Selenium Supplementation in Pregnant Women with Autoimmune Thyroiditis: A Practical Approach
Abstract
:1. Introduction
2. Selenium Intake and Status
3. Selenium Supplementation and Autoimmune Thyroiditis
4. Selenium Status, Autoimmune Thyroiditis, and Fertility
5. Autoimmune Thyroiditis in Pregnancy
6. Selenium Status, Thyroid, and Pregnancy
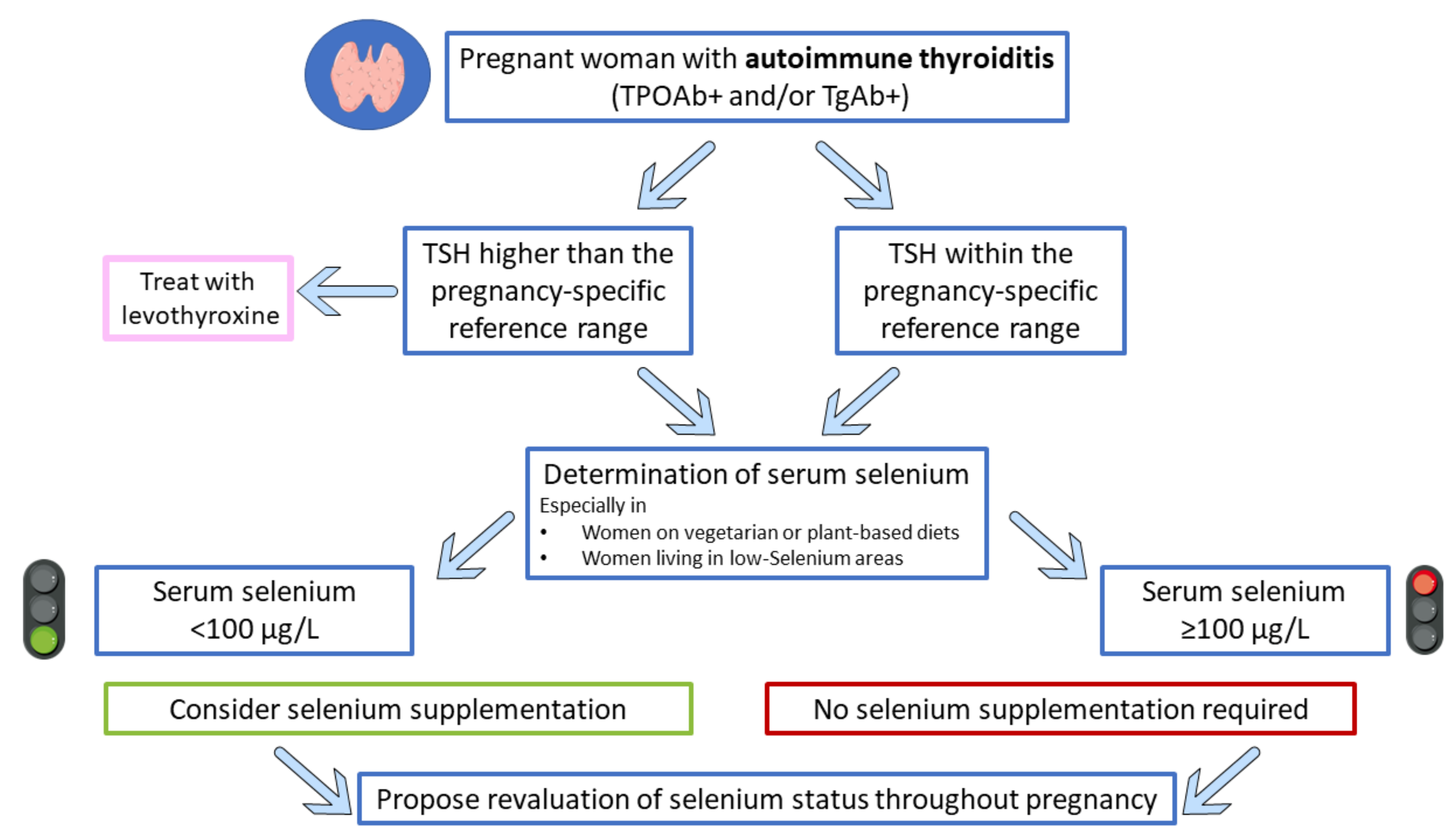
7. Selenium Supplementation in Pregnant Women with Autoimmune Thyroiditis: Has the Time Come?
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones (Athens) 2020, 19, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Winther, K.H.; Rayman, M.P.; Bonnema, S.J.; Hegedus, L. Selenium in thyroid disorders—Essential knowledge for clinicians. Nat. Rev. Endocrinol. 2020, 16, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Kohrle, J. Selenium in Endocrinology-Selenoprotein-Related Diseases, Population Studies, and Epidemiological Evidence. Endocrinology 2021, 162, bqaa228. [Google Scholar] [CrossRef] [PubMed]
- Kohrle, J.; Jakob, F.; Contempre, B.; Dumont, J.E. Selenium, the thyroid, and the endocrine system. Endocr. Rev. 2005, 26, 944–984. [Google Scholar] [CrossRef]
- Schomburg, L. Selenium Deficiency Due to Diet, Pregnancy, Severe Illness, or COVID-19-A Preventable Trigger for Autoimmune Disease. Int. J. Mol. Sci. 2021, 22, 8532. [Google Scholar] [CrossRef] [PubMed]
- Hogan, C.; Perkins, A.V. Selenoproteins in the Human Placenta: How Essential Is Selenium to a Healthy Start to Life? Nutrients 2022, 14, 628. [Google Scholar] [CrossRef]
- Nettore, I.C.; De Nisco, E.; Desiderio, S.; Passaro, C.; Maione, L.; Negri, M.; Albano, L.; Pivonello, R.; Pivonello, C.; Portella, G.; et al. Selenium supplementation modulates apoptotic processes in thyroid follicular cells. BioFactors 2017, 43, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Ruggeri, R.M.; D’Ascola, A.; Vicchio, T.M.; Campo, S.; Giani, F.; Giovinazzo, S.; Frasca, F.; Cannavo, S.; Campenni, A.; Trimarchi, F. Selenium exerts protective effects against oxidative stress and cell damage in human thyrocytes and fibroblasts. Endocrine 2020, 68, 151–162. [Google Scholar] [CrossRef]
- Santos, L.R.; Neves, C.; Melo, M.; Soares, P. Selenium and Selenoproteins in Immune Mediated Thyroid Disorders. Diagnostics 2018, 8, 70. [Google Scholar] [CrossRef] [Green Version]
- Santos, L.R.; Duraes, C.; Mendes, A.; Prazeres, H.; Alvelos, M.I.; Moreira, C.S.; Canedo, P.; Esteves, C.; Neves, C.; Carvalho, D.; et al. A polymorphism in the promoter region of the selenoprotein S gene (SEPS1) contributes to Hashimoto’s thyroiditis susceptibility. J. Clin. Endocrinol. Metab. 2014, 99, E719–E723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucharzewski, M.; Braziewicz, J.; Majewska, U.; Gozdz, S. Concentration of selenium in the whole blood and the thyroid tissue of patients with various thyroid diseases. Biol. Trace Elem. Res. 2002, 88, 25–30. [Google Scholar] [CrossRef]
- Balazs, C.; Kaczur, V. Effect of Selenium on HLA-DR Expression of Thyrocytes. Autoimmune Dis. 2012, 2012, 374635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Feng, W.; Chen, H.; Shi, H.; Jiang, L.; Zheng, X.; Liu, X.; Zhang, W.; Ge, Y.; Liu, Y.; et al. Effect of selenium on thyroid autoimmunity and regulatory T cells in patients with Hashimoto’s thyroiditis: A prospective randomized-controlled trial. Clin. Transl. Sci. 2021, 14, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Blazejak, S. Current Knowledge on the Importance of Selenium in Food for Living Organisms: A Review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [Green Version]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef]
- Johnson, C.C.; Fordyce, F.M.; Rayman, M.P. Symposium on ‘Geographical and geological influences on nutrition’: Factors controlling the distribution of selenium in the environment and their impact on health and nutrition. Proc. Nutr. Soc. 2010, 69, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Hill, K.E.; Byrne, D.W.; Xu, J.; Burk, R.F. Effectiveness of selenium supplements in a low-selenium area of China. Am. J. Clin. Nutr. 2005, 81, 829–834. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.Q.; Xia, Y.M. Studies on human dietary requirements and safe range of dietary intakes of selenium in China and their application in the prevention of related endemic diseases. Biomed. Environ. Sci. 1995, 8, 187–201. [Google Scholar]
- Ashton, K.; Hooper, L.; Harvey, L.J.; Hurst, R.; Casgrain, A.; Fairweather-Tait, S.J. Methods of assessment of selenium status in humans: A systematic review. Am. J. Clin. Nutr. 2009, 89, 2025S–2039S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Dato, C.; Gianfrilli, D.; Greco, E.; Astolfi, M.; Canepari, S.; Lenzi, A.; Isidori, A.M.; Giannetta, E. Profiling of selenium absorption and accumulation in healthy subjects after prolonged L-selenomethionine supplementation. J. Endocrinol. Investig. 2017, 40, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Hill, K.E.; Li, P.; Xu, J.; Zhou, D.; Motley, A.K.; Wang, L.; Byrne, D.W.; Burk, R.F. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: A placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am. J. Clin. Nutr. 2010, 92, 525–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pop, V.; Krabbe, J.; Maret, W.; Rayman, M. Plasma mineral (selenium, zinc or copper) concentrations in the general pregnant population, adjusted for supplement intake, in relation to thyroid function. Br. J. Nutr. 2021, 125, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine; Food and Nutrition Board; Panel on Dietary Antioxidants and Related Compounds; Subcommittee on Upper Reference Levels of Nutrients; Subcommittee on Interpretation and Uses of Dietary Reference Intakes; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000. [Google Scholar] [CrossRef]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohe, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H.; German Nutrition, S. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izquierdo Alvarez, S.; Castanon, S.G.; Ruata, M.L.; Aragues, E.F.; Terraz, P.B.; Irazabal, Y.G.; Gonzalez, E.G.; Rodriguez, B.G. Updating of normal levels of copper, zinc and selenium in serum of pregnant women. J. Trace Elem. Med. Biol. 2007, 21 (Suppl. S1), 49–52. [Google Scholar] [CrossRef] [PubMed]
- Grieger, J.A.; Grzeskowiak, L.E.; Wilson, R.L.; Bianco-Miotto, T.; Leemaqz, S.Y.; Jankovic-Karasoulos, T.; Perkins, A.V.; Norman, R.J.; Dekker, G.A.; Roberts, C.T. Maternal Selenium, Copper and Zinc Concentrations in Early Pregnancy, and the Association with Fertility. Nutrients 2019, 11, 1609. [Google Scholar] [CrossRef] [Green Version]
- Stoffaneller, R.; Morse, N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Yang, L.; Kong, C.; Wang, J.; Li, Y. Selenium Nutritional Status of Rural Residents and Its Correlation with Dietary Intake Patterns in a Typical Low-Selenium Area in China. Nutrients 2020, 12, 3816. [Google Scholar] [CrossRef]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [Green Version]
- Casey, C.E.; Guthrie, B.E.; Friend, G.M.; Robinson, M.F. Selenium in human tissues from New Zealand. Arch. Environ. Health 1982, 37, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Fallon, N.; Dillon, S.A. Low Intakes of Iodine and Selenium Amongst Vegan and Vegetarian Women Highlight a Potential Nutritional Vulnerability. Front. Nutr. 2020, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Hemmelgarn, B.; Klarenbach, S.; Field, C.; Manns, B.; Thadhani, R.; Gill, J.; Alberta Kidney Disease, N. Trace elements in hemodialysis patients: A systematic review and meta-analysis. BMC Med. 2009, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Yang, G.Q.; Wang, S.Z.; Zhou, R.H.; Sun, S.Z. Endemic selenium intoxication of humans in China. Am. J. Clin. Nutr. 1983, 37, 872–881. [Google Scholar] [CrossRef]
- Yang, G.; Zhou, R. Further observations on the human maximum safe dietary selenium intake in a seleniferous area of China. J. Trace Elem. Electrolytes Health Dis. 1994, 8, 159–165. [Google Scholar]
- Pearce, E.N.; Farwell, A.P.; Braverman, L.E. Thyroiditis. N. Engl. J. Med. 2003, 348, 2646–2655. [Google Scholar] [CrossRef]
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367. [Google Scholar] [CrossRef]
- Hawkes, W.C.; Keim, N.L. Dietary selenium intake modulates thyroid hormone and energy metabolism in men. J. Nutr. 2003, 133, 3443–3448. [Google Scholar] [CrossRef]
- Duffield, A.J.; Thomson, C.D.; Hill, K.E.; Williams, S. An estimation of selenium requirements for New Zealanders. Am. J. Clin. Nutr. 1999, 70, 896–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duntas, L.H.; Mantzou, E.; Koutras, D.A. Effects of a six month treatment with selenomethionine in patients with autoimmune thyroiditis. Eur. J. Endocrinol. 2003, 148, 389–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurst, R.; Armah, C.N.; Dainty, J.R.; Hart, D.J.; Teucher, B.; Goldson, A.J.; Broadley, M.R.; Motley, A.K.; Fairweather-Tait, S.J. Establishing optimal selenium status: Results of a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2010, 91, 923–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gartner, R.; Gasnier, B.C.; Dietrich, J.W.; Krebs, B.; Angstwurm, M.W. Selenium supplementation in patients with autoimmune thyroiditis decreases thyroid peroxidase antibodies concentrations. J. Clin. Endocrinol. Metab. 2002, 87, 1687–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Mao, J.; Zhao, J.; Lu, J.; Yan, L.; Du, J.; Lu, Z.; Wang, H.; Xu, M.; Bai, X.; et al. Decreased Thyroid Peroxidase Antibody Titer in Response to Selenium Supplementation in Autoimmune Thyroiditis and the Influence of a Selenoprotein P Gene Polymorphism: A Prospective, Multicenter Study in China. Thyroid 2018, 28, 1674–1681. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, L.; Xu, E.; Bi, Y.; Hu, X.; Pei, X.; Jin, G. Levothyroxine monotherapy versus levothyroxine and selenium combination therapy in chronic lymphocytic thyroiditis. J. Endocrinol. Investig. 2017, 40, 1243–1250. [Google Scholar] [CrossRef]
- Esposito, D.; Rotondi, M.; Accardo, G.; Vallone, G.; Conzo, G.; Docimo, G.; Selvaggi, F.; Cappelli, C.; Chiovato, L.; Giugliano, D.; et al. Influence of short-term selenium supplementation on the natural course of Hashimoto’s thyroiditis: Clinical results of a blinded placebo-controlled randomized prospective trial. J. Endocrinol. Investig. 2017, 40, 83–89. [Google Scholar] [CrossRef]
- Pirola, I.; Gandossi, E.; Agosti, B.; Delbarba, A.; Cappelli, C. Selenium supplementation could restore euthyroidism in subclinical hypothyroid patients with autoimmune thyroiditis. Endokrynol. Pol. 2016, 67, 567–571. [Google Scholar] [CrossRef] [Green Version]
- Pilli, T.; Cantara, S.; Schomburg, L.; Cenci, V.; Cardinale, S.; Heid, E.C.; Kuhn, E.C.; Cevenini, G.; Sestini, F.; Fioravanti, C.; et al. IFNgamma-Inducible Chemokines Decrease upon Selenomethionine Supplementation in Women with Euthyroid Autoimmune Thyroiditis: Comparison between Two Doses of Selenomethionine (80 or 160 mug) versus Placebo. Eur. Thyroid J. 2015, 4, 226–233. [Google Scholar] [CrossRef] [Green Version]
- De Farias, C.R.; Cardoso, B.R.; de Oliveira, G.M.; de Mello Guazzelli, I.C.; Catarino, R.M.; Chammas, M.C.; Cozzolino, S.M.; Knobel, M. A randomized-controlled, double-blind study of the impact of selenium supplementation on thyroid autoimmunity and inflammation with focus on the GPx1 genotypes. J. Endocrinol. Investig. 2015, 38, 1065–1074. [Google Scholar] [CrossRef]
- Eskes, S.A.; Endert, E.; Fliers, E.; Birnie, E.; Hollenbach, B.; Schomburg, L.; Kohrle, J.; Wiersinga, W.M. Selenite supplementation in euthyroid subjects with thyroid peroxidase antibodies. Clin. Endocrinol. 2014, 80, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Toulis, K.A.; Nisianakis, P.; Goulis, D.G.; Kampas, L.; Valeri, R.M.; Oikonomou, D.; Tzellos, T.G.; Delaroudis, S. Selenomethionine treatment in patients with autoimmune thyroiditis: A prospective, quasi-randomised trial. Int. J. Clin. Pract. 2012, 66, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H. Selenium and the thyroid: A close-knit connection. J. Clin. Endocrinol. Metab. 2010, 95, 5180–5188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krysiak, R.; Okopien, B. Haemostatic effects of levothyroxine and selenomethionine in euthyroid patients with Hashimoto’s thyroiditis. Thromb. Haemost. 2012, 108, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Okopien, B. The effect of levothyroxine and selenomethionine on lymphocyte and monocyte cytokine release in women with Hashimoto’s thyroiditis. J. Clin. Endocrinol. Metab. 2011, 96, 2206–2215. [Google Scholar] [CrossRef] [Green Version]
- Nacamulli, D.; Mian, C.; Petricca, D.; Lazzarotto, F.; Barollo, S.; Pozza, D.; Masiero, S.; Faggian, D.; Plebani, M.; Girelli, M.E.; et al. Influence of physiological dietary selenium supplementation on the natural course of autoimmune thyroiditis. Clin. Endocrinol. 2010, 73, 535–539. [Google Scholar] [CrossRef]
- Wichman, J.; Winther, K.H.; Bonnema, S.J.; Hegedus, L. Selenium Supplementation Significantly Reduces Thyroid Autoantibody Levels in Patients with Chronic Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis. Thyroid 2016, 26, 1681–1692. [Google Scholar] [CrossRef]
- Karanikas, G.; Schuetz, M.; Kontur, S.; Duan, H.; Kommata, S.; Schoen, R.; Antoni, A.; Kletter, K.; Dudczak, R.; Willheim, M. No immunological benefit of selenium in consecutive patients with autoimmune thyroiditis. Thyroid 2008, 18, 7–12. [Google Scholar] [CrossRef]
- Mazokopakis, E.E.; Papadakis, J.A.; Papadomanolaki, M.G.; Batistakis, A.G.; Giannakopoulos, T.G.; Protopapadakis, E.E.; Ganotakis, E.S. Effects of 12 months treatment with L-selenomethionine on serum anti-TPO Levels in Patients with Hashimoto’s thyroiditis. Thyroid 2007, 17, 609–612. [Google Scholar] [CrossRef]
- Turker, O.; Kumanlioglu, K.; Karapolat, I.; Dogan, I. Selenium treatment in autoimmune thyroiditis: 9-month follow-up with variable doses. J. Endocrinol. 2006, 190, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Xu, S.; Zhang, H.; Cao, W.; Wang, K.; Chen, G.; Di, H.; Cao, M.; Liu, C. Selenium supplementation for autoimmune thyroiditis: A systematic review and meta-analysis. Int. J. Endocrinol. 2014, 2014, 904573. [Google Scholar] [CrossRef] [PubMed]
- Vissenberg, R.; Manders, V.D.; Mastenbroek, S.; Fliers, E.; Afink, G.B.; Ris-Stalpers, C.; Goddijn, M.; Bisschop, P.H. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum. Reprod. Update 2015, 21, 378–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seungdamrong, A.; Steiner, A.Z.; Gracia, C.R.; Legro, R.S.; Diamond, M.P.; Coutifaris, C.; Schlaff, W.D.; Casson, P.; Christman, G.M.; Robinson, R.D.; et al. Preconceptional antithyroid peroxidase antibodies, but not thyroid-stimulating hormone, are associated with decreased live birth rates in infertile women. Fertil. Steril. 2017, 108, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korevaar, T.I.M.; Minguez-Alarcon, L.; Messerlian, C.; de Poortere, R.A.; Williams, P.L.; Broeren, M.A.; Hauser, R.; Souter, I.C. Association of Thyroid Function and Autoimmunity with Ovarian Reserve in Women Seeking Infertility Care. Thyroid 2018, 28, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Kilic, S.; Tasdemir, N.; Yilmaz, N.; Yuksel, B.; Gul, A.; Batioglu, S. The effect of anti-thyroid antibodies on endometrial volume, embryo grade and IVF outcome. Gynecol. Endocrinol. 2008, 24, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Birjandi, B.; Ramezani Tehrani, F.; Amouzegar, A.; Tohidi, M.; Bidhendi Yarandi, R.; Azizi, F. The association between subclinical hypothyroidism and TPOAb positivity with infertility in a population-based study: Tehran thyroid study (TTS). BMC Endocr. Disord. 2021, 21, 108. [Google Scholar] [CrossRef]
- Ahsan, U.; Kamran, Z.; Raza, I.; Ahmad, S.; Babar, W.; Riaz, M.H.; Iqbal, Z. Role of selenium in male reproduction—A review. Anim. Reprod. Sci. 2014, 146, 55–62. [Google Scholar] [CrossRef]
- Basini, G.; Tamanini, C. Selenium stimulates estradiol production in bovine granulosa cells: Possible involvement of nitric oxide. Domest. Anim. Endocrinol. 2000, 18, 1–17. [Google Scholar] [CrossRef]
- Paszkowski, T.; Traub, A.I.; Robinson, S.Y.; McMaster, D. Selenium dependent glutathione peroxidase activity in human follicular fluid. Clin. Chim. Acta 1995, 236, 173–180. [Google Scholar] [CrossRef]
- Khera, A.; Dong, L.F.; Holland, O.; Vanderlelie, J.; Pasdar, E.A.; Neuzil, J.; Perkins, A.V. Selenium supplementation induces mitochondrial biogenesis in trophoblasts. Placenta 2015, 36, 863–869. [Google Scholar] [CrossRef]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korevaar, T.I.M.; Medici, M.; Visser, T.J.; Peeters, R.P. Thyroid disease in pregnancy: New insights in diagnosis and clinical management. Nat. Rev. Endocrinol. 2017, 13, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, R.; Liberati, M.; Silvi, C.; D’Antonio, F. Levothyroxine Supplementation in Euthyroid Pregnant Women With Positive Autoantibodies: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 759064. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.; Shan, Z.; Patil-Sisodia, K.; Cooper, D.S. Hypothyroidism in pregnancy. Lancet Diabetes Endocrinol. 2013, 1, 228–237. [Google Scholar] [CrossRef]
- Man, E.B.; Jones, W.S.; Holden, R.H.; Mellits, E.D. Thyroid function in human pregnancy. 8. Retardation of progeny aged 7 years; relationships to maternal age and maternal thyroid function. Am. J. Obstet. Gynecol. 1971, 111, 905–916. [Google Scholar] [CrossRef]
- Haddow, J.E.; Palomaki, G.E.; Allan, W.C.; Williams, J.R.; Knight, G.J.; Gagnon, J.; O’Heir, C.E.; Mitchell, M.L.; Hermos, R.J.; Waisbren, S.E.; et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N. Engl. J. Med. 1999, 341, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negro, R.; Formoso, G.; Mangieri, T.; Pezzarossa, A.; Dazzi, D.; Hassan, H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: Effects on obstetrical complications. J. Clin. Endocrinol. Metab. 2006, 91, 2587–2591. [Google Scholar] [CrossRef]
- Stagnaro-Green, A.; Roman, S.H.; Cobin, R.H.; el-Harazy, E.; Alvarez-Marfany, M.; Davies, T.F. Detection of at-risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. JAMA 1990, 264, 1422–1425. [Google Scholar] [CrossRef]
- Prummel, M.F.; Wiersinga, W.M. Thyroid autoimmunity and miscarriage. Eur. J. Endocrinol. 2004, 150, 751–755. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Jiang, L.; Sadhukhan, A.; Yang, S.; Yao, Q.; Zhou, P.; Rao, J.; Jin, M. Effect of antithyroid antibodies on women with recurrent miscarriage: A meta-analysis. Am. J. Reprod. Immunol. 2020, 83, e13238. [Google Scholar] [CrossRef] [Green Version]
- Beneventi, F.; De Maggio, I.; Bellingeri, C.; Cavagnoli, C.; Spada, C.; Boschetti, A.; Magri, F.; Spinillo, A. Thyroid autoimmunity and adverse pregnancy outcomes: A prospective cohort study. Endocrine 2022, 76, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, R. of antithyroid antibodies on women with: A meta-analysis. Clin. Endocrinol. 2011, 74, 513–519. [Google Scholar] [CrossRef]
- Thangaratinam, S.; Tan, A.; Knox, E.; Kilby, M.D.; Franklyn, J.; Coomarasamy, A. Association between thyroid autoantibodies and miscarriage and preterm birth: Meta-analysis of evidence. BMJ 2011, 342, d2616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, C.C.; Pearce, E.N. An update on thyroid disorders in the postpartum period. J. Endocrinol. Investig. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stagnaro-Green, A.; Roman, S.H.; Cobin, R.H.; el-Harazy, E.; Wallenstein, S.; Davies, T.F. A prospective study of lymphocyte-initiated immunosuppression in normal pregnancy: Evidence of a T-cell etiology for postpartum thyroid dysfunction. J. Clin. Endocrinol. Metab. 1992, 74, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Stagnaro-Green, A. Approach to the patient with postpartum thyroiditis. J. Clin. Endocrinol. Metab. 2012, 97, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, M.M.; Vissenberg, R.; Fliers, E.; van der Post, J.A.M.; van der Hoorn, M.P.; de Weerd, S.; Kuchenbecker, W.K.; Hoek, A.; Sikkema, J.M.; Verhoeve, H.R.; et al. Levothyroxine in euthyroid thyroid peroxidase antibody positive women with recurrent pregnancy loss (T4LIFE trial): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2022, 10, 322–329. [Google Scholar] [CrossRef]
- Duntas, L.H. Selenium and at-risk pregnancy: Challenges and controversies. Thyroid Res. 2020, 13, 16. [Google Scholar] [CrossRef]
- Arikan, T.A. Plasma Selenium Levels in First Trimester Pregnant Women with Hyperthyroidism and the Relationship with Thyroid Hormone Status. Biol. Trace Elem. Res. 2015, 167, 194–199. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, L.; Xu, J.; Liu, Z.; Liu, J.; Yan, C. Prenatal Maternal Low Selenium, High Thyrotropin, and Low Birth Weights. Biol. Trace Elem. Res. 2021, 199, 18–25. [Google Scholar] [CrossRef]
- Ambroziak, U.; Hybsier, S.; Shahnazaryan, U.; Krasnodebska-Kiljanska, M.; Rijntjes, E.; Bartoszewicz, Z.; Bednarczuk, T.; Schomburg, L. Severe selenium deficits in pregnant women irrespective of autoimmune thyroid disease in an area with marginal selenium intake. J. Trace Elem. Med. Biol. 2017, 44, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Hofstee, P.; James-McAlpine, J.; McKeating, D.R.; Vanderlelie, J.J.; Cuffe, J.S.M.; Perkins, A.V. Low serum selenium in pregnancy is associated with reduced T3 and increased risk of GDM. J. Endocrinol. 2021, 248, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Mirone, M.; Giannetta, E.; Isidori, A.M. Selenium and reproductive function. A systematic review. J. Endocrinol. Investig. 2013, 36, 28–36. [Google Scholar]
- Negro, R.; Greco, G.; Mangieri, T.; Pezzarossa, A.; Dazzi, D.; Hassan, H. The influence of selenium supplementation on postpartum thyroid status in pregn.nant women with thyroid peroxidase autoantibodies. J. Clin. Endocrinol. Metab. 2007, 92, 1263–1268. [Google Scholar] [CrossRef]
- Mao, J.; Pop, V.J.; Bath, S.C.; Vader, H.L.; Redman, C.W.; Rayman, M.P. Effect of low-dose selenium on thyroid autoimmunity and thyroid function in UK pregnant women with mild-to-moderate iodine deficiency. Eur. J. Nutr. 2016, 55, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, G.; Isidori, A.M.; Moretti, C.; Di Dato, C.; Greco, E.; Ciolli, P.; Bonomi, M.; Petrone, L.; Fumarola, A.; Campagna, G.; et al. Selenium supplementation in the management of thyroid autoimmunity during pregnancy: Results of the “SERENA study”, a randomized, double-blind, placebo-controlled trial. Endocrine 2019, 66, 542–550. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, W.K.; Robinson, K.A.; Smallridge, R.C.; Ladenson, P.W.; Powe, N.R. Prevalence of postpartum thyroid dysfunction: A quantitative review. Thyroid 2006, 16, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Negro, R.; Attanasio, R.; Grimaldi, F.; Marcocci, C.; Guglielmi, R.; Papini, E. A 2016 Italian Survey about the Clinical Use of Selenium in Thyroid Disease. Eur. Thyroid J. 2016, 5, 164–170. [Google Scholar] [CrossRef] [Green Version]

| Food | Se Content (µg/g Fresh Weight) |
|---|---|
| Brazil nuts | ~0.85–7 |
| Tuna (in oil) | ~0.8 |
| Chicken | ~0.6 |
| Sardines | ~0.6 |
| Lamb | ~0.3-0.4 |
| Shellfish | ~0.4–1.3 |
| Beef | ~0.35–0.5 |
| Salmon | ~0.2–0.4 |
| Ham | ~0.2 |
| Eggs | ~0.2 |
| Milk products | ~0.1–0.5 |
| First Author, Year | Country | Study Design | Study Population | No. of Patients | Gestational Age | Outcomes | Main Results |
|---|---|---|---|---|---|---|---|
| Arikan et al. 2015 [90] | Turkey | Cross-sectional study | Healthy pregnant vs. hyperthyroid pregnant | 107: 70 healthy (group 1); 37 hyperthyroid (group 2) | First trimester | TSH, FT3, FT4, serum Se | Significantly higher FT3 and FT4 levels and significantly lower TSH and Se levels in group 2 than in group 1; positive correlation between Se and FT4 in group 1 and with TSH in group 2 |
| Ambroziak et al. 2017 [92] | Poland | Prospective study | Healthy pregnant and pregnant women with AITD | 74: 45 healthy; 39 AITD | First, second and third trimester | TSH, FT3, FT4, TPOAb, TgAb, thyroid US, serum Se and SELENOP | Relatively low serum Se and SELENOP levels in both healthy and AITD; from first to third trimester TPOAb and TgAb declined in AITD, but this was unrelated to Se status |
| Guo et al. 2021 [91] | China | Prospective cohort study | Pregnant | 1931 | 28–36 wks | TSH, serum Se, birth weight and length | Serum Se levels <103.7 μg/L, each unit increase significantly associated with a decrease of 0.014 μIU/mL in TSH; maternal TSH levels inversely associated with infant birth weights |
| Hofstee et al. 2021 [93] | Australia | Retrospective cross-sectional study | Pregnant euthyroid | 63: 21 with low Se; 21 with mean Se; 21 with optimal Se | 26–30 wks | TSH, FT3, FT4, TPOAb, serum Se | Females with low Se concentrations showed reduced FT3 and increased TPOAb and incidence of pregnancy disorders; Se levels positively correlated with FT3 and negatively correlated with TPOAb |
| Pop et al. 2021 [24] | The Netherlands | Longitudinal prospective study | Pregnant | 2041: 1479 taking mineral supplements; 544 not taking mineral supplements | 12 wks | TSH, FT4, TPOAb, serum Se, Zn and Cu | Negative correlation between Se levels and logFT4; positive correlation between Se levels and logTSH; women taking supplements were 1.46 times less likely to have elevated TPOAb at 12 wks |
| First Author, Year | Country | No. of Patients (Selenium/Placebo/Control) | Selenium Supplementation | Duration of Treatment (Months) | Patients Requiring LT4 during Pregnancy (%) | Outcomes | Main Results |
|---|---|---|---|---|---|---|---|
| Negro et al. 2007 [95] | Italy | 232 (77/74/81) | selenomethionine 200 μg/day | 18 | 33 (14.2) | TSH, FT4, TPOAb, thyroid US, PPT | Lower prevalence of PPT and permanent hypothyroidism |
| Mao et al. 2016 [96] | UK | 230 (120/110) | selenium 60 μg/day | 6 | None | TSH, FT4, TPOAb, TgAb | Decrease in TSH and FT4 during pregnancy but no effect on TPOAb |
| Mantovani et al. 2019 [97] | Italy | 45 (21/24) | selenomethionine 83 μg/day | 12 | 13 * (28.9) | TSH, FT3, FT4, TPOAb, TgAb, thyroid US, HRQoL | Decrease in TPOAb and TgAb in the Se group but increase in the PLB group at PP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minnetti, M.; Sada, V.; Feola, T.; Giannetta, E.; Pozza, C.; Gianfrilli, D.; Isidori, A.M.; Cozzolino, A. Selenium Supplementation in Pregnant Women with Autoimmune Thyroiditis: A Practical Approach. Nutrients 2022, 14, 2234. https://doi.org/10.3390/nu14112234
Minnetti M, Sada V, Feola T, Giannetta E, Pozza C, Gianfrilli D, Isidori AM, Cozzolino A. Selenium Supplementation in Pregnant Women with Autoimmune Thyroiditis: A Practical Approach. Nutrients. 2022; 14(11):2234. https://doi.org/10.3390/nu14112234
Chicago/Turabian StyleMinnetti, Marianna, Valentina Sada, Tiziana Feola, Elisa Giannetta, Carlotta Pozza, Daniele Gianfrilli, Andrea M. Isidori, and Alessia Cozzolino. 2022. "Selenium Supplementation in Pregnant Women with Autoimmune Thyroiditis: A Practical Approach" Nutrients 14, no. 11: 2234. https://doi.org/10.3390/nu14112234
APA StyleMinnetti, M., Sada, V., Feola, T., Giannetta, E., Pozza, C., Gianfrilli, D., Isidori, A. M., & Cozzolino, A. (2022). Selenium Supplementation in Pregnant Women with Autoimmune Thyroiditis: A Practical Approach. Nutrients, 14(11), 2234. https://doi.org/10.3390/nu14112234






