Abstract
To assess the relationship between fat mass percentage (FMP) and glucose metabolism in children aged 0–18 years we performed a systematic review of the literature on Medline/PubMed, SinoMed, Embase and Cochrane Library using the PRISMA 2020 guidelines up to 12 October 2021 for observational studies that assessed the relationship of FMP and glucose metabolism. Twenty studies with 18,576 individuals were included in the meta-analysis. The results showed that FMP was significantly associated with fasting plasma glucose (FPG) (r = 0.08, 95% confidence interval (CI): 0.04–0.13, p < 0.001), fasting plasma insulin (INS) (r = 0.48, 95% CI: 0.37–0.57, p < 0.001), and homeostasis model assessment (HOMA)- insulin resistance (IR) (r = 0.44, 95% CI: 0.33–0.53, p < 0.001). The subgroup analysis according to country or overweight and obesity indicated that these associations remained significant between FMP and INS or HOMA-IR. Our results demonstrated that there is a positive relationship between FMP and FPG. Moreover, subgroup analysis according to country or overweight and obesity indicated that FMP is significantly associated with INS and HOMA-IR. This is the first known systematic review and meta-analysis to determine the associations of FMP with glucose metabolism in children and adolescents.
1. Introduction
In 2021, approximately 537 million adults (20–79 years) are living with diabetes, which has become one of the most common metabolic disorders [1]. Type 2 diabetes (T2D) has been a global epidemic [2], and childhood obesity increases the risk of developing T2D [3]. Early prevention of childhood obesity may be critical for decreasing the risk of T2D [4].
Glucose metabolism disorders are frequently found among children with obesity [5], and adipose tissue is an underlying mechanism for the relationship between birth weight and T2D [6]. Significantly higher mean levels of glucose and insulin were observed among overweight and obese children than controls [7]. Meanwhile, the prevalence of prediabetes and diabetes was high among overweight and obese children [8]. Recently, it has been considered that the evaluation of body fat mass has a certain value in predicting abnormal glucose metabolism and metabolic syndrome in children [9,10]. Fat mass percentage (FMP) was positively associated with homeostasis model assessment insulin resistance (HOMA-IR) and glycosylated hemoglobin A1c (HbA1c), and South Asian children are more metabolically sensitive to adiposity [4]. It has been demonstrated that total and central adiposity are also positively associated with insulin resistance (IR) [11], which is a component of T2D and represents the most common metabolic disorder associated with obesity [12,13]. Abdominal adipose tissue is a powerful marker for inducing IR in obese children [14] and is a more significant predictor of metabolic syndrome traits in children than in adults [15]. Although fat mass is positively related to IR and T2D, the precise role of fat mass in glucose metabolism has not been fully elucidated.
Presently, body mass index (BMI) is commonly used to measure obesity, but it cannot comprehensively assess the type and location of obesity because it cannot differentiate muscle mass and fat mass.
Due to the important role of body fat mass in predicting glucose metabolism disorders and the lack of a meta-analysis assessing the relationship between FMP and glucose metabolism (blood glucose and IR) in children, the aim of the present study is to assess the relationship between FMP and glucose metabolism in children aged 0–18 years.
2. Materials and Methods
The protocol used for the systematic review is based on PRISMA 2020 (Preferred Reporting Items for Systematic reviews and Meta-Analyses) [16] and is registered in PROSPERO (International Prospective Register of Systematic Reviews; http://www.crd.york.ac.uk/prospero; accessed on 28 April 2020) under the registration number CRD42020150180.
The literature search was conducted using MEDLINE/PubMed, EMBASE, SinoMed and the Cochrane Library. We restricted the search to (1) studies published in English or Chinese and (2) studies with human (aged less than 18 years old) samples only.
Observational studies (cross-sectional or longitudinal design) relating FMP and glucose metabolism in children and adolescents were selected. To be included, studies were required to meet the following criteria: (1) FMP was measured by the direct method, BIA/DXA/ADP/the isotopic dilution method/the four-compartment (4C) model of the body composition method, etc.; (2) assessed at least one glucose metabolism indicator (fasting glucose, IR, HOMA-IR, or HbA1c.
Studies were not included if (a) the study outcomes only focused on surrogate indicators of body composition or physical fitness rather than directly measured indicators; (b) they assessed the relationship in adults or nonhumans; (c) they involved children with severe physical limitations; (d) they referred to children with disorders that could limit generalizability (mitochondrial dysfunction, Prader–Willi syndrome, nonalcoholic fatty liver disease, polycystic ovarian syndrome, and mental disorders including attention-deficit/hyperactivity disorder (ADHD), conduct or neuropsychiatric disorders including schizophrenia, or any detected delay in communication, adaptive, cognition or socioemotional domains); and (e) they referred to a particular population group, such as an aboriginal group, immigrant group, economic status or nonhealthy child.
2.1. Data Extraction (Selection and Coding)
The abstracts of the retrieved studies were screened by two review team members, Fangfang Chen and Junting Liu. Any disagreements over the data collection were resolved by consensus, with a third researcher, Dongqing Hou, being asked if consensus could not be reached. Studies that provided the association between FMP and glucose metabolism in children and adolescents were selected and recorded. The indices of glucose metabolism included blood glucose, insulin, HbA1c, and HOMA-IR. The association described as correlation coefficient (r) and regression coefficient (β) and odds ratio (OR) values in the studies will be extracted and recorded as the main data. Other data will be extracted, including author, publication year, study year, study location, study design, body composition assessment method, sample size, age, and gender of the study population.
2.2. Risk of Bias (Quality) Assessment
‘Agency for Healthcare Research and Quality (AHRQ, Rockville, MD, USA)’ quality assessment forms recommended for cross-sectional/prevalence studies, which have 11-item checklists, were used to assess the methodological quality of the selected studies independently by two researchers (Fangfang Chen and Junting Liu). Any disagreements over the assessment of the risk of bias will be discussed, and a consensus will be reached, with a third researcher (Dongqing Hou) being consulted if consensus cannot be reached.
An item was scored ‘0’ if it was answered ‘NO’ or ‘UNCLEAR’; if it was answered ‘YES’, then the item scored ‘1’. Article quality was assessed as follows: low quality = 0–3; moderate quality = 4–7; and high quality = 8–11.
2.3. Strategy for Data Synthesis
The amount the observed variance reflecting real differences in the effect size across the included trials was graded with the I2 statistic, with values representing mild, moderate, and severe heterogeneity (<25%, 25–75%, and >75%, respectively) [17]. Subgroup analysis clustered by gender was processed according to the results of the heterogeneity test and the quality of the studies. The correlation coefficient (r) and regression coefficient (β) values of FMP and glucose metabolism (fasting plasma glucose (FPG), fasting plasma insulin (INS), and HOMA-IR) were synthesized. HOMA-IR = (INS (μU/mL) * FPG (mmol/L))/22.5. OR values were also extracted when variables were categorized and OR values were provided. Tables were created to summarize the characteristics of the selected studies, and the feasibility of conducting a meta-analysis was determined after the data were extracted.
R software was used to combine the pooled data. A fixed-effect model was used if there was no evidence of heterogeneity; otherwise, a random-effects model was used.
2.4. Analysis of Subgroups or Subsets
Subgroup and meta-regression analyses were performed with respect to the main factors causing heterogeneity, such as the measurement of FMP in different studies, the gender of the study participants, the year of study, the study country, and the study population. Furthermore, the design and methodological quality of the studies were considered for additional subgroup analyses.
2.5. Analysis of Publication Bias
We used funnel plots to assess publication bias, when ≥10 studies were included in the meta-analysis. Asymmetry of the funnel plot was measured via Egger’s test, with a p value < 0.05 indicating statistical significance.
3. Results
3.1. Identification and Selection of Studies
The original search identified 4423 studies. After removal of duplicates and elimination of papers based on eligibility criteria, 20 studies remained (Figure 1). Of the 20 studies included, 11 studies assessed the relationship between FMP and FPG, 14 studies assessed it between FMP and INS, 17 studies focused on the relationship between FMP and HOMA-IR, and 1 study calculated the correlation coefficient (r) between FMP and HbA1c.
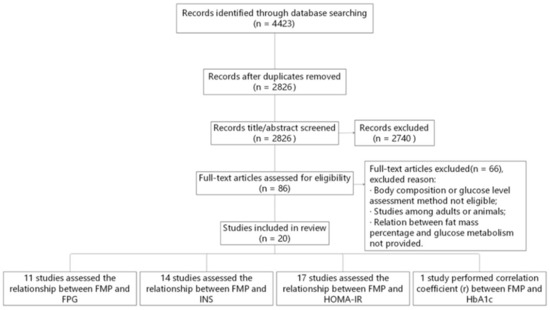
Figure 1.
Flow diagram of the literature search and paper selection process. FMP, fat mass percentage; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin A1c; HOMA-IR, homeostasis model assessment insulin resistance; INS, fasting plasma insulin.
3.2. Study Characteristics
The characteristics of all 20 studies [4,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] are shown in Table 1. The papers were based on cross-sectional study or cohort study, and were conducted in France, South Africa, the U.S.A., Guatemala, Brazil, England, China, Malaysia, Mexico, and Italy. Table 2 shows the quality evaluation of the methodology according to AHRQ quality assessment forms (US 2004). In the case of AHRQ scoring, there were 1 study with 10 points, 1 study with 9 points, 1 study with 8 points, 3 studies with 7 points, 5 studies with 6 points, 5 studies with 5 points, 3 studies with 4 points, and 1 study with 2 points. There were 3 high-quality trials with an average score of 9 points, 16 moderate-quality trials with an average score of 5.5 points, and 1 low-quality trial with 2 points.

Table 1.
Characteristics of the included trials.

Table 2.
The quality evaluation of the methodology according to Agency for Healthcare Research and Quality (AHRQ, US 2004) assessment forms *.
3.3. Relationship between FMP and FPG
Referring to the relationship between FMP and FPG, seven studies provided the correlation coefficient (r) (study ID: 1–7) (Figure 2A). Parrett et al. (study ID: 1) and Zheng et al. (study ID: 2) reported that FMP was negatively associated with FPG, but other studies (study ID: 3–7) reported that FMP was positively associated with FPG. The data of 7 studies (n = 2002) were pooled, and the results showed that after data pooling using a fixed-effect model, FMP was significantly associated with FPG (Figure 2A) (r = 0.08, 95% confidence interval (CI): 0.04–0.13, p < 0.001). There was stratified analysis of the relationship between FMP and FPG in 3 studies (study ID: 2, 6, 7). After data pooling using a fixed-effect model (Figure 2B), FMP was significantly associated with FPG in females (r = 0.10, 95% CI: 0.03–0.18, p = 0.006), and there was no significant association between FMP and FPG in males (r = 0.06, 95% CI: −0.01–0.14, p = 0.09).
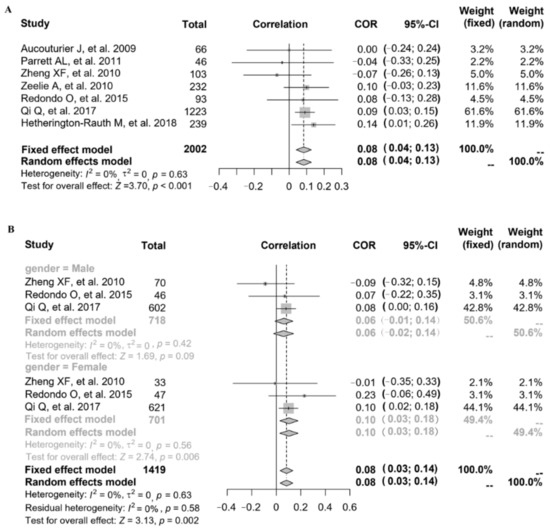
Figure 2.
The relationship between FMP and FPG: (A) 7 studies (study ID: 1–7) [18,19,20,21,22,23,24] provided the correlation coefficient (r); (B) stratified by gender in 3 studies (study ID: 2, 6, 7) [19,23,24].
Three studies also gave the regression coefficient (β) through linear analysis (study ID: 8, 9, 10) (Table 1). Coutinho et al. (study ID: 8) reported that FMP was positively associated with FPG (β = 0.09). Nightingale et al. (study ID: 10) also reported the positive relationship between FMP and FPG (β were expressed in 1 standard deviation (SD, 1SD = 9.1) increase in FMP; β = 0.69, 95% CI: 0.38–1.01, in males; β = 0.41, 95% CI: 0.09–0.73, in females). Faria et al. (study ID: 9) reported consistent results (BMI-age, P25–P75: β = 0.25; BMI-age, >P85: β = −0.22).
3.4. Relationship between FMP and INS
Referring to the relationship between FMP and INS, eleven studies provided the correlation coefficient (r) (study ID: 1–7, 12, 14–16) (Figure 3). The data of 11 studies (n = 2460) were pooled (heterogeneity, I2 = 86%), and the results showed that after data pooling using a random-effect model, FMP was significantly associated with fasting plasma insulin levels (Figure 3A) (r = 0.48, 95% CI: 0.37–0.57, p < 0.001).
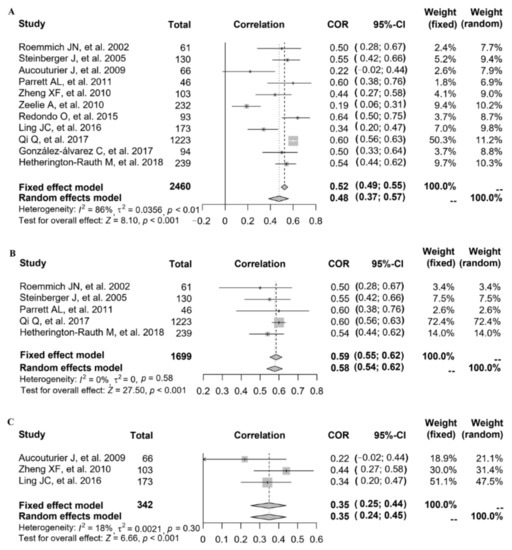
Figure 3.
The relationship between FMP and INS: (A) 11 studies (study ID: 1–7, 12, 14–16) [18,19,20,21,22,23,24,28,30,31,32] provided the correlation coefficient (r); (B) stratified by countries in 5 studies (study ID: 1, 5, 6, 12, 16) [18,22,23,28,32]; (C) stratified by overweight and obesity in 3 studies (study ID: 2, 3, 14) [19,20,30].
After excluding each study in turn, it was found that after removing the study with the greatest heterogeneity (study ID: 6), the heterogeneity slightly decreased but remained at a high level. Since no significant reduction in heterogeneity was observed, we explored the source of study heterogeneity. The heterogeneity was assessed using the I2 statistic, and the results showed that compared with age, the measurement of FMP in different studies, as well as the year of study, the study country and the study population might explain sources of heterogeneity better. When stratified by country, after data pooling using a fixed-effect model (study ID: 1, 5, 6, 12, 16; heterogeneity, I2 = 0%), FMP was significantly associated with INS in the American population (Figure 3B) (r = 0.59, 95% CI: 0.55–0.62, p < 0.001). When stratified by overweight and obesity, after data pooling using a fixed-effect model (study ID: 2, 3, 14; heterogeneity, I2 = 18%), we also found that FMP was significantly associated with INS (Figure 3C) (r = 0.35, 95% CI: 0.25–0.44, p < 0.001).
Four studies also gave the regression coefficient (β) through linear analysis (study ID: 2, 8, 9, 13) (Table 1). Zheng et al. (study ID: 2) reported that FMP was positively associated with INS (β = 0.149, in males; β = 0.226, in females). Coutinho et al. (study ID: 8, β = 0.38), Faria et al. (study ID: 9, BMI-age P25–P75: β = 0.003; BMI-age > P85: β = 0.009), and Ouyang et al. (study ID: 13, β = 0.16, in males; β = 0.14, in females) reported consistent results.
3.5. Relationship between FMP and HOMA-IR
Referring to the relationship between FMP and HOMA-IR, 11 studies provided the correlation coefficient (r) (study ID: 1–7, 14–17) (Figure 4). The data of 11 studies (n = 2409) were pooled (heterogeneity, I2 = 86%), and the results showed that after data pooling using a random-effect model, FMP was significantly associated with HOMA-IR (Figure 4A) (r = 0.44, 95% CI: 0.33–0.53, p < 0.001).
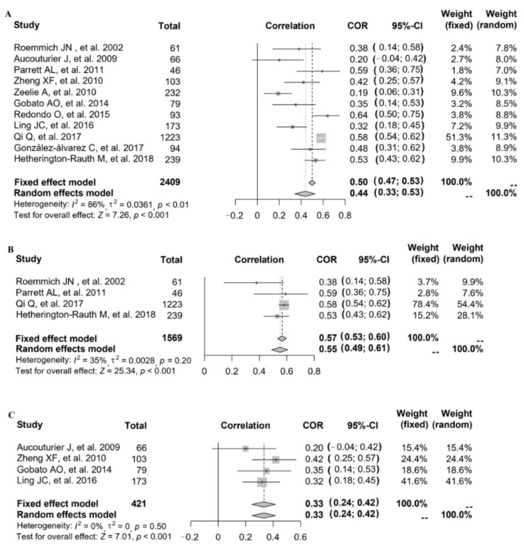
Figure 4.
The relationship between FMP and HOMA-IR: (A) 11 studies (study ID: 1–7, 14–17) [18,19,20,21,22,23,24,30,31,32,33] provided the correlation coefficient (r); (B) stratified by countries in 4 studies (study ID: 1, 5, 6, 16) [18,22,23,32]; (C) stratified by overweight and obesity in 4 studies.
After excluding each study in turn, it was found that after removing one study with the greatest heterogeneity (study ID: 6), the heterogeneity slightly decreased but remained at a high level. Since no significant reduction in heterogeneity was observed, we explored the source of study heterogeneity. The heterogeneity was assessed using the I2 statistic, and the results showed that compared with age, the measurement of FMP in different studies, as well as the year of study, the study country, and the study population might explain sources of heterogeneity better. When stratified by country, after data pooling using a fixed-effect model (study ID: 1, 5, 6, 16; heterogeneity, I2 = 35%), FMP was significantly associated with HOMA-IR in the American population (Figure 4B) (r = 0.57, 95% CI: 0.53–0.60, p < 0.001). When stratified by overweight and obesity, after data pooling using a fixed-effect model (study ID: 2, 3, 14, 17; heterogeneity, I2 = 0%), we also found that FMP was significantly associated with HOMA-IR (Figure 4C) (r = 0.33, 95% CI: 0.24–0.42, p < 0.001).
Seven studies also gave the regression coefficient (β) through linear analysis (study ID: 2, 8–10, 18–20) (Table 1). Among these studies, six studies (study ID: 2, 8–10, 18, 19) reported that FMP was positively associated with HOMA-IR, and one study (study ID: 20) reported that FMP was negatively associated with HOMA-IR.
In addition, Chen et al. (study ID: 11) reported that FMP was positively associated with impaired fasting glucose (IFG) (IFG: FPG ≥ 5.6 mmol/L; ORs were expressed in 1 SD increase in FMP; OR = 1.18, 95% CI: 1.11–1.26, in males; OR = 1.17, 95% CI: 1.09–1.26, in females). Nightingale et al. (study ID: 10) reported the positive relationship between FMP and HbA1c (β were expressed in 1 SD (1SD = 9.1) increase in FMP; β = 4.07, 95% CI: 2.77–5.36, in males; β = 2.65, 95% CI: 1.34–3.96, in females).
3.6. Publication Bias Evaluation
When ≥10 studies were included in the meta-analysis, publication bias was assessed using funnel plots and Egger’s test. The results showed that the distribution of included studies on both sides of the funnel plot was asymmetric. A publication bias was detected between FMP and INS or HOMA-IR (Supplementary Figure S1), which was mainly derived from between-research heterogeneity.
4. Discussion
Considering that fat mass plays a critical role in glucose metabolism, most previous research concerns the positive relationship of fat mass with IR and T2D. However, the precise role of fat mass in glucose metabolism has not been fully elucidated. There has been no meta-analysis to assess the relationship between FMP and glucose metabolism in children.
To exclude the influence of confounding factors, such as age and degenerative disease, the study of the pediatric population could be better to clarify the relationship between FMP and glucose metabolism. Our research is the first meta-analysis of the relationship between FMP and glucose metabolism in children and adolescents. The results demonstrated that FMP is positively associated with FPG in children and adolescents. Moreover, subgroup analysis according to country or overweight and obesity indicated that FMP is significantly associated with INS and HOMA-IR.
Interestingly, after subgroup analysis stratified by gender, the results showed that FMP was significantly associated with FPG in females, but there was no significant association between FMP and FPG in males. This suggests that there might be gender differences in the relationship between FMP and FPG. Muscles have estrogen receptors, which can increase the rate of glucose uptake into the muscle when they are activated. It is well known that skeletal muscle is an important component of body composition and a metabolic sink for glucose disposal [37]. There are also differences between genders in where fat is stored and the characteristics of that fat. These factors cause differences in metabolism between men and women. A recent study reported that the positive correlation between FMP and insulin in girls was more pronounced than that in boys [38]. This might be one of the reasons why there were gender differences in the relationship between FMP and FPG.
Skeletal muscle also plays an important role in the regulation of blood glucose levels and affects the development of IR and T2D [39]. A cross-sectional study revealed that trunk muscle quality was related to glucose tolerance [40], and a longitudinal study showed a positive association between abdominal muscle quantity and T2D [41]. A previous study reported that decreased skeletal muscle mass is associated with deterioration of insulin sensitivity [42]. Thus, targeting the mechanism(s) underlying skeletal muscle activity in glucose metabolism may prevent or delay IR and T2D.
Our literature search was conducted using MEDLINE/PubMed, EMBASE, SinoMed, and the Cochrane Library, and the original search identified 4423 studies. After removal of duplicates and elimination of papers based on eligibility criteria, 20 studies remained. Of the 20 studies included, 11 studies (study ID: 1–11) assessed the relationship between FMP and FPG, 14 studies (ID: 1–9, 12–16) assessed it between FMP and INS, and 17 studies (study ID: 1–10, 14–20) focused on the relationship between FMP and HOMA-IR. There were some studies of the associations of FMP with FPG, INS, and HOMA-IR in adult populations. For example, Borschmann et al. reported that reducing sedentary time and fat mass within 6 months of stroke might improve glucose tolerance and insulin resistance [43]. Zeng et al. showed that high fat mass increased fasting glucose, HOMA-IR, triglycerides, decreased high-density lipoprotein cholesterol, and high fat-free mass reduced HOMA-IR, triglycerides, and low-density lipoprotein cholesterol [44]. Park et al. reported that appendicular fat (%) had a negative correlation with glucose, log insulin, and HbA1c [45]. Müller et al. showed that small decreases and increases in fat mass were associated with corresponding decreases and increases in insulin secretion as well as increases and decreases in insulin sensitivity [46]. However, these studies could not exclude the influence of confounding factors such as age and degenerative disease.
Childhood obesity increases the risk of abnormal glucose metabolism. Anthropometric measures, such as BMI and waist-to-height ratio, are widely used to evaluate obesity, but high levels of these indicators do not equal high FMP. BMI and waist-to-height ratio are widely used to evaluate adiposity owing to their feasibility and low cost, but their use is limited because they do not discriminate for muscle mass and fat mass and cannot comprehensively assess the type and location of obesity [47]. A higher BMI may arise not only from greater body fat, but also from either higher lean mass, bone mass, or both, making it an imperfect measure of adiposity [48,49]. The evaluation of body fat mass has a certain value in predicting abnormal glucose metabolism and metabolic syndrome in children. A recent study also suggests that the associations of body fat and bone parameters with insulin were clearer when FMP was used instead of BMI to classify obesity [38]. Since FMP plays an important role in glucose metabolism, it is important to study the relationship between FMP and glucose metabolism in children.
Previous studies showed that sex hormones are significantly correlated with glucose and lipid metabolism. Premenopausal women exhibit increased insulin sensitivity compared with men, but this advantage disappears after menopause, in part owing to a reduction of 17β-estradiol (E2). Xu et al. reported that serum E2 and sex hormone binding globulin (SHBG) could influence HbA1c levels in non-diabetic populations [50]. Kim et al. showed that E2, testosterone, and SHBG levels are associated with lipid subfractions in adults [51]. Similar studies have been conducted in children. Adolescent glucose metabolism may be influenced by testosterone, perhaps partially via skeletal muscle mass [52]. Total testosterone was negatively associated with fasting glucose, insulin, and HOMA-IR, but these associations were attenuated by additional adjustment for skeletal mass index or FMP.
There was a strong relationship between insulin and SHBG, and SHBG could be a marker for either hyperinsulinemia, IR, or both, in obese children (aged 6–9 years) [53]. Ortega et al. also showed that SHBG is related to insulin and HOMA-IR independently of age in both sexes [54]. A recent study reported that E2 was negatively correlated with leptin in normal-weight boys, whereas testosterone was negatively correlated with leptin in overweight/obese boys [55].
Sex hormone levels and SHBG also have important effects on high-density lipoprotein (HDL) and IR among children and adolescents [56]. E2 drives a typically atheroprotective lipid profile through upregulation of HDL/ApoA1 [57]. SHBG levels are related to a decrease in HDL and ApoA1 levels during puberty in boys and to a decrease in triglycerides levels during puberty in both sexes [58].
Obesity is known to impact reproductive hormones during puberty. Youth with obesity have lower SHBG than those with normal weight [59]. During weight loss, SHBG and insulin sensitivity increased significantly in obese children, and the changes were independent of gender, puberty, and the changes in BMI [60].
However, several limitations should also be addressed. First, due to limited conditions, only MEDLINE/PubMed, EMBASE, SinoMed, and the Cochrane Library were searched, and only studies published in English or Chinese were included. There have been limited studies on FMP and glucose metabolism in children and adolescents, and there might be omissions. Second, in this study, funnel plot analysis was performed on FMP and INS or HOMA-IR. The results showed that the distribution of included studies on both sides of the funnel plot was asymmetric, indicating that there was potential publication bias in FMP and INS or HOMA-IR, which might be mainly derived from study heterogeneity. Nevertheless, our findings could avoid some potential bias because the results of stratification according to confounders, such as the study country and the study population, showed significant associations of FMP with INS and HOMA-IR. Third, heterogeneity among the studies was significant, which could be partly explained by the study country and the study population. Fourth, this is a systematic literature review examining the association between FMP and glucose metabolism through observational studies. The search selection criteria for research design included cross-sectional studies, and most of the articles actually searched are cross-sectional studies. In statistical analysis, correlation analysis was also attempted. However, meta-analysis data on correlation using cross-sectional studies have limitations in identifying causation.
5. Conclusions
From this systematic review and meta-analysis, we demonstrated that there is a positive relationship between FMP and FPG. Moreover, subgroup analysis according to country or overweight and obesity indicated that FMP is significantly associated with INS and HOMA-IR. This is the first known systematic review and meta-analysis to determine the associations of FMP with glucose metabolism in children and adolescents. Our results provide novel information to fill a gap in the literature. Further examination of these relationships requires more cohort studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14112272/s1, Figure S1: The funnel plots of the associations of FMP with INS (A) and HOMA-IR (B).
Author Contributions
Conceptualization, F.C.; methodology, F.C., J.L. and D.H.; software, F.C., J.L. and D.H.; validation, F.C., J.L. and D.H.; formal analysis, F.C., J.L. and D.H.; investigation, F.C., J.L. and D.H.; resources, F.C., J.L. and D.H.; data curation, F.C., J.L. and D.H.; writing—original draft preparation, L.W. and F.C.; writing—review and editing, F.C., L.W., T.L., Y.C. and Z.L.; visualization, L.W. and F.C.; supervision, F.C.; project administration, F.C.; funding acquisition, F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China, grant number 2016YFC1300100, public service development and reform pilot project of Beijing Medical Research Institute, grant number BMR2019-11, and Beijing Municipal Administration of Hospitals Incubating Program, grant number Px2022052.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- International Diabetic Federation. Diabetes Atlas, 10th Edition. 2021. Available online: https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html (accessed on 22 May 2022).
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Valaiyapathi, B.; Gower, B.; Ashraf, A.P. Pathophysiology of Type 2 Diabetes in Children and Adolescents. Curr. Diabetes Rev. 2020, 16, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, C.M.; Rudnicka, A.R.; Owen, C.G.; Wells, J.C.; Sattar, N.; Cook, D.G.; Whincup, P.H. Influence of adiposity on insulin resistance and glycemia markers among U.K. Children of South Asian, black African-Caribbean, and white European origin: Child heart and health study in England. Diabetes Care 2013, 36, 1712–1719. [Google Scholar] [CrossRef]
- Brufani, C.; Ciampalini, P.; Grossi, A.; Fiori, R.; Fintini, D.; Tozzi, A.; Cappa, M.; Barbetti, F. Glucose tolerance status in 510 children and adolescents attending an obesity clinic in Central Italy. Pediatr. Diabetes 2010, 11, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yu, X.; Li, Y.; Li, G.; Cheng, H.; Xiao, X.; Mi, J.; Gao, S.; Willi, S.; Li, M. Adipose tissue mediates associations of birth weight with glucose metabolism disorders in children. Obesity 2019, 27, 746–755. [Google Scholar] [CrossRef]
- Alissa, E.M.; Sutaih, R.H.; Kamfar, H.Z.; Alagha, A.E.; Marzouki, Z.M. Relationship between pediatric adiposity and cardiovascular risk factors in Saudi children and adolescents. Arch. Pediatr. 2020, 27, 135–139. [Google Scholar] [CrossRef]
- Al Amiri, E.; Abdullatif, M.; Abdulle, A.; Al Bitar, N.; Afandi, E.Z.; Parish, M.; Darwiche, G. The prevalence, risk factors, and screening measure for prediabetes and diabetes among Emirati overweight/obese children and adolescents. BMC Public Health 2015, 15, 1298. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Cheng, H.; Hou, D.Q.; Gao, A.Y.; Zhu, Z.X.; Yu, Z.C.; Wang, H.J.; Zhao, X.Y.; Xiao, P.; Huang, G.M.; et al. Value of body fat mass measured by bioelectrical impedance analysis in predicting abnormal blood pressure and abnormal glucose metabolism in children. Chin. J. Contemp. Pediatrics 2020, 22, 17–23. [Google Scholar]
- Calcaterra, V.; Verduci, E.; De Silvestri, A.; Magenes, V.C.; Siccardo, F.; Schneider, L.; Vizzuso, S.; Bosetti, A.; Zuccotti, G. Predictive ability of the estimate of fat mass to detect early-onset metabolic syndrome in prepubertal children with obesity. Children 2021, 8, 966. [Google Scholar] [CrossRef]
- Krekoukia, M.; Nassis, G.P.; Psarra, G.; Skenderi, K.; Chrousos, G.P.; Sidossis, L.S. Elevated total and central adiposity and low physical activity are associated with insulin resistance in children. Metabolism 2007, 56, 206–213. [Google Scholar] [CrossRef]
- Kindler, J.M.; Lobene, A.J.; A Vogel, K.; Martin, B.R.; McCabe, L.D.; Peacock, M.; Warden, S.J.; McCabe, G.P.; Weaver, C.M. Adiposity, insulin resistance, and bone mass in children and adolescents. J. Clin. Endocrinol. Metab. 2019, 104, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Tagi, V.M.; Chiarelli, F. Obesity and insulin resistance in children. Curr. Opin. Pediatr. 2020, 32, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhao, X.; He, H.; Mao, X.; Sheng, J.; Zou, J.; Tang, Q.; Cai, W. The effect of different adiposity factors on insulin resistance in obese children and adolescents. Clin. Endocrinol. 2021, 94, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Cerjak, D.; Kent, J.W.; James, R.; Blangero, J.; Zhang, Y. Obesity, central adiposity and cardiometabolic risk factors in children and adolescents: A family-based study. Pediatr. Obes. 2014, 9, e58–e62. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Parrett, A.L.; Valentine, R.J.; Arngrímsson, S.A.; Castelli, D.M.; Evans, E.M. Adiposity and aerobic fitness are associated with metabolic disease risk in children. Appl. Physiol. Nutr. Metab. 2011, 36, 72–79. [Google Scholar] [CrossRef]
- Zheng, X.F.; Tang, Q.Y.; Tao, Y.X.; Lu, W.; Cai, W. Clinical value of methods for analyzing the abdominal fat levels of obese children and adolescents. Obes. Metab. 2010, 6, 105–110. [Google Scholar]
- Aucouturier, J.; Meyer, M.; Thivel, D.; Taillardat, M.; Duché, P. Effect of android to gynoid fat ratio on insulin resistance in obese youth. Arch. Pediatr. Adolesc. Med. 2009, 163, 826–831. [Google Scholar] [CrossRef]
- Zeelie, A.; Moss, S.J.; Kruger, H.S. The relationship between body composition and selected metabolic syndrome markers in black adolescents in South Africa: The PLAY study. Nutrition 2010, 26, 1059–1064. [Google Scholar] [CrossRef]
- Hetherington-Rauth, M.; Bea, J.W.; Lee, V.R.; Blew, R.M.; Funk, J.L.; Lohman, T.G.; Going, S.B. Relationship between fat distribution and cardiometabolic risk in Hispanic girls. Am. J. Hum. Biol. 2018, 30, e23149. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Hua, S.; Perreira, K.M.; Cai, J.; van Horn, L.; Schneiderman, N.; Thyagarajan, B.; Delamater, A.M.; Kaplan, R.C.; Isasi, C.R. Sex differences in associations of adiposity measures and insulin resistance in US Hispanic/Latino Youth: The Hispanic Community Children’s Health Study/Study of Latino Youth (SOL Youth). J. Clin. Endocrinol. Metab. 2017, 102, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Redondo, O.; Villamor, E.; Valdés, J.; Bilal, U.; Caballero, B.; Roche, D.; Kroker, F.; Ramírez-Zea, M.; Franco, M. Validation of a BMI cut-off point to predict an adverse cardiometabolic profile with adiposity measurements by dual-energy X-ray absorptiometry in Guatemalan children. Public Health Nutr. 2015, 18, 951–958. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coutinho, P.R.; Leite, N.; Lopes, W.A.; Da Silva, L.R.; Consentino, C.M.; Araújo, C.T.; Moraes, F.B., Jr.; De Jesus, I.C.; Cavaglieri, C.R.; Radominski, R.B. Association between adiposity indicators, metabolic parameters and inflammatory markers in a sample of female adolescents. Arch. Endocrinol. Metab. 2015, 59, 325–334. [Google Scholar] [CrossRef]
- Faria, F.R.; Faria, E.R.; Cecon, R.S.; Júnior, D.A.B.; Franceschini, S.D.C.C.; Peluzio, M.D.C.G.; Ribeiro, A.Q.; Lira, P.I.C.; Cecon, P.R.; Priore, S.E. Body fat equations and electrical bioimpedance values in prediction of cardiovascular risk factors in eutrophic and overweight adolescents. Int. J. Endocrinol. 2013, 2013, 501638. [Google Scholar] [CrossRef]
- Chen, F.; Liu, J.; Yan, Y.; Mi, J.; on behalf of the China Child and Adolescent Cardiovascular Health (CCACH) Study Group. Abnormal metabolic phenotypes among urban Chinese children: Epidemiology and the impact of DXA-measured body composition. Obesity 2019, 27, 837–844. [Google Scholar] [CrossRef]
- Steinberger, J.; Jacobs, D.R.; Raatz, S.; Moran, A.; Hong, C.P.; Sinaiko, A.R. Comparison of body fatness measurements by BMI and skinfolds vs dual energy X-ray absorptiometry and their relation to cardiovascular risk factors in adolescents. Int. J. Obes. 2005, 29, 1346–1352. [Google Scholar] [CrossRef]
- Ouyang, F.; Christoffel, K.K.; Brickman, W.J.; Zimmerman, N.; Wang, B.; Xing, H.; Zhang, S.; Arguelles, L.M.; Wang, G.; Liu, R.; et al. Adiposity is inversely related to insulin sensitivity in relatively lean Chinese adolescents: A population-based twin study. Am. J. Clin. Nutr. 2010, 91, 662–671. [Google Scholar] [CrossRef]
- Ling, J.C.; Mohamed, M.N.; Jalaludin, M.Y.; Rampal, S.; Zaharan, N.L.; Mohamed, Z. Determinants of high fasting insulin and insulin resistance among overweight/obese adolescents. Sci. Rep. 2016, 6, 36270. [Google Scholar] [CrossRef]
- González-Álvarez, C.; Ramos-Ibáñez, N.; Azprioz-Leehan, J.; Ortiz-Hernández, L. Intra-abdominal and subcutaneous abdominal fat as predictors of cardiometabolic risk in a sample of Mexican children. Eur. J. Clin. Nutr. 2017, 71, 1068–1073. [Google Scholar] [CrossRef]
- Roemmich, J.N.; Clark, P.A.; Lusk, M.; Friel, A.; Weltman, A.; Epstein, L.H.; Rogol, A.D. Pubertal alterations in growth and body composition. VI. Pubertal insulin resistance: Relation to adiposity, body fat distribution and hormone release. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Gobato, A.O.; Vasques, A.C.; Zambon, M.P.; Barros Filho Ade, A.; Hessel, G. Metabolic syndrome and insulin resistance in obese adolescents. Rev. Paul. Pediatr. 2014, 32, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Cortes, L.; Villasis-Keever, M.A.; Del Prado-Manriquez, M.; Lopez-Alarcon, M. Adiposity and insulin resistance in children from a rural community in Mexico. Arch. Med. Res. 2015, 46, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.C.; Cintra Ide, P.; Fisberg, M.; Martini, L.A. Body trunk fat and insulin resistance in post-pubertal obese adolescents. Sao Paulo Med. J. 2008, 126, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Gastaldelli, A.; Agosti, F.; De Col, A.; Marazzi, N.; Mazzilli, G.; Saezza, A.; Sartorio, A. Impact of percent body fat on oral glucose tolerance testing: A cross-sectional study in 1512 obese children. J. Endocrinol. Invest. 2012, 35, 893–896. [Google Scholar]
- Smith, A.G.; Muscat, G.E. Skeletal muscle and nuclear hormone receptors: Implications for cardiovascular and metabolic disease. Int. J. Biochem. Cell Biol. 2005, 37, 2047–2063. [Google Scholar] [CrossRef]
- Khwanchuea, R.; Punsawad, C. Sex differences in the relationship between body composition and biomarkers of bone and fat metabolism in obese boys and girls. Bone Rep. 2021, 14, 101087. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef]
- Maltais, A.; Alméras, N.; Lemieux, I.; Tremblay, A.; Bergeron, J.; Poirier, P.; Després, J.P. Trunk muscle quality assessed by computed tomography: Association with adiposity indices and glucose tolerance in men. Metabolism 2018, 85, 205–212. [Google Scholar] [CrossRef]
- Granados, A.; Gebremariam, A.; Gidding, S.; Terry, J.G.; Carr, J.J.; Steffen, L.M.; Jacobs, D.R.; Lee, J.M. Association of abdominal muscle composition with prediabetes and diabetes: The CARDIA study. Diabetes Obes. Metab. 2019, 21, 267–275. [Google Scholar] [CrossRef]
- Srikanthan, P.; Karlamangla, A.S. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J. Clin. Endocrinol. Metab. 2011, 96, 2898–2903. [Google Scholar] [CrossRef] [PubMed]
- Borschmann, K.N.; Ekinci, E.I.; Iuliano, S.; Churilov, L.; Pang, M.Y.; Bernhardt, J. Reducing sedentary time and fat mass may improve glucose tolerance and insulin sensitivity in adults surviving 6 months after stroke: A phase I pilot study. Eur. Stroke J. 2017, 2, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Lin, C.; Wang, S.; Zheng, Y.; Gao, X. Genetically predicted body composition in relation to cardiometabolic traits: A Mendelian randomization study. Eur. J. Epidemiol. 2021, 36, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kwon, K.Y.; Kim, J.H.; Choi, H.H.; Han, K.H.; Han, J.H. Association between appendicular fat mass and metabolic risk factors. Korean J. Fam. Med. 2014, 35, 182–189. [Google Scholar] [CrossRef][Green Version]
- Müller, M.J.; Lagerpusch, M.; Enderle, J.; Schautz, B.; Heller, M.; Bosy-Westphal, A. Beyond the body mass index: Tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obes. Rev. 2012, 13 (Suppl. S2), 6–13. [Google Scholar] [CrossRef]
- Yajnik, C.S.; Yudkin, J.S. The Y-Y paradox. Lancet 2004, 363, 163. [Google Scholar] [CrossRef]
- Wells, J.C.K. Toward body composition reference data for infants, children, and adolescents. Adv. Nutr. 2014, 5, 320S–329S. [Google Scholar] [CrossRef]
- Javed, A.; Jumean, M.; Murad, M.H.; Okorodudu, D.; Kumar, S.; Somers, V.K.; Sochor, O.; Lopez-Jimenez, F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity in children and adolescents: A systematic review and meta-analysis. Pediatr. Obes. 2015, 10, 234–244. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, W.; Shen, Y.; Tang, J.; Wang, Y.; Ma, X.; Bao, Y. The relationship between sex hormones and glycated hemoglobin in a non-diabetic middle-aged and elderly population. BMC Endocr. Disord. 2022, 22, 91. [Google Scholar] [CrossRef]
- Kim, C.; Kong, S.; Krauss, R.M.; Stanczyk, F.Z.; Reddy, S.T.; Needham, B.; Kanaya, A.M. Endogenous sex steroid hormones, lipid subfractions, and ectopic adiposity in Asian Indians. Metab. Syndr. Relat. Disord. 2015, 13, 445–452. [Google Scholar] [CrossRef]
- Hou, W.W.; Tse, M.A.; Lam, T.H.; Leung, G.M.; Schooling, C.M. Adolescent testosterone, muscle mass and glucose metabolism: Evidence from the ‘Children of 1997’ birth cohort in Hong Kong. Diabet. Med. 2015, 32, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Gascón, F.; Valle, M.; Martos, R.; Ruz, F.J.; Ríos, R.; Montilla, P.; Canete, R. Sex hormone-binding globulin as a marker for hyperinsulinemia and/or insulin resistance in obese children. Eur. J. Endocrinol. 2000, 143, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Ortega, L.; Garcia-Anguita, A.; Riestra, P.; Ortega, H.; Soriano-Guillén, L.; Lasunción, M.A.; de Oya, M.; Garces, C. Plasma non-esterified fatty acid levels in children and their relationship with sex steroids. Steroids 2014, 88, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Peng, X.; Wang, Y.; Cao, R.; Zhang, Z.; Fu, L. What is the relationship between body mass index, sex hormones, leptin, and Irisin in children and adolescents? A path analysis. Front. Pediatr. 2022, 10, 823424. [Google Scholar] [CrossRef]
- Agirbasli, M.; Agaoglu, N.B.; Orak, N.; Caglioz, H.; Ocek, T.; Karabag, T.; Baykan, O.A. Sex hormones, insulin resistance and high-density lipoprotein cholesterol levels in children. Horm. Res. Paediatr. 2010, 73, 166–174. [Google Scholar] [CrossRef]
- Robinson, G.A.; Peng, J.; Peckham, H.; Radziszewska, A.; Butler, G.; Pineda-Torra, I.; Jury, E.C.; Ciurtin, C. Sex hormones drive changes in lipoprotein metabolism. iScience 2021, 24, 103257. [Google Scholar] [CrossRef]
- Garcés, C.; de Oya, I.; Lasunción, M.A.; López-Simón, L.; Cano, B.; de Oya, M. Sex hormone-binding globulin and lipid profile in pubertal children. Metabolism 2010, 59, 166–171. [Google Scholar] [CrossRef]
- Nokoff, N.; Thurston, J.; Hilkin, A.; Pyle, L.; Zeitler, P.S.; Nadeau, K.J.; Santoro, N.; Kelsey, M.M. Sex differences in effects of obesity on reproductive hormones and glucose metabolism in early puberty. J. Clin. Endocrinol. Metab. 2019, 104, 4390–4397. [Google Scholar] [CrossRef]
- Birkebæk, N.H.; Lange, A.; Holland-Fischer, P.; Kristensen, K.; Rittig, S.; Vilstrup, H.; Handberg, A.; Gronbaek, H. Effect of weight reduction on insulin sensitivity, sex hormone-binding globulin, sex hormones and gonadotrophins in obese children. Eur. J. Endocrinol. 2010, 163, 895–900. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).