Diallyl Trisulfide Promotes Placental Angiogenesis by Regulating Lipid Metabolism and Alleviating Inflammatory Responses in Obese Pregnant Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Animal Experiment
2.3. Sample Collection
2.4. Intraperitoneal Glucose Tolerance Test (IPGTT)
2.5. Analysis of Lipid Levels in Maternal Serum and Placenta
2.6. Analysis of Hydrogen Sulfide in Maternal Serum and Placenta
2.7. RNA Extraction and Quantitative Real-Time PCR
2.8. Hematoxylin and Eosin (H&E) Staining and Immunohistochemistry
2.9. Statistical Analysis
3. Results
3.1. Diallyl Trisulfide Treatment Improved Litter Performance and Reduced Fat Deposition in Obese Mice during Pregnancy
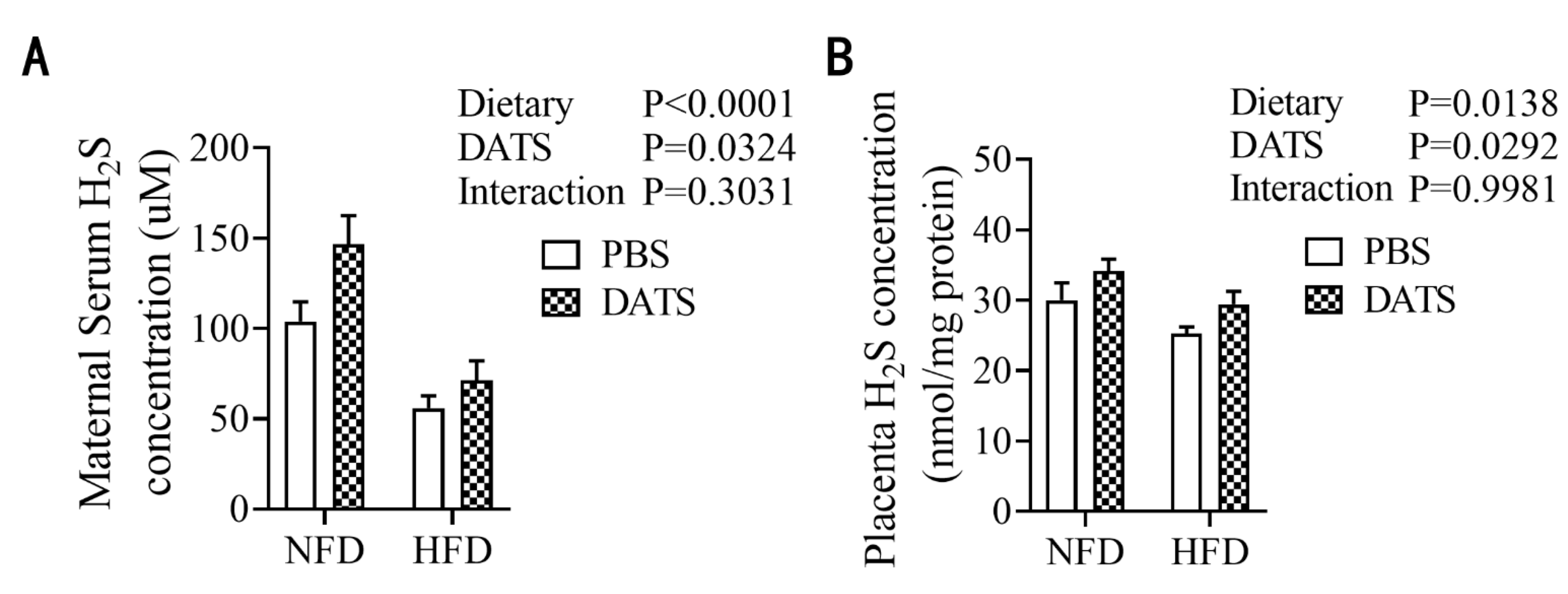
3.2. Diallyl Trisulfide Treatment Increased the Content of Hydrogen Sulfide in Obese Mice during Pregnancy
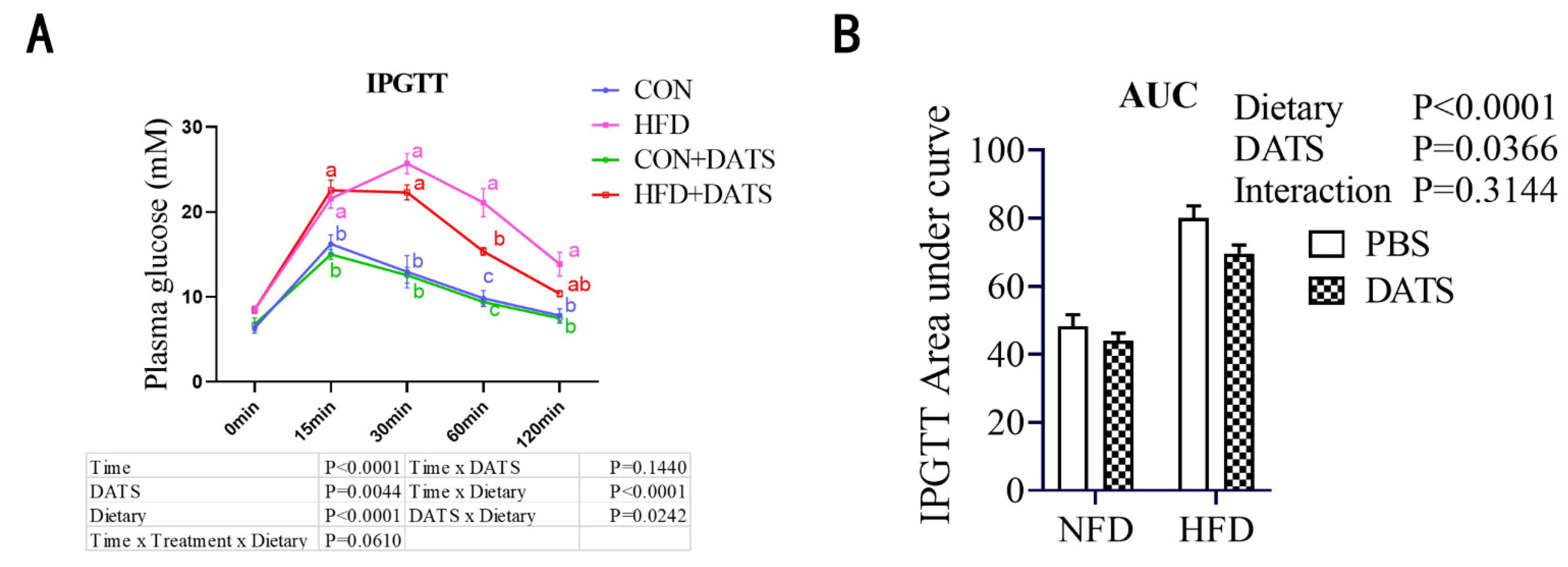
3.3. Diallyl Trisulfide Treatment Improved Glucose Tolerance of Obese Mice
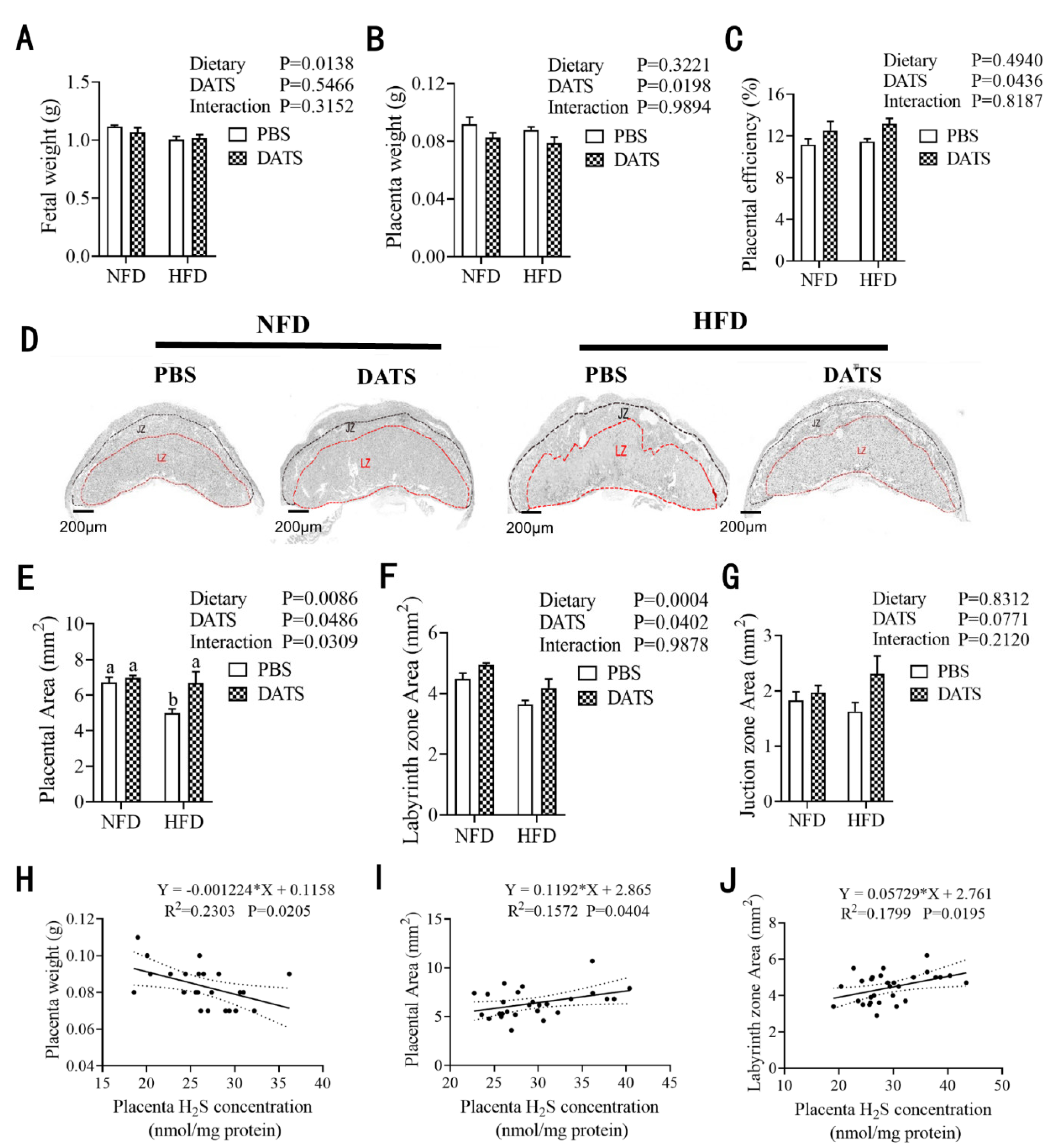
3.4. Diallyl Trisulfide Treatment Promoted Placental Development
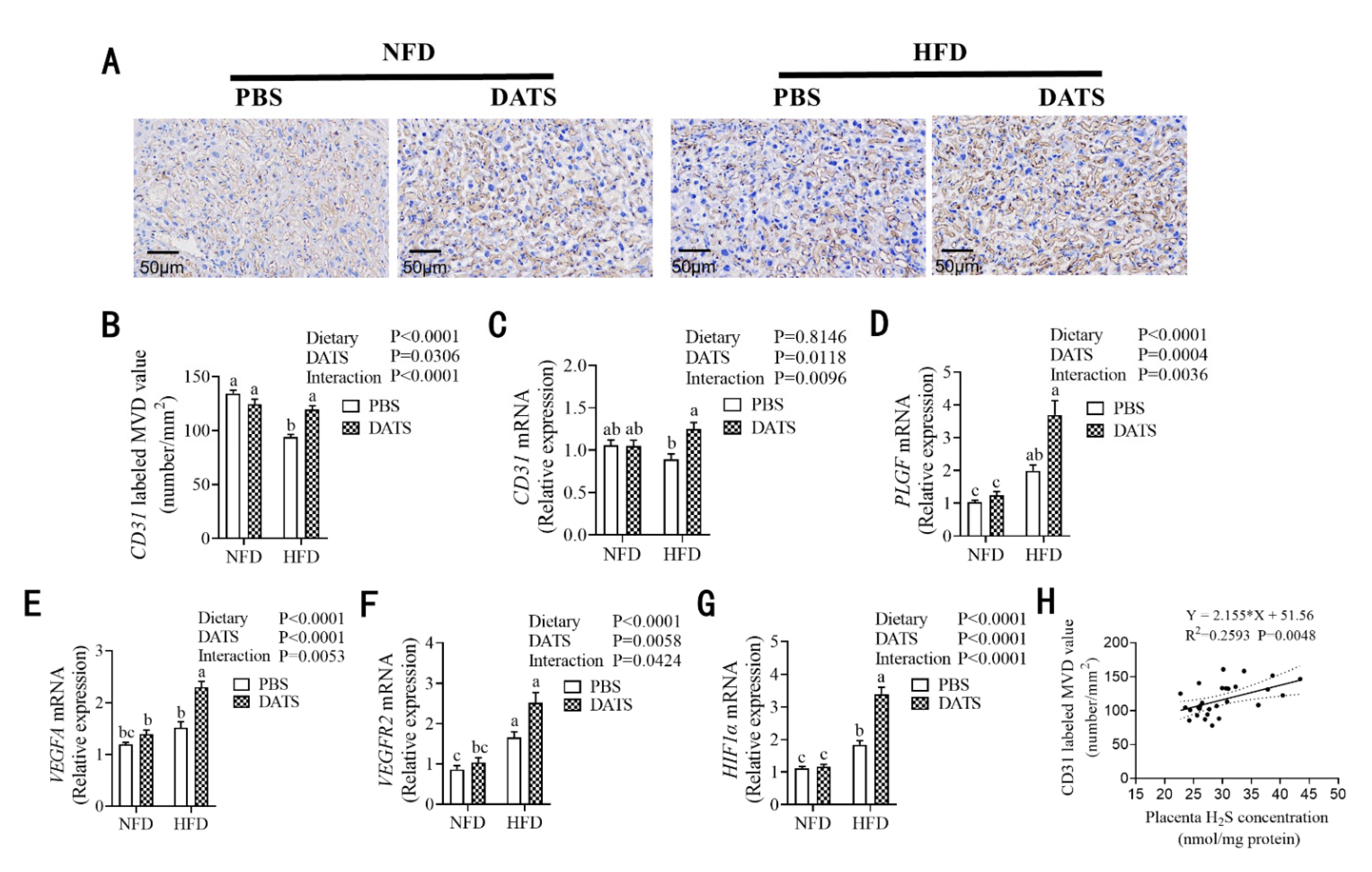
3.5. Diallyl Trisulfide Treatment Was Beneficial to Placental Angiogenesis
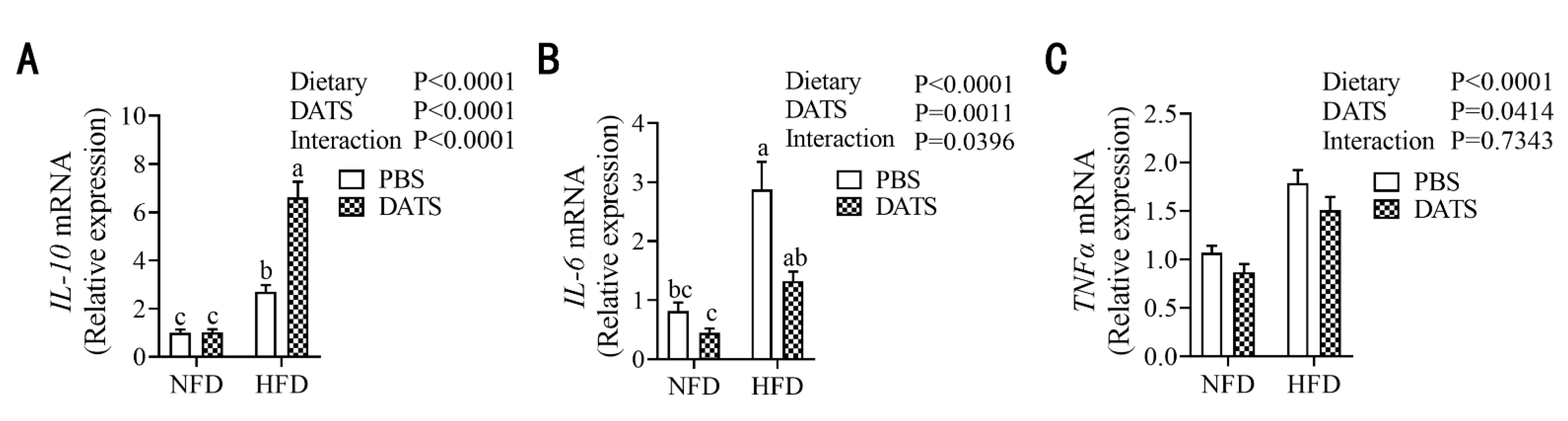
3.6. Diallyl Trisulfide Treatment Can Alleviate the Level of Placental Inflammation in Obese Mice during Pregnancy
3.7. Diallyl Trisulfide Treatment Improved the Lipid Metabolism in Placental Tissue of Obese Mice during Pregnancy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hruby, A.; Manson, J.E.; Qi, L.; Malik, V.S.; Rimm, E.B.; Sun, Q.; Willett, W.C.; Hu, F.B. Determinants and Consequences of Obesity. Am. J. Public Health 2016, 106, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.; Anderson, C.; Lippman, S.M. Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. Lancet Oncol. 2017, 18, E457–E471. [Google Scholar] [CrossRef]
- Maria Leiva, A.; Adela Martinez, M.; Cristi-Montero, C.; Salas, C.; Ramirez-Campillo, R.; Diaz Martinez, X.; Aguilar-Farias, N.; Celis-Morales, C. Sedentary lifestyle is associated with metabolic and cardiovascular risk factors independent of physical activity. Rev. Med. Chile 2017, 145, 458–467. [Google Scholar]
- Myatt, L.; Maloyan, A. Obesity and Placental Function. Semin. Reprod. Med. 2016, 34, 42–49. [Google Scholar] [PubMed] [Green Version]
- Avagliano, L.; Garo, C.; Marconi, A.M. Placental amino acids transport in intrauterine growth restriction. J. Pregnancy 2012, 2012, 972562. [Google Scholar] [CrossRef]
- Meher, A.; Sundrani, D.; Joshi, S. Maternal Nutrition Influences Angiogenesis in the Placenta Through Peroxisome Proliferator Activated Receptors: A Novel Hypothesis. Mol. Reprod. Dev. 2015, 82, 726–734. [Google Scholar] [CrossRef]
- Stuart, T.J.; O’Neill, K.; Condon, D.; Sasson, I.; Sen, P.; Xia, Y.; Simmons, R.A. Diet-induced obesity alters the maternal metabolome and early placenta transcriptome and decreases placenta vascularity in the mouse. Biol. Reprod. 2018, 98, 795–809. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef] [Green Version]
- Song, T.; Lu, J.; Deng, Z.; Xu, T.; Yang, Y.; Wei, H.; Li, S.; Jiang, S.; Peng, J. Maternal obesity aggravates the abnormality of porcine placenta by increasing N-6-methyladenosine. Int. J. Obes. 2018, 42, 1812–1820. [Google Scholar] [CrossRef]
- Bairagi, S.; Quinn, K.E.; Crane, A.R.; Ashley, R.L.; Borowicz, P.P.; Caton, J.S.; Redden, R.R.; Grazul-Bilska, A.T.; Reynolds, L.P. Maternal environment and placental vascularization in small ruminants. Theriogenology 2016, 86, 288–305. [Google Scholar] [CrossRef] [Green Version]
- Kondo, K.; Bhushan, S.; King, A.L.; Prabhu, S.D.; Hamid, T.; Koenig, S.; Murohara, T.; Predmore, B.L.; Gojon, G., Sr.; Gojon, G., Jr.; et al. H2S Protects Against Pressure Overload-Induced Heart Failure via Upregulation of Endothelial Nitric Oxide Synthase. Circulation 2013, 127, 1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabo, C.; Papapetropoulos, A. Hydrogen sulphide and angiogenesis: Mechanisms and applications. Br. J. Pharmacol. 2011, 164, 853–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.-H.; Cui, L.-B.; Wu, K.; Zheng, X.-L.; Cayabyab, F.S.; Chen, Z.-W.; Tang, C.-K. Hydrogen sulfide as a potent cardiovascular protective agent. Clin. Chim. Acta 2014, 437, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-G.; Li, W. Hydrogen sulfide improves vessel formation of the ischemic adductor muscle and wound healing in diabetic db/db mice. Iran. J. Basic Med. Sci. 2019, 22, 1192–1197. [Google Scholar] [PubMed]
- Chen, D.-B.; Feng, L.; Hodges, J.K.; Lechuga, T.J.; Zhang, H. Human trophoblast-derived hydrogen sulfide stimulates placental artery endothelial cell angiogenesis. Biol. Reprod. 2017, 97, 478–489. [Google Scholar] [CrossRef]
- Zhou, X.; An, G.; Chen, J. Inhibitory effects of hydrogen sulphide on pulmonary fibrosis in smoking rats via attenuation of oxidative stress and inflammation. J. Cell. Mol. Med. 2014, 18, 1098–1103. [Google Scholar] [CrossRef]
- Shi Xe Zhou, X.; Chu, X.; Wang, J.; Xie, B.; Ge, J.; Guo, Y.; Li, X.; Yang, G. Allicin Improves Metabolism in High-Fat Diet-Induced Obese Mice by Modulating the Gut Microbiota. Nutrients 2019, 11, 2909. [Google Scholar]
- Challier, J.C.; Basu, S.; Bintein, T.; Minium, J.; Hotmire, K.; Catalano, P.M.; Mouzon, S.H.-D. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 2008, 29, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Farley, D.; Tejero, M.E.; Comuzzie, A.G.; Higgins, P.B.; Cox, L.; Werner, S.L.; Jenkins, S.L.; Li, C.; Choi, J.; Dick, E.J., Jr.; et al. Feto-placental Adaptations to Maternal Obesity in the Baboon. Placenta 2009, 30, 752–760. [Google Scholar] [CrossRef] [Green Version]
- Frias, A.E.; Morgan, T.K.; Evans, A.E.; Rasanen, J.; Oh, K.Y.; Thornburg, K.L.; Grove, K.L. Maternal High-Fat Diet Disturbs Uteroplacental Hemodynamics and Increases the Frequency of Stillbirth in a Nonhuman Primate Model of Excess Nutrition. Endocrinology 2011, 152, 2456–2464. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.Y.; Jung, E.; Kim, E.N.; Kim, C.J.; Lee, J.Y.; Hwang, J.H.; Song, W.S.; Lee, B.S.; Kim, E.A.-R.; Kim, K.-S. Chronic Placental Inflammation as a Risk Factor of Severe Retinopathy of Prematurity. J. Pathol. Transl. Med. 2018, 52, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.M.; Messaoudi, I. The impact of maternal obesity during pregnancy on offspring immunity. Mol. Cell. Endocrinol. 2015, 418, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Kohn, C.; Dubrovska, G.; Huang, Y.; Gollasch, M. Hydrogen sulfide: Potent regulator of vascular tone and stimulator of angiogenesis. Int. J. Biomed. Sci. 2012, 8, 81–86. [Google Scholar]
- Sun, X.; Wang, W.; Dai, J.; Jin, S.; Huang, J.; Guo, C.; Wang, C.; Pang, L.; Wang, Y. A Long-Term and Slow-Releasing Hydrogen Sulfide Donor Protects against Myocardial Ischemia/Reperfusion Injury. Sci. Rep. 2017, 7, 3541. [Google Scholar] [CrossRef]
- Aye, I.L.M.H.; Rosario, F.J.; Powell, T.L.; Janssona, T. Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. Proc. Natl. Acad. Sci. USA 2015, 112, 12858–12863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Liang, X.; Sun, X.; Zhang, L.; Fu, X.; Rogers, C.J.; Berim, A.; Zhang, S.; Wang, S.; Wang, B.; et al. AMPK/alpha-Ketoglutarate Axis Dynamically Mediates DNA Demethylation in the Prdm16 Promoter and Brown Adipogenesis. Cell Metab. 2016, 24, 542–554. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.P.; Liu, K.C.; Huang, W.W.; Chueh, F.S.; Ko, Y.C.; Chiu, T.H.; Lin, J.P.; Kuo, J.H.; Yang, J.S.; Chung, J.G. Diallyl trisulfide (DATS) inhibits mouse colon tumor in mouse CT-26 cells allograft model in vivo. Phytomedicine 2011, 18, 672–676. [Google Scholar] [CrossRef]
- Andrikopoulos, S.; Blair, A.R.; Deluca, N.; Fam, B.C.; Proietto, J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1323–E1332. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-J.; Li, Q.; Du, H.-P.; Wang, Y.-L.; You, S.-J.; Wang, F.; Xu, X.-S.; Cheng, J.; Cao, Y.-J.; Liu, C.-F.; et al. Homocysteine Triggers Inflammatory Responses in Macrophages through Inhibiting CSE-H2S Signaling via DNA Hypermethylation of CSE Promoter. Int. J. Mol. Sci. 2015, 16, 12560–12577. [Google Scholar] [CrossRef]
- Peng, J.; Xia, M.; Xiong, J.; Cui, C.; Huang, N.; Zhou, Y.; Wei, H.; Peng, J. Effect of Sows Gestational Methionine/Lysine Ratio on Maternal and Placental Hydrogen Sulfide Production. Animals 2020, 10, 251. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Pan, L.; Zhuo, Y.; Gong, Q.; Rose, P.; Zhu, Y. Hypoxia-Inducible Factor-1 alpha Is Involved in the Pro-angiogenic Effect of Hydrogen Sulfide under Hypoxic Stress. Biol. Pharm. Bull. 2010, 33, 1550–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Xu, T.; Cai, A.; Wu, Y.; Wei, H.; Jiang, S.; Peng, J. Excessive backfat of sows at 109 d of gestation induces lipotoxic placental environment and is associated with declining reproductive performance. J. Anim. Sci. 2018, 96, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Dubova, E.A.; Pavlov, K.A.; Borovkova, E.I.; Bayramova, M.A.; Makarov, I.O.; Shchegolev, A.I. Vascular Endothelial Growth Factor and its Receptors in the Placenta of Pregnant Women with Obesity. Bull. Exp. Biol. Med. 2011, 151, 253–258. [Google Scholar] [CrossRef]
- Son, J.S.; Liu, X.; Tian, Q.; Zhao, L.; Chen, Y.; Hu, Y.; Chae, S.A.; de Avila, J.M.; Zhu, M.-J.; Du, M. Exercise prevents the adverse effects of maternal obesity on placental vascularization and fetal growth. J. Physiol. 2019, 597, 3333–3347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Du, J.; Tang, C.; Huang, Y.; Jin, H. H2S-Induced Sulfhydration: Biological Function and Detection Methodology. Front. Pharmacol. 2017, 8, 608. [Google Scholar] [CrossRef] [Green Version]
- Velmurugan, G.V.; Huang, H.; Sun, H.; Candela, J.; Jaiswal, M.K.; Beaman, K.D.; Yamashita, M.; Prakriya, M.; White, C. Depletion of H2S during obesity enhances store-operated Ca2+ entry in adipose tissue macrophages to increase cytokine production. Sci. Signal. 2015, 8, ra128. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Gao, B.; Li, M.; Yao, L.; Wang, S.; Chen, M.; Li, H.; Ma, C.; Ji, A.; Li, Y. Hydrogen Sulfide Mitigates Kidney Injury in High Fat Diet-Induced Obese Mice. Oxidative Med. Cell. Longev. 2016, 2016, 2715718. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Yang, Y.; Sun, J.; Zhang, Y.; Luo, T.; Li, B.; Jiang, Y.; Shi, Y.; Le, G. Dietary methionine restriction ameliorates the impairment of learning and memory function induced by obesity in mice. Food Funct. 2019, 10, 1411–1425. [Google Scholar] [CrossRef]
- Kretschmer, T.; Turnwald, E.-M.; Janoschek, R.; Zentis, P.; Bae-Gartz, I.; Beers, T.; Handwerk, M.; Wohlfarth, M.; Ghilav, M.; Bloch, W.; et al. Maternal high fat diet-induced obesity affects trophoblast differentiation and placental function in mice. Biol. Reprod. 2020, 103, 1260–1274. [Google Scholar] [CrossRef]
- Li, M.; Mao, J.; Zhu, Y. New Therapeutic Approaches Using Hydrogen Sulfide Donors in Inflammation and Immune Response. Antioxid. Redox Signal. 2021, 35, 341–356. [Google Scholar] [CrossRef]
- Su, Y.; Wang, Y.; Liu, M.; Chen, H. Hydrogen sulfide attenuates renal I/R-induced activation of the inflammatory response and apoptosis via regulating Nrf2-mediated NLRP3 signaling pathway inhibition. Mol. Med. Rep. 2021, 24, 518. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Huang, J.-T.; Chen, W.-L.; Wang, R.-H.; Kao, M.-C.; Pan, Y.-R.; Chan, S.-H.; Tsai, K.-W.; Kung, H.-J.; Lin, K.-T.; et al. Dysregulation of cystathionine gamma-lyase promotes prostate cancer progression and metastasis. Embo Rep. 2019, 20, e45986. [Google Scholar] [CrossRef] [PubMed]
- Bai, A.P.; Qin, O.Y.; Hu, R.W. Diallyl trisulfide inhibits tumor necrosis factor-alpha expression in inflammed mucosa of ulcerative colitis. Dig. Dis. Sci. 2005, 50, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Calabuig-Navarro, V.; Haghiac, M.; Minium, J.; Glazebrook, P.; Ranasinghe, G.C.; Hoppel, C.; de-Mouzon, S.H.; Catalano, P.; O’Tierney-Ginn, P. Effect of Maternal Obesity on Placental Lipid Metabolism. Endocrinology 2017, 158, 2543–2555. [Google Scholar] [CrossRef]
- Hastie, R.; Lappas, M. The effect of pre-existing maternal obesity and diabetes on placental mitochondrial content and electron transport chain activity. Placenta 2014, 35, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Malti, N.; Merzouk, H.; Merzouk, S.A.; Loukidi, B.; Karaouzene, N.; Malti, A.; Narce, M. Oxidative stress and maternal obesity: Feto-placental unit interaction. Placenta 2014, 35, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Saben, J.; Lindsey, F.; Zhong, Y.; Thakali, K.; Badger, T.M.; Andres, A.; Gomez-Acevedo, H.; Shankar, K. Maternal obesity is associated with a lipotoxic placental environment. Placenta 2014, 35, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Zheng, N.; Qi, K.; Cheng, H.; Sun, Z.; Gao, B.; Zhang, Y.; Pang, W.; Huangfu, C.; Ji, S.; et al. Exogenous hydrogen sulfide mitigates the fatty liver in obese mice through improving lipid metabolism and antioxidant potential. Med. Gas Res. 2015, 5, 1. [Google Scholar] [CrossRef] [Green Version]

| NFD + PBS | NFD + DATS | HFD + PBS | HFD + DATS | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Dietary | DATS | Interaction | ||||||
| No. | 9 | 11 | 11 | 10 | ||||
| Maternal weights at E0.5 (g) | 20.61 ± 0.23 | 21.22 ± 0.33 | 32.47 ± 0.46 | 33.09 ± 0.42 | <0.01 | 0.12 | 1.00 | |
| Maternal weights at E18.5 (g) | 34.07 ± 0.73 | 34.41 ± 0.68 | 40.40 ± 0.74 | 39.55 ± 0.69 | <0.01 | 0.73 | 0.42 | |
| Gestational weight gain (g) | 13.46 ± 0.56 | 13.18 ± 0.64 | 8.43 ± 0.37 | 6.47 ± 0.63 | <0.01 | 0.09 | 0.07 | |
| Gestational total feed intake (g) | 57.67 ± 0.60 | 56.79 ± 0.66 | 41.45 ± 1.87 | 39.73 ± 0.79 | <0.01 | 0.27 | 0.97 | |
| Gestational daily feed intake (g) | 3.21 ± 0.03 | 3.16 ± 0.04 | 2.31 ± 0.10 | 2.21 ± 0.04 | <0.01 | 0.26 | 0.97 | |
| Gestational daily energy intake (kcal) | 11.85 ± 0.12 | 11.67 ± 0.16 | 11.51 ± 0.52 | 11.04 ± 0.22 | 0.05 | 0.30 | 0.80 | |
| iWAT Percentage (%) 2 | 2.29 ± 0.09 | 1.76 ± 0.09 | 4.64 ± 0.15 | 3.98 ± 0.44 | <0.01 | 0.01 | 0.77 | |
| Inguinal white adipose tissue weight (g) | 0.77 ± 0.03 c | 0.62 ± 0.03 c | 1.92 ± 0.05 a | 1.44 ± 0.14 b | <0.01 | 0.00 | 0.03 | |
| Litter size (No.) | 7.78 ± 0.36 | 8.60 ± 0.43 | 8.64 ± 0.36 | 9.44 ± 0.24 | 0.02 | 0.03 | 0.98 | |
| Alive litter size (No.) | 6.78 ± 0.55 | 8.40 ± 0.40 | 8.00 ± 0.47 | 8.50 ± 0.38 | 0.16 | 0.03 | 0.23 | |
| Embryo resorption rate (%) 3 | 6.50 ± 4.16 | 3.64 ± 2.44 | 7.47 ± 3.54 | 4.73 ± 2.23 | 0.76 | 0.40 | 0.99 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Wang, Z.; Miao, Y.; Wei, H.; Peng, J.; Zhou, Y. Diallyl Trisulfide Promotes Placental Angiogenesis by Regulating Lipid Metabolism and Alleviating Inflammatory Responses in Obese Pregnant Mice. Nutrients 2022, 14, 2230. https://doi.org/10.3390/nu14112230
Wang M, Wang Z, Miao Y, Wei H, Peng J, Zhou Y. Diallyl Trisulfide Promotes Placental Angiogenesis by Regulating Lipid Metabolism and Alleviating Inflammatory Responses in Obese Pregnant Mice. Nutrients. 2022; 14(11):2230. https://doi.org/10.3390/nu14112230
Chicago/Turabian StyleWang, Miaomiao, Zhaoyu Wang, Yueyue Miao, Hongkui Wei, Jian Peng, and Yuanfei Zhou. 2022. "Diallyl Trisulfide Promotes Placental Angiogenesis by Regulating Lipid Metabolism and Alleviating Inflammatory Responses in Obese Pregnant Mice" Nutrients 14, no. 11: 2230. https://doi.org/10.3390/nu14112230
APA StyleWang, M., Wang, Z., Miao, Y., Wei, H., Peng, J., & Zhou, Y. (2022). Diallyl Trisulfide Promotes Placental Angiogenesis by Regulating Lipid Metabolism and Alleviating Inflammatory Responses in Obese Pregnant Mice. Nutrients, 14(11), 2230. https://doi.org/10.3390/nu14112230






