Reduction of De Novo Lipogenesis Mediates Beneficial Effects of Isoenergetic Diets on Fatty Liver: Mechanistic Insights from the MEDEA Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Experimental Procedures
2.3. Laboratory Methods
2.4. Calculations
2.5. Statistical Analysis
3. Results
3.1. Dietary Effects on Metabolic Outcomes
3.2. Dietary Effects on the Fatty Acid Composition of Serum Triglycerides
3.3. Dietary Effects on de novo Lipogenesis, Desaturase Activity, and β-oxidation
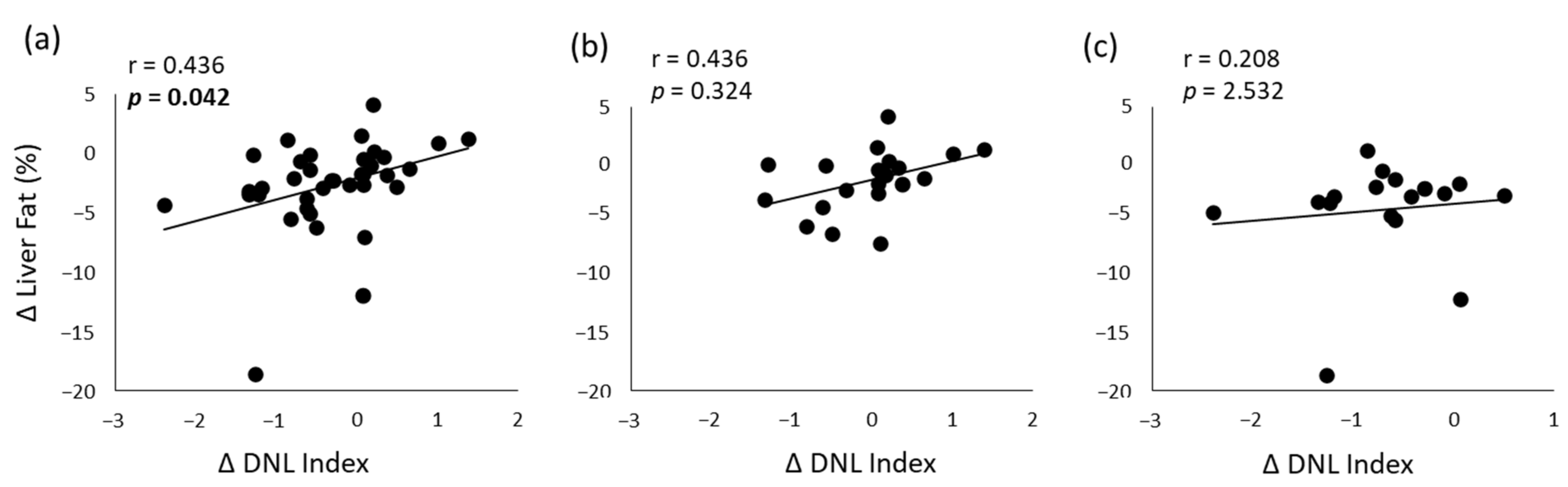
3.4. Correlation Analyses
4. Discussion
4.1. De Novo Lipogenesis
4.2. Fatty Acid Composition of Serum Triglycerides
4.3. Stearoyl-CoA Desaturase-1 Activity
4.4. Beta-Oxidation
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haas, J.T.; Francque, S.; Staels, B. Pathophysiology and Mechanisms of Nonalcoholic Fatty Liver Disease. Annu. Rev. Physiol. 2016, 78, 181–205. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Della Pepa, G.; Vetrani, C.; Lombardi, G.; Bozzetto, L.; Annuzzi, G.; Rivellese, A.A. Isocaloric Dietary Changes and Non-Alcoholic Fatty Liver Disease in High Cardiometabolic Risk Individuals. Nutrients 2017, 9, 1065. [Google Scholar] [CrossRef] [Green Version]
- Roumans, K.H.M.; Basset Sagarminaga, J.; Peters, H.P.F.; Schrauwen, P.; Schrauwen-Hinderling, V.B. Liver fat storage pathways: Methodologies and dietary effects. Curr. Opin. Lipidol. 2021, 32, 9–15. [Google Scholar] [CrossRef]
- Hudgins, L.C.; Seidman, C.E.; Diakun, J.; Hirsch, J. Human fatty acid synthesis is reduced after the substitution of dietary starch for sugar. Am. J. Clin. Nutr. 1998, 67, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Green, C.J.; Pramfalk, C.; Charlton, C.A.; Gunn, P.J.; Cornfield, T.; Pavlides, M.; Karpe, F.; Hodson, L. Hepatic de novo lipogenesis is suppressed and fat oxidation is increased by omega-3 fatty acids at the expense of glucose metabolism. BMJ Open Diabetes Res. Care 2020, 8, e000871. [Google Scholar] [CrossRef] [Green Version]
- Hodson, L.; Rosqvist, F.; Parry, S.A. The influence of dietary fatty acids on liver fat content and metabolism. Proc. Nutr. Soc. 2020, 79, 30–41, Erratum in Proc. Nutr. Soc. 2019, 78, 473. [Google Scholar] [CrossRef]
- Bozzetto, L.; Costabile, G.; Luongo, D.; Naviglio, D.; Cicala, V.; Piantadosi, C.; Patti, L.; Cipriano, P.; Annuzzi, G.; Rivellese, A.A. Reduction in liver fat by dietary MUFA in type 2 diabetes is helped by enhanced hepatic fat oxidation. Diabetologia 2016, 59, 2697–2701. [Google Scholar] [CrossRef] [Green Version]
- Bozzetto, L.; Annuzzi, G.; Ragucci, M.; Di Donato, O.; Della Pepa, G.; Della Corte, G.; Griffo, E.; Anniballi, G.; Giacco, A.; Mancini, M.; et al. Insulin resistance, postprandial GLP-1 and adaptive immunity are the main predictors of NAFLD in a homogeneous population at high cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 623–629. [Google Scholar] [CrossRef]
- Della Pepa, G.; Vetrani, C.; Brancato, V.; Vitale, M.; Monti, S.; Annuzzi, G.; Lombardi, G.; Izzo, A.; Tommasone, M.; Cipriano, P.; et al. Effects of a multifactorial ecosustainable isocaloric diet on liver fat in patients with type 2 diabetes: Randomized clinical trial. BMJ Open Diabetes Res. Care 2020, 8, e001342. [Google Scholar] [CrossRef]
- Bozzetto, L.; Prinster, A.; Annuzzi, G.; Costagliola, L.; Mangione, A.; Vitelli, A.; Mazzarella, R.; Longobardo, M.; Mancini, M.; Vigorito, C.; et al. Liver fat is reduced by an isoenergetic MUFA diet in a controlled randomized study in type 2 diabetic patients. Diabetes Care 2012, 35, 1429–1435. [Google Scholar] [CrossRef] [Green Version]
- Burdge, G.C.; Wright, P.; Jones, A.E.; Wootton, S.A. A method for separation of phosphatidylcholine, triacylglycerol, non-esterified fatty acids and cholesterol esters from plasma by solid-phase extraction. Br. J. Nutr. 2000, 84, 781–787. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Hudgins, L.C.; Hellerstein, M.; Seidman, C.; Neese, R.; Diakun, J.; Hirsch, J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J. Clin. Investig. 1996, 97, 2081–2091. [Google Scholar] [CrossRef]
- Peter, A.; Cegan, A.; Wagner, S.; Lehmann, R.; Stefan, N.; Königsrainer, A.; Königsrainer, I.; Häring, H.U.; Schleicher, E. Hepatic lipid composition and stearoyl-coenzyme A desaturase 1 mRNA expression can be estimated from plasma VLDL fatty acid ratios. Clin. Chem. 2009, 55, 2113–2120. [Google Scholar] [CrossRef] [Green Version]
- Mardinoglu, A.; Wu, H.; Bjornson, E.; Zhang, C.; Hakkarainen, A.; Räsänen, S.M.; Lee, S.; Mancina, R.M.; Bergentall, M.; Pietiläinen, K.H.; et al. An Integrated Understanding of the Rapid Metabolic Benefits of a Carbohydrate-Restricted Diet on Hepatic Steatosis in Humans. Cell Metab. 2018, 27, 559–571.e5. [Google Scholar] [CrossRef] [Green Version]
- Konrad, S.D.; Cook, S.L.; Goh, Y.K.; French, M.A.; Clandinin, M.T. Use of deuterium oxide to measure de novo fatty acid synthesis in normal subjects consuming different dietary fatty acid composition1. Biochim. Biophys. Acta 1998, 1393, 143–152. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Sädevirta, S.; Zhou, Y.; Kayser, B.; Ali, A.; Ahonen, L.; Lallukka, S.; Pelloux, V.; Gaggini, M.; Jian, C.; et al. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care 2018, 41, 1732–1739. [Google Scholar] [CrossRef] [Green Version]
- Vetrani, C.; Maukonen, J.; Bozzetto, L.; Della Pepa, G.; Vitale, M.; Costabile, G.; Riccardi, G.; Rivellese, A.A.; Saarela, M.; Annuzzi, G. Diets naturally rich in polyphenols and/or long-chain n-3 polyunsaturated fatty acids differently affect microbiota composition in high-cardiometabolic-risk individuals. Acta Diabetol. 2020, 57, 853–860. [Google Scholar] [CrossRef]
- Lambert, J.E.; Ramos-Roman, M.A.; Browning, J.D.; Parks, E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Roumans, K.H.M.; Lindeboom, L.; Veeraiah, P.; Remie, C.M.E.; Phielix, E.; Havekes, B.; Bruls, Y.M.H.; Brouwers, M.C.G.J.; Ståhlman, M.; Alssema, M.; et al. Hepatic saturated fatty acid fraction is associated with de novo lipogenesis and hepatic insulin resistance. Nat. Commun. 2020, 11, 1891. [Google Scholar] [CrossRef] [PubMed]
- Bjermo, H.; Iggman, D.; Kullberg, J.; Dahlman, I.; Johansson, L.; Persson, L.; Berglund, J.; Pulkki, K.; Basu, S.; Uusitupa, M.; et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1003–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridén, M.; Rosqvist, F.; Kullberg, J.; Ahlström, H.; Lind, L.; Risérus, U. Associations between fatty acid composition in serum cholesteryl esters and liver fat, basal fat oxidation, and resting energy expenditure: A population-based study. Am. J. Clin. Nutr. 2021, 114, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Wiest, M.M.; Cheung, O.; Mirshahi, F.; Sargeant, C.; Min, H.K.; Contos, M.J.; Sterling, R.K.; Fuchs, M.; Zhou, H.; et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology 2009, 50, 1827–1838. [Google Scholar] [CrossRef] [Green Version]
- von Loeffelholz, C.; Döcke, S.; Lock, J.F.; Lieske, S.; Horn, P.; Kriebel, J.; Wahl, S.; Singmann, P.; de Las Heras Gala, T.; Grallert, H.; et al. Increased lipogenesis in spite of upregulated hepatic 5’AMP-activated protein kinase in human non-alcoholic fatty liver. Hepatol. Res. 2017, 47, 890–901. [Google Scholar] [CrossRef]
- Hodson, L.; Fielding, B.A. Stearoyl-CoA desaturase: Rogue or innocent bystander? Prog. Lipid Res. 2013, 52, 15–42. [Google Scholar] [CrossRef]
- Davidson, M.H. Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am. J. Cardiol. 2006, 98, 27i–33i. [Google Scholar] [CrossRef]
- Croci, I.; Byrne, N.M.; Choquette, S.; Hills, A.P.; Chachay, V.S.; Clouston, A.D.; O’Moore-Sullivan, T.M.; Macdonald, G.A.; Prins, J.B.; Hickman, I.J. Whole-body substrate metabolism is associated with disease severity in patients with non-alcoholic fatty liver disease. Gut 2013, 62, 1625–1633. [Google Scholar] [CrossRef] [Green Version]
- Kotronen, A.; Seppänen-Laakso, T.; Westerbacka, J.; Kiviluoto, T.; Arola, J.; Ruskeepää, A.L.; Oresic, M.; Yki-Järvinen, H. Hepatic stearoyl-CoA desaturase (SCD)-1 activity and diacylglycerol but not ceramide concentrations are increased in the nonalcoholic human fatty liver. Diabetes 2009, 58, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Bugianesi, E.; Gastaldelli, A.; Vanni, E.; Gambino, R.; Cassader, M.; Baldi, S.; Ponti, V.; Pagano, G.; Ferrannini, E.; Rizzetto, M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: Sites and mechanisms. Diabetologia 2005, 48, 634–642. [Google Scholar] [CrossRef] [Green Version]
- Luukkonen, P.K.; Qadri, S.; Ahlholm, N.; Porthan, K.; Männistö, V.; Sammalkorpi, H.; Penttilä, A.K.; Hakkarainen, A.; Lehtimäki, T.E.; Gaggini, M.; et al. Distinct contributions of metabolic dysfunction and genetic risk factors in the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2022, 76, 526–535. [Google Scholar] [CrossRef]
- Rosqvist, F.; McNeil, C.A.; Pramfalk, C.; Parry, S.A.; Low, W.S.; Cornfield, T.; Fielding, B.A.; Hodson, L. Fasting hepatic de novo lipogenesis is not reliably assessed using circulating fatty acid markers. Am. J. Clin Nutr. 2019, 109, 260–268. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]

| MUFA Diet (n = 20) | Multifactorial Diet (n = 17) | p-Value Diet × Time † | |||
|---|---|---|---|---|---|
| Baseline | 8th Week | Baseline | 8th Week | ||
| Body weight (kg) | 84 (15) | 83 (15) * | 84 (9) | 83 (9) * | 0.860 |
| BMI (kg/m2) | 31 (3) | 30 (3) * | 32 (4) | 31 (4) * | 0.535 |
| Waist circumference (cm) | 105 (10) | 104 (11) | 106 (10) | 105 (10) | 0.986 |
| Plasma total cholesterol (mg/dL) | 144 (29) | 140 (26) | 144 (25) | 145 (32) | 0.478 |
| HDL cholesterol (mg/dL) | 39 (8) | 38 (8) | 41 (10) | 38 (8) | 0.088 |
| LDL cholesterol (mg/dL) | 84 (24) | 81 (21) | 82 (21) | 85 (26) | 0.407 |
| Plasma triglycerides (mg/dL) | 109 (35) | 107 (44) | 105 (38) | 110 (40) | 0.783 |
| Plasma glucose (mg/dL) | 130 (18) | 128 (17) | 123 (14) | 124 (19) | 0.606 |
| HbA1c (%) | 6.5 (0.6) | 6.3 (0.7) * | 6.5 (0.4) | 6.3 (0.6) * | 0.681 |
| Plasma insulin (µU/mL) | 19 (10) | 20 (11) | 19 (9) | 15 (8) | 0.090 |
| HOMA-IR | 6.0 (3.0) | 6.4 (3.4) | 5.8 (2.8) | 4.6 (2.4) | 0.216 |
| Liver fat (%) | 9.9 (9.0) | 8.4 (9.0) * | 9.5 (7.9) | 5.4 (4.9) * | 0.040 |
| MUFA Diet (n = 20) | Multifactorial Diet (n = 17) | p-Value Diet × Time † | |||
|---|---|---|---|---|---|
| Baseline | 8th Week | Baseline | 8th Week | ||
| Myristic acid (%) | 1.9 (0.6) | 2.1 (0.9) | 2.4 (0.7) ‡ | 1.8 (0.7) * | 0.003 |
| Palmitic acid (%) | 21.0 (5.7) | 19.2 (3.8) | 21.4 (3.9) | 17.6 (4.6) * | 0.262 |
| Stearic acid (%) | 6.9 (3.8) | 6.1 (2.3) | 6.3 (2.1) | 6.1 (1.6) | 0.692 |
| Palmitoleic acid (%) | 2.7 (1.2) | 2.2 (0.8) | 2.8 (1) | 1.8 (0.6) * | 0.087 |
| Oleic acid (%) | 30.2 (6.9) | 33.9 (6.2) * | 30.8 (6.9) | 34.7 (6.9) * | 0.917 |
| n-6 PUFA (%) | 13.8 (3.6) | 13.4 (3.4) | 12.5 (3.8) | 14.1(3.2) | 0.121 |
| Linoleic acid (%) | 12.2 (3.5) | 11.6 (4.2) | 10.9 (3.9) | 12.1 (3.5) | 0.230 |
| γ-Linolenic acid (%) | 0.5 (0.3) | 0.6 (0.5) | 0.6 (0.3) | 0.7 (0.5) | 0.220 |
| Arachidonic acid (%) | 1.1 (0.5) | 1.3 (0.6) | 1.0 (0.4) | 1.2 (0.5) | 0.147 |
| n-3 PUFA (%) | 2.9 (1.0) | 3.3 (1.1) * | 2.5 (0.8) | 4.4 (1.7) * | 0.001 |
| α-Linolenic acid (%) | 0.7 (0.3) | 0.8 (0.4) | 0.7 (0.4) | 1.1 (0.6) * | 0.092 |
| Eicosapentaenoic acid (%) | 0.7 (0.4) | 0.8 (0.5) | 0.6 (0.4) | 1.3 (0.8) * | 0.009 |
| Docosapentaenoic acid (%) | 0.7 (0.4) | 0.8 (0.5) | 0.5 (0.3) | 1.0 (0.8) * | 0.115 |
| Docosahexaenoic acid (%) | 0.8 (0.5) | 1.0 (0.6) | 0.7 (0.6) | 1.3 (0.9) * | 0.046 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costabile, G.; Della Pepa, G.; Salamone, D.; Luongo, D.; Naviglio, D.; Brancato, V.; Cavaliere, C.; Salvatore, M.; Cipriano, P.; Vitale, M.; et al. Reduction of De Novo Lipogenesis Mediates Beneficial Effects of Isoenergetic Diets on Fatty Liver: Mechanistic Insights from the MEDEA Randomized Clinical Trial. Nutrients 2022, 14, 2178. https://doi.org/10.3390/nu14102178
Costabile G, Della Pepa G, Salamone D, Luongo D, Naviglio D, Brancato V, Cavaliere C, Salvatore M, Cipriano P, Vitale M, et al. Reduction of De Novo Lipogenesis Mediates Beneficial Effects of Isoenergetic Diets on Fatty Liver: Mechanistic Insights from the MEDEA Randomized Clinical Trial. Nutrients. 2022; 14(10):2178. https://doi.org/10.3390/nu14102178
Chicago/Turabian StyleCostabile, Giuseppina, Giuseppe Della Pepa, Dominic Salamone, Delia Luongo, Daniele Naviglio, Valentina Brancato, Carlo Cavaliere, Marco Salvatore, Paola Cipriano, Marilena Vitale, and et al. 2022. "Reduction of De Novo Lipogenesis Mediates Beneficial Effects of Isoenergetic Diets on Fatty Liver: Mechanistic Insights from the MEDEA Randomized Clinical Trial" Nutrients 14, no. 10: 2178. https://doi.org/10.3390/nu14102178
APA StyleCostabile, G., Della Pepa, G., Salamone, D., Luongo, D., Naviglio, D., Brancato, V., Cavaliere, C., Salvatore, M., Cipriano, P., Vitale, M., Corrado, A., Rivellese, A. A., Annuzzi, G., & Bozzetto, L. (2022). Reduction of De Novo Lipogenesis Mediates Beneficial Effects of Isoenergetic Diets on Fatty Liver: Mechanistic Insights from the MEDEA Randomized Clinical Trial. Nutrients, 14(10), 2178. https://doi.org/10.3390/nu14102178










