [Green Version]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Muto, Y.; Moriwaki, H.; Yamato, M. Effect of long-term oral supplementation with branched-chain amino acid granules on the prognosis of liver cirrhosis. Gastroenterol. Jpn. 1989, 24, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Bianchi, G.; Merli, M.; Amodio, P.; Panella, C.; Loguercio, C.; Rossi Fanelli, F.; Abbiati, R.; Italian BCAA Study Group. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: A double-blind, randomized trial. Gastroenterology 2003, 124, 1792–1801. [Google Scholar] [CrossRef]
- Gluud, L.L.; Dam, G.; Les, I.; Marchesini, G.; Borre, M.; Aagaard, N.K.; Vilstrup, H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst. Rev. 2015, 9, CD001939. [Google Scholar]
- Nissen, S.; Sharp, R.; Ray, M.; Rathmacher, J.A.; Rice, D.; Fuller, J.C., Jr.; Connelly, A.S.; Abumrad, N. Effect of leucine metabolite beta-hydroxy-beta-methylbutyrate on muscle metabolism during resistance-exercise training. J. Appl. Physiol. 1996, 81, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Bear, D.E.; Langan, A.; Dimidi, E.; Wandrag, L.; Harridge, S.D.R.; Hart, N.; Connolly, B.; Whelan, K. β-Hydroxy-β-methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 109, 1119–1132. [Google Scholar] [CrossRef] [Green Version]
- Holeček, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle 2017, 8, 529–541. [Google Scholar] [CrossRef]
- Silva, V.R.; Belozo, F.L.; Micheletti, T.O.; Conrado, M.; Stout, J.R.; Pimentel, G.D.; Gonzalez, A.M. β-hydroxy-β-methylbutyrate free acid supplementation may improve recovery and muscle adaptations after resistance training: A systematic review. Nutr. Res. 2017, 45, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Martinez, J.; Santos-Lozano, A.; Garcia-Hermoso, A.; Sadarangani, K.P.; Cristi-Montero, C. Effects of beta-hydroxy-beta-methylbutyrate supplementation on strength and body com-position in trained and competitive athletes: A meta-analysis of randomized controlled trials. J. Sci. Med. Sport 2018, 21, 727–735. [Google Scholar] [CrossRef]
- Gepner, Y.; Varanoske, A.N.; Boffey, D.; Hoffman, J.R. Benefits of β-hydroxy-β-methylbutyrate supplementation in trained and untrained individuals. Res. Sport Med. 2019, 27, 204–218. [Google Scholar] [CrossRef]
- Portal, S.; Zadik, Z.; Rabinowitz, J.; Pilz-Burstein, R.; Adler-Portal, D.; Meckel, Y.; Cooper, D.M.; Eliakim, A.; Nemet, D. The effect of HMB supplementation on body composition, fitness, hormonal and inflammatory mediators in elite adolescent volleyball players: A prospective randomized, double-blind, placebo-controlled study. Eur. J. Appl. Physiol. 2011, 111, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xia, Y.; Jiang, J.; Du, H.; Guo, X.; Liu, X.; Li, C.; Huang, G.; Niu, K. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2015, 61, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Oktaviana, J.; Zanker, J.; Vogrin, S.; Duque, G. The effect of β-hydroxy-β-methylbutyrate (HMB) on sarcopenia and functional frailty in older persons: A systematic review. J. Nutr. Health Aging 2019, 23, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, B.; Bruni, A.; Di Cola, S.; Molfino, A.; De Santis, A.; Muscaritoli, M.; Merli, M. The Effects of 12-Week Beta-Hydroxy-Beta-Methylbutyrate Supplementation in Patients with Liver Cirrhosis: Results from a Randomized Controlled Single-Blind Pilot Study. Nutrients 2021, 13, 2296. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.H.; Feleke, G.; Din, M.; Yasmin, T.; Singh, G.; Khan, F.A.; Rathmacher, J.A. Nutritional treatment for acquired immunodeficiency virus-associated wasting using β-hydroxy β-methylbutyrate, glutamine, and arginine: A randomized, double-blind, placebo-controlled study. J. Parenter. Enter. Nutr. 2000, 24, 133–139. [Google Scholar] [CrossRef]
- Rathmacher, J.A.; Nissen, S.; Panton, L.; Clark, R.H.; Eubanks May, P.; Barber, A.E.; D’Olimpio, J.; Abumrad, N.N. Supplementation with a combination of beta-hydroxy-beta-methylbutyrate (HMB), arginine, and glutamine is safe and could improve hematological parameters. JPEN J. Parenter. Enter. Nutr. 2004, 28, 65–75. [Google Scholar] [CrossRef]
- May, P.E.; Barber, A.; TD’Olimpio, J.; Hourihane, A.; Abumrad, N.N. Reversal of cancer-related wasting using oral supplementation with a combination of β-hydroxy-β-methylbutyrate, argi-nine, and glutamine. Am. J. Surg. 2002, 183, 471–479. [Google Scholar] [CrossRef]
- Berk, L.; James, J.; Schwartz, A.; Hug, E.; Mahadevan, A.; Samuels, M.; Kachnic, L. A randomized, double-blind, placebo-controlled trial of a β-hydroxyl β-methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG 0122). Support. Care Cancer 2008, 16, 1179–1188. [Google Scholar] [CrossRef]
- Detsky, A.S.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? J. Parenter. Enter. Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Hopewell, S.; Clarke, M.; Moher, D.; Wager, E.; Middleton, P.; Altman, D.G.; Schulz, K.F. CONSORT for reporting randomised trials in journal and conference abstracts. Lancet 2008, 371, 281–283. [Google Scholar] [CrossRef]
- Holeček, M. Branched-chain amino acid supplementation in treatment of liver cirrhosis: Updated views on how to attenuate their harmful effects on cataplerosis and ammonia formation. Nutrition 2017, 41, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.N.H.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Weissenborn, K.; Ennen, J.C.; Schomerus, H.; Rückert, N.; Hecker, H. Neuropsychological characterization of hepatic encephalopathy. J. Hepatol. 2001, 34, 768–773. [Google Scholar] [CrossRef]
- Figueiredo, F.A.F.; Perez, R.D.; Kondo, M. Effect of liver cirrhosis on body composition: Evidence of significant depletion even in mild disease. J. Gastroenterol. Hepatol. 2005, 20, 209–216. [Google Scholar] [CrossRef]
- Morgan, M.Y.; Madden, A.M. The assessment of body composition in patients with cirrhosis. Eur. J. Nucl. Med. 1996, 23, 213–225. [Google Scholar] [CrossRef]
- Garcia-Almeida, J.M.; Vegas-Aguilar, I.; Rioja-Vazquez, R.; Lopez-Medina, J.A.; Cardona-Diaz, F.; Alcaide, J.; Tinahones-Madueño, F. MON-PP112: Effects on Nutritional and Functional Recovery in Patients with Protein-Energy Malnutrition after Prolonged Hospitalization: Effects of a Specific Protein-Calorie Supplementation Enriched in Calcium Hydroxymethyl Butyrate (HMB). Clin. Nutr. 2015, 34, S169–S170. [Google Scholar] [CrossRef]
- Peng, L.N.; Cheng, Y.C.; Yu, P.C.; Lee, W.J.; Lin, M.H.; Chen, L.K. Oral Nutritional Supplement with β-hydroxy-β-methylbutyrate (HMB) Improves Nutrition, Physical Performance and Ameliorates Intramuscular Adiposity in Pre-Frail Older Adults: A Randomized Controlled Trial. J. Nutr. Health Aging 2021, 25, 767–773. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Ramirez, M.; Camprubi-Robles, M.; Rueda, R.; Vegas-Aguilar, I.M.; Garcia-Almeida, J.M. Effect on an Oral Nutritional Supplement with β-Hydroxy-β-methylbutyrate and Vitamin D on Morphofunctional Aspects, Body Composition, and Phase Angle in Mal-nourished Patients. Nutrients 2021, 13, 4355. [Google Scholar] [CrossRef]
- Lattanzi, B.; Giusto, M.; Albanese, C.; Mennini, G.; D’Ambrosio, D.; Farcomeni, A.; Ginanni Corradini, S.; Rossi, M.; Merli, M. The Effect of 12 Weeks of β-Hydroxy-β-Methyl-Butyrate Supplementation after Liver Transplantation: A Pilot Randomized Controlled Study. Nutrients 2019, 11, 2259. [Google Scholar] [CrossRef] [Green Version]
- Chew, S.T.H.; Tan, N.C.; Cheong, M.; Oliver, J.; Baggs, G.; Choe, Y.; How, C.H.; Chow, W.L.; Tan, C.Y.L.; Kwan, S.C.; et al. Impact of specialized oral nutritional supplement on clinical, nutritional, and functional outcomes: A randomized, placebo-controlled trial in community-dwelling older adults at risk of malnutrition. Clin. Nutr. 2021, 40, 1879–1892. [Google Scholar] [CrossRef] [PubMed]
- Ooi, P.H.; Gilmour, S.M.; Yap, J.; Mager, D.R. Effects of branched chain amino acid supplementation on patient care outcomes in adults and children with liver cirrhosis: A systematic review. Clin. Nutr. ESPEN 2018, 28, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Espina, S.; Gonzalez-Irazabal, Y.; Sanz-Paris, A.; Lopez-Yus, M.; Garcia-Sobreviela, M.P.; Del Moral-Bergos, R.; Garcia-Rodriguez, B.; Fuentes-Olmo, J.; Bernal-Monterde, V.; Arbones-Mainar, J.M. Amino Acid Profile in Malnourished Patients with Liver Cirrhosis and Its Modification with Oral Nutritional Supplements: Implications on Minimal Hepatic Encephalopathy. Nutrients 2021, 13, 3764. [Google Scholar] [CrossRef] [PubMed]
- Olveira, G.; Olveira, C.; Doña, E.; Palenque, F.J.; Porras, N.; Dorado, A.; Godoy, A.M.; Rubio-Martínez, E.; Rojo-Martínez, G.; Martín-Valero, R. Oral supplement enriched in HMB combined with pulmonary rehabilitation improves body composition and health related quality of life in patients with bronchiectasis (Prospective, Randomised Study). Clin. Nutr. 2016, 35, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Molenberghs, G.; Thijs, H.; Jansen, I.; Beunckens, C.; Kenward, M.G.; Mallinckrodt, C.; Carroll, R.J. Analyzing incomplete longitudinal clinical trial data. Biostatistics 2004, 5, 445–464. [Google Scholar] [CrossRef] [PubMed]

| HMB Group (n = 22) | HP Group (n = 21) | p | |
|---|---|---|---|
| Age (Years) | 60.4 ± 8.61 | 61.4 ± 9.27 | 0.711 |
| Sex (Men/Female) | 14 (63.6%)/8 (26.4%) | 13 (61.9%)/8 (38.1%) | 1.000 |
| Etiology n (%) | |||
| Alcohol | 17 (77.3%) | 11 (52.4%) | 0.624 |
| HCV | 2 (9.09%) | 3 (14.3%) | |
| Autoimmune | 2 (9.09%) | 2 (9.52%) | |
| NAFLD | 1 (4.55%) | 2 (9.52%) | |
| HBV + NAFLD | 0 (0%) | 1 (4.76%) | |
| PBC | 0 (0%) | 1 (4.76%) | |
| Hemochromatosis | 0 (0%) | 1 (4.76%) | |
| Ascites | 12 (54.5%) | 9 (42.9%) | 0.645 |
| Refractory ascites | 4 (18.3%) | 0 (0%) | 0.108 |
| Previous encephalopathy | 2 (9.09%) | 3 (14.3%) | 0.664 |
| MHE (PHES) ¹ | 8 (36.4%) | 4 (19%) | 0.355 |
| Child-Pugh | 0.398 | ||
| Class A | 9 (40.9%) | 10 (47.6%) | |
| Class B | 11 (50%) | 11 (52.3%) | |
| Class C | 2 (9.09%) | 0 (0%) | |
| MELD | 12.7 ± 5.31 | 13 ± 4.7 | 0.835 |
| SGA | 0.355 | ||
| Class B | 14 (63.6%) | 17 (81.0%) | |
| Class C | 8 (36.4%) | 4 (19.0%) |
| HMB Group | HP Group | plong | plong* treatment | |||||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | |||
| BMI (Kg/m²) | 25.4 [22.5;28.8] | 27.4 [22.0;30.5] | 26.9 [21.4;30.6] | 26.3 [23.8;28.7] | 27.1 [24.4;29.0] | 25.6 [25.1;28.5] | 0.002 | 0.517 |
| FM (Kg) | 13.4 [3.45;20.8] | 15.8 [12.0;23.4] | 16.3 [5.65;20.5] | 14.2 [6.40;19.0] | 17.5 [12.9;18.8] | 16.4 [10.9;21.0] | 0.024 | 0.651 |
| FMI (Kg/m²) | 4.85 [1.25;7.15] | 6.35 [3.92;8.40] | 6.20 [1.95;7.50] | 5.30 [2.20;7.20] | 6.05 [4.67;7.47] | 6.10 [3.75;8.10] | 0.014 | 0.692 |
| %FM | 18.4 [6.15;25.6] | 25.5 [15.1;27.9] | 21.1 [8.45;26.6] | 14.2 [6.40;19.0] | 17.5 [12.9;18.8] | 16.4 [10.9;21.0] | 0.029 | 0.684 |
| FFM (Kg) | 62.1 [48.5;64.5] | 56.1 [46.6;66.3] | 57.9 [46.8;64.9] | 56.9 [50.3;68.1] | 55.8 [51.5;62.5] | 55.7 [52.3;59.6] | 0.841 | 0.963 |
| FFMI (Kg/m²) | 21.4 [19.9;22.6] | 22.2 [18.5;22.9] | 19.8 [19.1;22.4] | 21.7 [19.2;23.4] | 20.6 [19.2;21.8] | 20.3 [19.2;22.8] | 0.718 | 0.916 |
| %FFM | 81.5 [74.5;93.8] | 74.5 [72.0;84.9] | 78.9 [73.3;91.6] | 81.2 [73.9;91.6] | 77.8 [73.2;82.9] | 77.0 [71.2;86.3] | 0.029 | 0.684 |
| TBW (L) | 46.5 [35.5;50.4] | 44.4 [34.5;50.1] | 43.2 [34.5;49.6] | 41.0 [35.6;47.2] | 42.0 [37.1;47.9] | 41.8 [38.9;44.9] | 0.819 | 0.194 |
| BCM (Kg) | 33.5 [27.5;37.4] | 30.9 [24.3;37.8] | 31.2 [23.0;38.3] | 32.9 [25.1;46.1] | 29.9 [23.9;40.1] | 27.3 [25.5;35.5] | 0.069 | 0.529 |
| Body weight (Kg) | 71.8 [58.5;82.9] | 73.0 [57.8;88.5] | 70.0 [59.5;85.5] | 72.0 [64.5;79.0] | 72.5 [66.9;77.8] | 72.0 [66.3;76.2] | 0.002 | 0.619 |
| Biceps SF (mm) | 6.00 [4.50;10.0] | 6.00 [4.62;8.38] | 7.00 [4.75;11.0] | 8.00 [6.00;10.0] | 8.00 [6.25;8.50] | 7.00 [6.00;9.50] | 0.335 | 0.077 |
| Triceps SF (mm) | 12.5 [8.50;14.5] | 12.5 [10.2;19.0] | 11.0 [9.00;18.0] | 15.0 [8.50;20.0] | 11.5 [8.75;18.0] | 16.0 [9.50;20.5] | 0.015 | 0.922 |
| MUAC (cm) | 26.0 [23.2;29.8] | 27.2 [24.1;29.6] | 27.5 [23.8;29.5] | 28.0 [25.0;30.0] | 27.5 [25.5;30.2] | 28.0 [25.5;30.8] | 0.111 | 0.956 |
| MAMC (cm) | 21.3 [19.2;25.0] | 21.2 [18.0;24.6] | 21.7 [20.2;24.2] | 22.8 [20.7;25.0] | 23.3 [20.7;25.9] | 22.4 [21.2;24.9] | 0.688 | 0.344 |
| Calf circumference (cm) | 35.0 [32.9;38.0] | 34.8 [30.4;36.8] | 35.0 [31.0;37.2] | 36.0 [33.0;38.0] | 36.0 [33.2;38.2] | 35.0 [33.8;38.0] | 0.037 | 0.303 |
| Handgrip (Kg) | 26.5 [23.2;34.0] | 29.5 [24.8;35.2] | 30.0 [24.5;33.0] | 32.0 [28.0;40.0] | 33.0 [26.5;38.0] | 33.0 [25.5;37.0] | 0.095 | 0.608 |
| HMB Group | HP Group | plong | plong* treatment | |||||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | |||
| Prealbumin (mg/dL) | 11.6 [6.38;15.2] | 12.8 [8.75;16.4] | 14.5 [9.49;22.8] | 9.18 [7.62;12.8] | 11.2 [8.67;14.6] | 10.9 [9.60;13.9] | <0.001 | 0.063 |
| Albumin (g/dL) | 3.55 [3.00;4.18] | 3.70 [3.27;4.12] | 3.70 [3.35;4.05] | 3.50 [3.20;4.00] | 3.60 [3.30;4.10] | 3.50 [3.35;3.85] | 0.695 | 0.632 |
| Plasma proteins (g/dL) | 7.20 [6.82;7.40] | 7.30 [6.77;7.82] | 7.40 [6.93;7.80] | 7.10 [6.60;7.43] | 7.10 [6.72;7.50] | 7.20 [6.45;7.40] | 0.551 | 0.358 |
| Transferrin (mg/dL) | 217 [201;259] | 216 [196;281] | 230 [197;316] | 203 [148;252] | 261 [212;303] | 268 [217;308] | <0.001 | 0.757 |
| Folic acid (ng/mL) | 7.74 [6.42;9.88] | 11.0 [8.39;12.0] | 10.5 [6.86;12.9] | 9.39 [6.69;11.6] | 12.8 [8.34;14.7] | 12.1 [10.3;15.5] | <0.001 | 0.816 |
| Vitamin B12 (pg/mL) | 488 [252;778] | 488 [292;673] | 406 [330;610] | 599 [389;756] | 648 [427;771] | 598 [438;791] | 0.942 | 0.432 |
| LDL-chol (mg/dL) | 100 [70.0;146] | 83.5 [64.0;132] | 102 [73.0;133] | 100 [86.0;119] | 94.0 [79.0;118] | 95.0 [73.0;114] | 0.002 | 0.894 |
| HDL-chol (mg/dL) | 50.0 [37.2;63.0] | 49.0 [34.8;56.2] | 50.0 [35.5;69.5] | 53.0 [36.0;66.0] | 59.0 [48.0;74.0] | 56.0 [48.0;72.0] | 0.114 | 0.619 |
| Total chol (mg/dL) | 166 [130;213] | 162 [118;206] | 170 [131;218] | 168 [139;208] | 170 [152;200] | 171 [137;198] | 0.078 | 0.687 |
| Triglicerides (mg/dL) | 68.0 [51.2;93.2] | 71.0 [55.5;93.2] | 80.0 [59.0;87.0] | 68.0 [54.0;106] | 66.0 [59.0;86.0] | 68.0 [64.0;98.0] | 0.858 | 0.679 |
| APO A1 (mg/dL) | 142 [108;168] | 128 [103;174] | 119 [107;171] | 148 [117;170] | 152 [134;185] | 142 [129;177] | 0.604 | 0.381 |
| APO B (mg/dL) | 72.3 [51.3;112] | 64.2 [46.1;104] | 70.9 [46.5;95.4] | 70.3 [63.8;86.1] | 63.8 [52.2;77.1] | 65.3 [55.8;71.8] | <0.001 | 0.519 |
| Lipoprotein a (mg/dL) | 9.75 [3.21;17.1] | 13.0 [3.23;23.6] | 9.75 [4.22;20.8] | 3.40 [2.06;7.79] | 5.17 [4.13;9.93] | 4.60 [3.40;10.2] | 0.303 | 0.79 |
| Bilirubin (mg/dL) | 1.69 [1.05;2.46] | 1.42 [0.84;2.31] | 1.42 [0.94;2.23] | 1.75 [1.31;2.57] | 1.49 [0.96;2.13] | 1.38 [1.00;2.08] | 0.022 | 0.218 |
| Creatinine (mg/dL) | 0.73 [0.58;0.91] | 0.75 [0.62;0.99] | 0.80 [0.68;0.92] | 0.69 [0.61;0.81] | 0.75 [0.61;0.90] | 0.74 [0.55;0.81] | 0.608 | 0.672 |
| Urea (mg/dL) | 29.0 [24.5;42.5] | 47.0 [28.2;66.8] | 45.0 [27.5;53.0] | 28.0 [25.0;34.0] | 38.0 [30.0;44.0] | 38.0 [25.5;41.5] | 0.048 | 0.097 |
| Ammonia (µM) | 56.0 [40.0;83.0] | 59.0 [50.5;85.2] | 67.0 [49.8;74.8] | 54.0 [39.8;78.0] | 62.0 [53.2;87.2] | 67.0 [55.5;75.5] | 0.11 | 0.689 |
| ALP (U/l) | 104 [93.8;140] | 107 [93.0;134] | 110 [88.5;176] | 131 [112;158] | 127 [101;171] | 126 [98.5;146] | 0.594 | 0.172 |
| GGT (U/l) | 64.5 [40.2;100] | 91.5 [50.2;146] | 80.0 [51.0;264] | 94.5 [58.8;120] | 84.0 [54.0;95.0] | 81.0 [62.0;102] | 0.01 | 0.023 |
| AST(U/l) | 40.5 [30.8;56.5] | 41.0 [27.5;61.5] | 45.0 [29.5;81.5] | 43.0 [32.0;88.0] | 45.0 [32.0;64.0] | 40.0 [34.0;64.0] | 0.039 | 0.004 |
| ALT (U/l) | 23.0 [15.0;28.5] | 20.5 [14.0;30.5] | 23.0 [17.5;37.0] | 30.0 [20.0;41.0] | 28.0 [23.0;33.0] | 26.0 [22.0;40.0] | 0.125 | 0.032 |
| CRP (mg/dL) | 0.38 [0.15;0.68] | 0.73 [0.28;1.71] | 0.39 [0.24;1.06] | 0.66 [0.17;1.55] | 0.54 [0.13;0.99] | 0.43 [0.16;0.62] | 0.044 | 0.314 |
| Osteocalcin (ng/mL) | 7.30 [6.15;11.9] | 9.40 [7.85;12.5] | 13.4 [10.6;19.0] | 6.95 [5.17;9.12] | 9.75 [6.65;14.4] | 11.8 [7.35;16.9] | <0.001 | 0.635 |
| Vitamin D (nmol/L) | 17.5 [10.8;38.2] | 43.6 [39.1;56.5] | 51.8 [39.7;71.5] | 34.0 [15.9;40.6] | 30.2 [23.0;43.0] | 29.6 [25.1;43.0] | <0.001 | <0.001 |
| HMB (µmol/L) | 3.26 [1.54;4.47] | 20.0 [6.67;28.2] | 5.73 [4.06;34.2] | 1.61 [1.19;4.58] | 3.73 [1.58;7.51] | 2.26 [1.30;5.66] | 0.001 | 0.003 |
| Leukocytes (1000/µL) | 5.2 [4.0;7.0] | 4.8 [4.0;5.8] | 4.3 [3.9;5.4] | 5.0 [3.5;5.7] | 4.2 [3.8;5.5] | 4.3 [3.3;5.3] | 0.055 | 0.058 |
| Hemoglobin (g/dL) | 12.4 [11.1;13.5] | 12.0 [10.9;14.0] | 12.8 [11.1;13.8] | 13.1 [11.3;13.7] | 12.9 [11.5;14.1] | 12.7 [11.6;13.7] | 0.987 | 0.735 |
| Platelets (1000/µL) | 100 [72;132] | 75 [68;101] | 86 [73;101] | 96 [78;112] | 105 [84;121] | 93 [74;120] | 0.556 | 0.68 |
| INR | 1.21 [1.08;1.35] | 1.20 [1.17;1.40] | 1.19 [1.13;1.31] | 1.23 [1.12;1.41] | 1.21 [1.11;1.29] | 1.22 [1.08;1.26] | 0.561 | 0.914 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espina, S.; Sanz-Paris, A.; Gonzalez-Irazabal, Y.; Pérez-Matute, P.; Andrade, F.; Garcia-Rodriguez, B.; Carpéné, C.; Zakaroff, A.; Bernal-Monterde, V.; Fuentes-Olmo, J.; et al. Randomized Clinical Trial: Effects of β-Hydroxy-β-Methylbutyrate (HMB)-Enriched vs. HMB-Free Oral Nutritional Supplementation in Malnourished Cirrhotic Patients. Nutrients 2022, 14, 2344. https://doi.org/10.3390/nu14112344
Espina S, Sanz-Paris A, Gonzalez-Irazabal Y, Pérez-Matute P, Andrade F, Garcia-Rodriguez B, Carpéné C, Zakaroff A, Bernal-Monterde V, Fuentes-Olmo J, et al. Randomized Clinical Trial: Effects of β-Hydroxy-β-Methylbutyrate (HMB)-Enriched vs. HMB-Free Oral Nutritional Supplementation in Malnourished Cirrhotic Patients. Nutrients. 2022; 14(11):2344. https://doi.org/10.3390/nu14112344
Chicago/Turabian StyleEspina, Silvia, Alejandro Sanz-Paris, Yolanda Gonzalez-Irazabal, Patricia Pérez-Matute, Fernando Andrade, Beatriz Garcia-Rodriguez, Christian Carpéné, Alexia Zakaroff, Vanesa Bernal-Monterde, Javier Fuentes-Olmo, and et al. 2022. "Randomized Clinical Trial: Effects of β-Hydroxy-β-Methylbutyrate (HMB)-Enriched vs. HMB-Free Oral Nutritional Supplementation in Malnourished Cirrhotic Patients" Nutrients 14, no. 11: 2344. https://doi.org/10.3390/nu14112344
APA StyleEspina, S., Sanz-Paris, A., Gonzalez-Irazabal, Y., Pérez-Matute, P., Andrade, F., Garcia-Rodriguez, B., Carpéné, C., Zakaroff, A., Bernal-Monterde, V., Fuentes-Olmo, J., & Arbones-Mainar, J. M. (2022). Randomized Clinical Trial: Effects of β-Hydroxy-β-Methylbutyrate (HMB)-Enriched vs. HMB-Free Oral Nutritional Supplementation in Malnourished Cirrhotic Patients. Nutrients, 14(11), 2344. https://doi.org/10.3390/nu14112344







