Effects of Resistant Dextrin from Potato Starch on the Growth Dynamics of Selected Co-Cultured Strains of Gastrointestinal Bacteria and the Activity of Fecal Enzymes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates
2.2. Growth Dynamics of the Intestinal Microbiota
2.3. Determination of Prebiotic Index
2.4. Enzymatic Assays
2.5. Statistical Analysis
3. Results
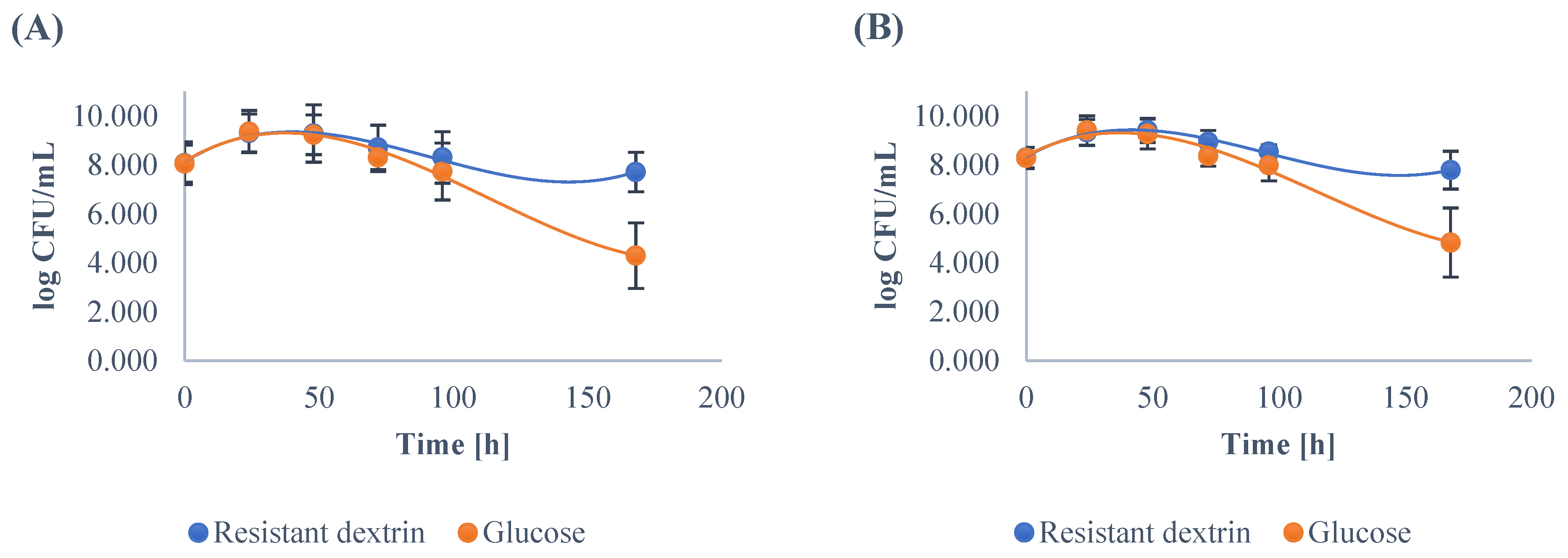
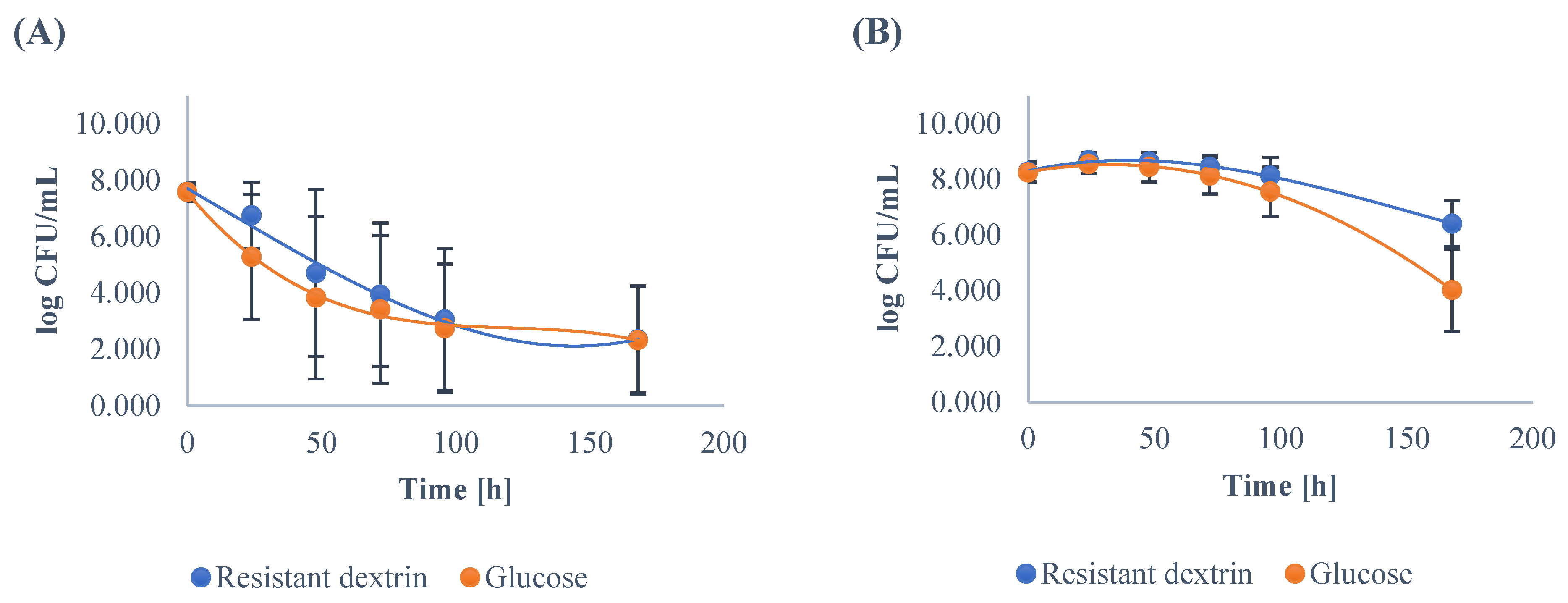
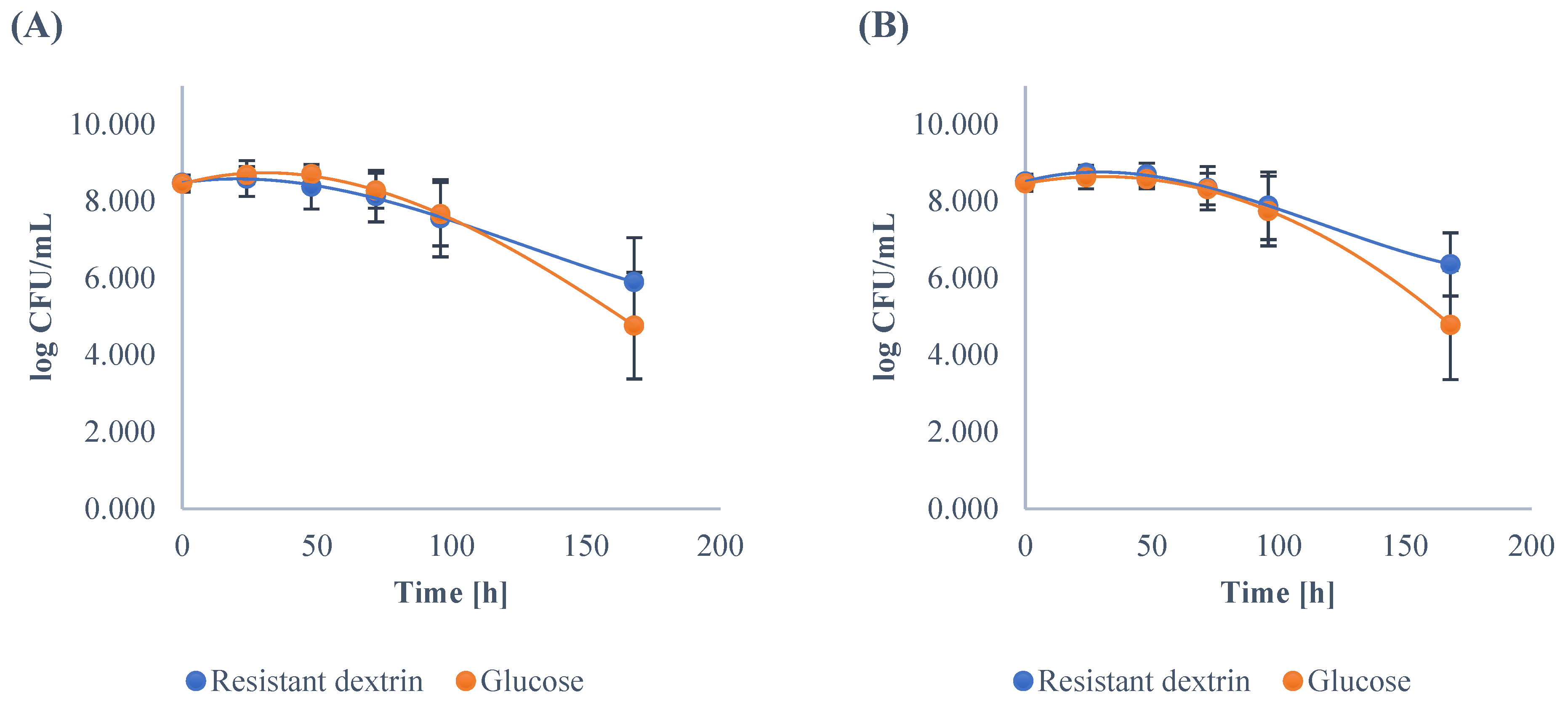
3.1. Growth Dynamics of the Gut Microbiota
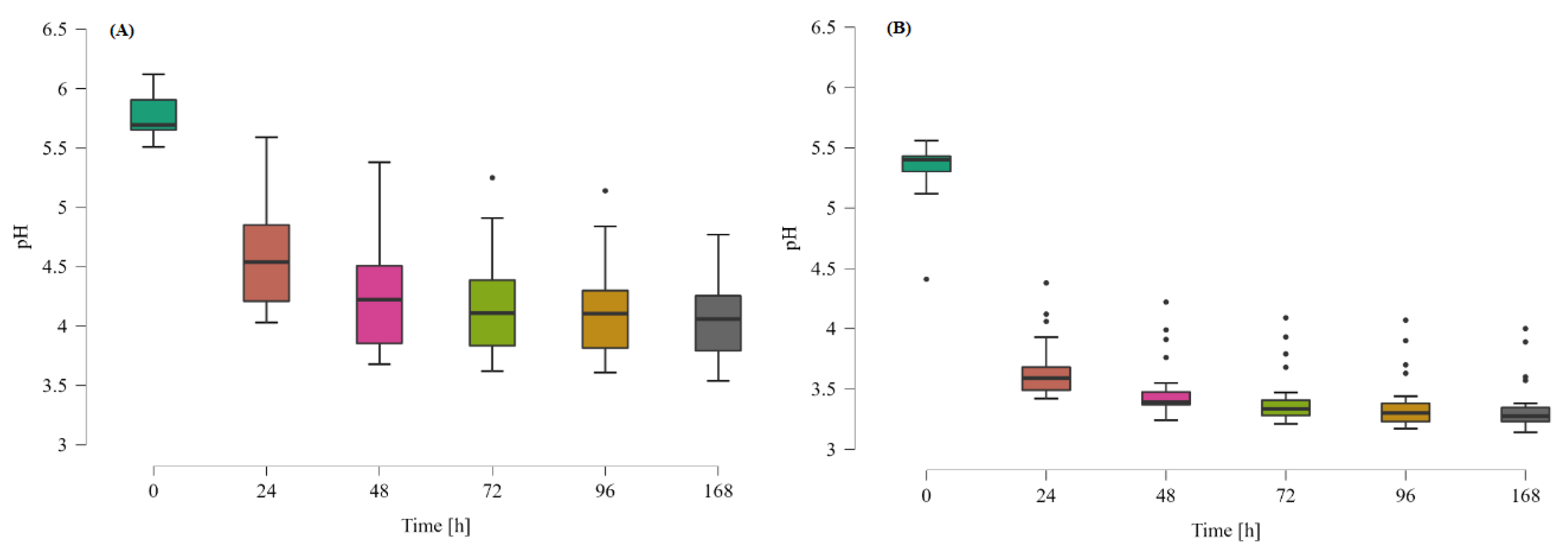
3.2. The Effect of Resistant Dextrin on the Activity of Fecal Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Geier, M.S.; Butler, R.N.; Howarth, G.S. Probiotics, prebiotics and synbiotics: A role in chemoprevention for colorectal cancer? Cancer Biol. Ther. 2006, 5, 1265–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peredo-Lovillo, A.; Romero-Luna, H.E.; Jiménez-Fernández, M. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Res. Int. 2020, 136, 109473. [Google Scholar] [CrossRef]
- Janssen, A.W.F.; Kersten, S. The role of the gut microbiota in metabolic health. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 3111–3123. [Google Scholar] [CrossRef] [Green Version]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Rendic, S.; Guengerich, F.P. Contributions of human enzymes in carcinogen metabolism. Chem. Res. Toxicol. 2012, 25, 1316–1383. [Google Scholar] [CrossRef]
- Bruno-Barcena, J.M.; Azcarate-Peril, M.A. Galacto-oligosaccharides and colorectal cancer: Feeding our intestinal probiome. J. Funct. Foods 2015, 12, 92–108. [Google Scholar] [CrossRef]
- Nakamura, J.; Kubota, Y.; Miyaoka, M.; Saitoh, T.; Mizuno, F.; Benno, Y. Comparison of Four Microbial Enzymes in Clostridia and Bacteroides Isolated from Human Feces. Microbiol. Immunol. 2002, 46, 487–490. [Google Scholar] [CrossRef] [Green Version]
- Dabek, M.; McCrae, S.I.; Stevens, V.J.; Duncan, S.H.; Louis, P. Distribution of β-glucosidase and β-glucuronidase activity and of β-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol. Ecol. 2008, 66, 487–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mroczyńska, M.; Galecka, M.; Szachta, P.; Kamoda, D.; Libudzisz, Z.; Roszak, D. Beta-glucuronidase and Beta-glucosidase activity in stool specimens of children with inflammatory bowel disease. Pol. J. Microbiol. 2013, 62, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Dudek-Wicher, R.K.; Junka, A.; Bartoszewicz, M. The influence of antibiotics and dietary components on gut microbiota. Prz. Gastroenterol. 2018, 13, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eslami, M.; Yousefi, B.; Kokhaei, P.; Hemati, M.; Nejad, Z.R.; Arabkari, V.; Namdar, A. Importance of probiotics in the prevention and treatment of colorectal cancer. J. Cell. Physiol. 2019, 234, 17127–17143. [Google Scholar] [CrossRef] [PubMed]
- Heyman, M.; Ménard, S. Probiotic microorganisms: How they affect intestinal pathophysiology. Cell. Mol. Life Sci. CMLS 2002, 59, 1151–1165. [Google Scholar] [CrossRef]
- Badgeley, A.; Anwar, H.; Modi, K.; Murphy, P.; Lakshmikuttyamma, A. Effect of probiotics and gut microbiota on anti-cancer drugs: Mechanistic perspectives. Biochim. Et Biophys. Acta (BBA) Rev. Cancer 2021, 1875, 188494. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Roviello, G.; Catalano, M.; Polom, K. Gut Microbiota Modulation in the Context of Immune-Related Aspects of Lactobacillus spp. and Bifidobacterium spp. in Gastrointestinal Cancers. Nutrients 2021, 13, 2674. [Google Scholar] [CrossRef]
- Legesse Bedada, T.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for cancer alternative prevention and treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar] [CrossRef]
- Śliżewska, K.; Markowiak-Kopeć, P.; Śliżewska, W. The Role of Probiotics in Cancer Prevention. Cancers 2021, 13, 20. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health Effects and Sources of Prebiotic Dietary Fiber. Curr. Dev. Nutr. 2018, 2, nzy005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, G.K.; Wang, L.; Nanavati, J.; Twose, C.; Singh, R.; Mullin, G. Dietary Alteration of the Gut Microbiome and Its Impact on Weight and Fat Mass: A Systematic Review and Meta-Analysis. Genes 2018, 9, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Włodarczyk, M.; Śliżewska, K. Efficiency of Resistant Starch and Dextrins as Prebiotics: A Review of the Existing Evidence and Clinical Trials. Nutrients 2021, 13, 3808. [Google Scholar] [CrossRef] [PubMed]
- Wynne, A.G.; McCartney, A.L.; Brostoff, J.; Hudspith, B.N.; Gibson, G.R. An in vitro assessment of the effects of broad-spectrum antibiotics on the human gut microflora and concomitant isolation of a Lactobacillus plantarum with anti-Candida activities. Anaerobe 2004, 10, 165–169. [Google Scholar] [CrossRef]
- Palframan, R.; Gibson, G.R.; Rastall, R.A. Development of a quantitative tool for the comparison of the prebiotic effect of dietary oligosaccharides. Lett. Appl. Microbiol. 2003, 37, 281–284. [Google Scholar] [CrossRef] [Green Version]
- Barczynska, R.; Slizewska, K.; Jochym, K.; Kapusniak, J.; Libudzisz, Z. The tartaric acid-modified enzyme-resistant dextrin from potato starch as potential prebiotic. J. Funct. Foods 2012, 4, 954–962. [Google Scholar] [CrossRef]
- Barczynska, R.; Zawierucha, I.; Bandurska, K.; Kapusniak, J. Lactose-free milk enriched with resistant dextrin. Postępy Hig. I Med. Doświadczalnej 2018, 72, 781–787. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Díez-Municio, M.; Herrero, M.; Martínez-Cuesta, M.C.; Peláez, C.; Requena, T.; Moreno, F.J. Selective fermentation of potential prebiotic lactose-derived oligosaccharides by probiotic bacteria. Int. Dairy J. 2014, 38, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Sliżewska, K. The citric acid-modified, enzyme-resistant dextrin from potato starch as a potential prebiotic. Acta Biochim. Pol. 2013, 60, 671–675. [Google Scholar] [CrossRef] [Green Version]
- Barczynska, R.; Jochym, K.; Slizewska, K.; Kapusniak, J.; Libudzisz, Z. The effect of citric acid-modified enzyme-resistant dextrin on growth and metabolism of selected strains of probiotic and other intestinal bacteria. J. Funct. Foods 2010, 2, 126–133. [Google Scholar] [CrossRef]
- Barczynska, R.; Slizewska, K.; Litwin, M.; Szalecki, M.; Zarski, A.; Kapusniak, J. The effect of dietary fibre preparations from potato starch on the growth and activity of bacterial strains belonging to the phyla Firmicutes, Bacteroidetes, and Actinobacteria. J. Funct. Foods 2015, 19, 661–668. [Google Scholar] [CrossRef]
- Lefranc-Millot, C.; Guérin-Deremaux, L.; Wils, D.; Neut, C.; Miller, L.E.; Saniez-Degrave, M.H. Impact of a Resistant Dextrin on Intestinal Ecology: How Altering the Digestive Ecosystem with NUTRIOSE®, a Soluble Fibre with Prebiotic Properties, May Be Beneficial for Health. J. Int. Med. Res. 2012, 40, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.M.; Solch, R.J.; Dennis-Wall, J.C.; Ukhanova, M.; Nieves, C.J.; Mai, V.; Christman, M.C.; Gordon, D.T.; Langkamp-Henken, B. In healthy adults, resistant maltodextrin produces a greater change in fecal bifidobacteria counts and increases stool wet weight: A double-blind, randomized, controlled crossover study. Nutr. Res. 2018, 60, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.M.; Champ, M.M.-J.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef] [PubMed]
- Hess, A.; Benítez-Páez, A.; Blædel, T.; Larsen, L.; Iglesias, J.; Madera, C.; Sanz, Y.; Larsen, T. The effect of inulin and resistant maltodextrin on weight loss during energy restriction: A randomised, placebo-controlled, double-blinded intervention. Eur. J. Nutr. 2020, 59, 2507–2524. [Google Scholar] [CrossRef]
- Barczynska, R.; Kapusniak, J.; Litwin, M.; Slizewska, K.; Szalecki, M. Dextrins from Maize Starch as Substances Activating the Growth of Bacteroidetes and Actinobacteria Simultaneously Inhibiting the Growth of Firmicutes, Responsible for the Occurrence of Obesity. Plant Foods Hum. Nutr. 2016, 71, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Sivaprakasam, S.; Bhutia, Y.D.; Yang, S.; Ganapathy, V. Short-Chain Fatty Acid Transporters: Role in Colonic Homeostasis. Compr. Physiol. 2017, 8, 299–314. [Google Scholar] [CrossRef]
- Azcárate-Peril, M.A.; Sikes, M.; Bruno-Bárcena, J.M. The intestinal microbiota, gastrointestinal environment and colorectal cancer: A putative role for probiotics in prevention of colorectal cancer? Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G401–G424. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Gao, R.; Wu, W.; Qin, H. The role of gut microbiota in the pathogenesis of colorectal cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2013, 34, 1285–1300. [Google Scholar] [CrossRef]
- Li, Z.; Xu, D.; Tong, X.; Shan, C. Inhibition of β-glucosidase overcomes gastric cancer chemoresistance through inducing lysosomal dysfunction. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101456. [Google Scholar] [CrossRef]
- Lin, C.; Cai, X.; Zhang, J.; Wang, W.; Sheng, Q.; Hua, H.; Zhou, X. Role of Gut Microbiota in the Development and Treatment of Colorectal Cancer. Digestion 2019, 100, 72–78. [Google Scholar] [CrossRef]
- Wei, X.; Li, J.; Yang, X.; Dong, B.; Geng, B.; Li, Z.; Hu, X.; Ding, B.; Zhang, J.; Yan, M. An enzyme-activated two-photon ratiometric fluorescent probe with lysosome targetability for imaging β-glucuronidase in colon cancer cells and tissue. Anal. Chim. Acta 2022, 1192, 339354. [Google Scholar] [CrossRef] [PubMed]
- Pasman, W.; Wils, D.; Saniez, M.-H.; Kardinaal, A. Long-term gastrointestinal tolerance of NUTRIOSE®FB in healthy men. Eur. J. Clin. Nutr. 2006, 60, 1024–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Heuvel, E.G.H.M.; Wils, D.; Pasman, W.J.; Saniez, M.-H.; Kardinaal, A.F.M. Dietary supplementation of differentdoses of NUTRIOSE®FB, a fermentabledextrin, alters the activity of faecalenzymes in healthy men. Eur. J. Nutr. 2005, 44, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Chaiongkarn, A.; Dathong, J.; Phatvej, W.; Saman, P.; Kuancha, C.; Chatanon, L.; Moonmungmee, S. Characterization of prebiotics and their synergistic activities with Lactobacillus probiotics for β-glucuronidase reduction. ScienceAsia 2019, 45, 538–546. [Google Scholar] [CrossRef] [Green Version]
- Chundakkattumalayil, H.C.; Kumar, S.; Narayanan, R.; Raghavan, K.T. Role of L. plantarum KX519413 as Probiotic and Acacia Gum as Prebiotic in Gastrointestinal Tract Strengthening. Microorganisms 2019, 7, 659. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.J.; Lee, N.-K.; Paik, H.-D. Probiotic characterization of Lactobacillus brevis KU15153 showing antimicrobial and antioxidant effect isolated from kimchi. Food Sci. Biotechnol. 2019, 28, 1521–1528. [Google Scholar] [CrossRef]
- Kim, H.; Chae, S.A.; Lee, M.; Yang, S.-Y.; Ban, O.-H.; Jung, Y.H.; Yang, J. Genomic and Toxicity Studies on Bifidobacterium longum IDCC 4101 and Bifidobacterium bifidum IDCC 4201 Isolated from Feces of Breast-Fed Infants. Food Suppl. Biomater. Health 2021, 1, e37. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.; Kim, D.; Cho, J.; Yang, J.; Chung, M.; Kim, K.; Ha, N. Inhibition of proliferation in colon cancer cell lines and harmful enzyme activity of colon bacteria by Bifidobacterium adolescentis SPM0212. Arch. Pharmacal Res. 2008, 31, 468. [Google Scholar] [CrossRef]
| Age [Years] | Participants | BMI ≤ 25 | BMI ≥ 25 | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| 3–7 | 7 | 6 | 100% | 100% | 0% | 0% |
| 8–12 | 5 | 6 | 60% | 83% | 40% | 17% |
| 13–17 | 5 | 8 | 40% | 12% | 60% | 88% |
| Total | 17 | 20 | 29% | 60% | 71% | 40% |
| Resistant Dextrin | Glucose | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactobacillus | ||||||||||||
| Time [h] | 0 | 24 | 48 | 72 | 96 | 168 | 0 | 24 | 48 | 72 | 96 | 168 |
| Minimum | 5.22 | 6.00 | 3.56 | 4.56 | 3.08 | 5.48 | 5.61 | 6.00 | 6.00 | 6.00 | 2.30 | 2.00 |
| Maximum | 8.79 | 9.94 | 9.99 | 9.83 | 8.96 | 8.73 | 8.92 | 9.96 | 9.99 | 8.84 | 8.68 | 7.00 |
| Mean | 8.07 | 9.30 | 9.29 | 8.72 A | 8.31 A | 7.71 A | 8.06 | 9.36 | 9.24 | 8.31 B | 7.73 B | 4.29 B |
| SD | 0.87 | 0.77 | 1.17 | 0.91 | 1.05 | 0.81 | 0.76 | 0.86 | 0.81 | 0.57 | 1.16 | 1.34 |
| Bifidobacterium | ||||||||||||
| Minimum | 7.15 | 8.26 | 8.36 | 7.92 | 7.85 | 6.00 | 7.06 | 8.24 | 7.60 | 7.34 | 6.00 | 2.30 |
| Maximum | 8.69 | 9.92 | 9.99 | 9.91 | 9.06 | 8.85 | 8.73 | 9.99 | 9.96 | 8.90 | 8.65 | 8.02 |
| Mean | 8.30 | 9.33 | 9.42 A | 8.94 A | 8.55 A | 7.79 A | 8.28 | 9.41 | 9.26 B | 8.36 B | 7.98 B | 4.83 B |
| SD | 0.42 | 0.53 | 0.49 | 0.47 | 0.28 | 0.77 | 0.43 | 0.60 | 0.61 | 0.41 | 0.63 | 1.41 |
| E. coli | ||||||||||||
| Minimum | 6.67 | 4.18 | 0.00 | 0.00 | 0.00 | 0.00 | 6.60 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Maximum | 8.09 | 8.34 | 8.00 | 7.50 | 7.13 | 5.62 | 8.15 | 8.16 | 7.28 | 6.83 | 5.90 | 5.37 |
| Mean | 7.59 | 6.76 A | 4.71 A | 3.94 A | 3.06 A | 2.35 | 7.59 | 5.28 B | 3.83 B | 3.42 B | 2.75 B | 2.32 |
| SD | 0.29 | 1.18 | 2.96 | 2.55 | 2.51 | 1.90 | 0.33 | 2.23 | 2.88 | 2.62 | 2.28 | 1.91 |
| Enterococcus | ||||||||||||
| Minimum | 7.48 | 7.93 | 7.54 | 7.11 | 6.30 | 5.00 | 7.51 | 7.68 | 6.60 | 6.00 | 5.81 | 0.00 |
| Maximum | 8.88 | 9.02 | 9.00 | 8.85 | 8.79 | 8.03 | 8.79 | 8.91 | 8.89 | 8.81 | 8.51 | 6.69 |
| Mean | 8.28 | 8.68 | 8.64 A | 8.44 A | 8.13 A | 6.41 A | 8.24 | 8.55 | 8.44 B | 8.13 B | 7.56 B | 4.02 B |
| SD | 0.38 | 0.28 | 0.34 | 0.42 | 0.66 | 0.82 | 0.33 | 0.34 | 0.53 | 0.66 | 0.89 | 1.48 |
| Clostridium | ||||||||||||
| Minimum | 7.93 | 7.05 | 6.77 | 6.18 | 5.53 | 4.22 | 8.05 | 8.29 | 8.27 | 6.89 | 5.66 | 2.00 |
| Maximum | 8.90 | 9.12 | 8.96 | 8.84 | 8.62 | 8.47 | 8.79 | 9.47 | 8.97 | 8.79 | 8.76 | 7.58 |
| Mean | 8.48 | 8.59 | 8.38 A | 8.13 A | 7.56 | 5.89 A | 8.46 | 8.68 | 8.70 B | 8.27 B | 7.66 | 4.76 B |
| SD | 0.21 | 0.47 | 0.58 | 0.67 | 1.00 | 1.15 | 0.22 | 0.22 | 0.16 | 0.45 | 0.82 | 1.39 |
| Bacteroides | ||||||||||||
| Minimum | 8.02 | 8.16 | 7.84 | 7.00 | 5.49 | 5.11 | 8.10 | 7.87 | 7.94 | 7.45 | 5.53 | 2.00 |
| Maximum | 8.86 | 9.00 | 9.06 | 9.06 | 8.92 | 8.18 | 8.87 | 9.46 | 9.03 | 8.76 | 8.95 | 7.62 |
| Mean | 8.51 | 8.74 A | 8.69 A | 8.34 | 7.88 A | 6.36 A | 8.47 | 8.62 B | 8.57 B | 8.31 | 7.74 B | 4.78 B |
| SD | 0.19 | 0.20 | 0.30 | 0.56 | 0.88 | 0.82 | 0.21 | 0.30 | 0.25 | 0.41 | 0.91 | 1.42 |
| Resistant Dextrin | Glucose | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| α-glucosidase | ||||||||||
| Time [h] | 24 | 48 | 72 | 96 | 168 | 24 | 48 | 72 | 96 | 168 |
| Minimum | 0.51 | 1.11 | 1.76 | 2.54 | 2.72 | 2.77 | 3.09 | 3.44 | 3.86 | 3.95 |
| Maximum | 5.41 | 5.72 | 6.07 | 6.49 | 6.58 | 5.62 | 5.94 | 6.28 | 6.79 | 6.92 |
| Mean | 3.31 C | 3.69 C | 4.10 C | 4.60 C | 4.71 C | 4.33 D | 4.61 D | 4.95 D | 5.32 D | 5.36 D |
| SD | 1.19 | 1.15 | 1.11 | 1.08 | 1.07 | 0.88 | 0.85 | 0.84 | 0.86 | 0.88 |
| β-glucosidase | ||||||||||
| Minimum | 0.04 | 0.15 | 0.19 | 0.16 | 0.16 | 0.19 | 0.30 | 0.34 | 0.31 | 0.30 |
| Maximum | 0.76 | 0.87 | 0.91 | 0.88 | 0.87 | 1.14 | 1.17 | 1.19 | 1.28 | 1.24 |
| Mean | 0.39 C | 0.52 C | 0.57 C | 0.55 C | 0.55 C | 0.65 D | 0.75 D | 0.78 D | 0.80 D | 0.79 D |
| SD | 0.22 | 0.21 | 0.21 | 0.21 | 0.21 | 0.28 | 0.24 | 0.23 | 0.26 | 0.25 |
| α-galactosidase | ||||||||||
| Minimum | 1.35 | 1.56 | 1.75 | 2.25 | 2.00 | 3.16 | 3.22 | 3.25 | 3.47 | 3.25 |
| Maximum | 4.42 | 4.48 | 4.51 | 4.73 | 4.51 | 5.79 | 5.89 | 5.95 | 6.17 | 5.87 |
| Mean | 3.44 C | 3.53 C | 3.60 C | 3.87 C | 3.66 C | 4.34 D | 4.44 D | 4.50 D | 4.76 D | 4.52 D |
| SD | 0.70 | 0.68 | 0.66 | 0.63 | 0.65 | 0.73 | 0.73 | 0.74 | 0.73 | 0.72 |
| β-galactosidase | ||||||||||
| Minimum | 0.41 | 0.49 | 0.56 | 0.57 | 0.51 | 0.60 | 0.66 | 0.68 | 0.74 | 0.70 |
| Maximum | 0.94 | 1.00 | 1.00 | 1.01 | 1.00 | 1.26 | 1.28 | 1.30 | 1.37 | 1.32 |
| Mean | 0.73 C | 0.75 C | 0.77 C | 0.78 C | 0.76 C | 0.92 D | 0.95 D | 0.97 D | 0.99 D | 0.97 D |
| SD | 0.14 | 0.14 | 0.13 | 0.12 | 0.13 | 0.19 | 0.19 | 0.19 | 0.20 | 0.19 |
| β-glucuronidase | ||||||||||
| Minimum | 0.43 | 0.45 | 0.75 | 1.10 | 1.23 | 2.35 | 2.61 | 2.72 | 2.79 | 2.84 |
| Maximum | 4.19 | 4.59 | 4.82 | 5.04 | 5.13 | 5.73 | 5.87 | 6.68 | 6.71 | 6.94 |
| Mean | 2.55 C | 2.84 C | 2.99 C | 3.13 C | 3.20 C | 4.02 D | 4.27 D | 4.67 D | 4.76 D | 4.89 D |
| SD | 0.93 | 0.96 | 0.95 | 0.94 | 0.94 | 0.83 | 0.83 | 0.96 | 0.96 | 1.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarczyk, M.; Śliżewska, K.; Barczyńska, R.; Kapuśniak, J. Effects of Resistant Dextrin from Potato Starch on the Growth Dynamics of Selected Co-Cultured Strains of Gastrointestinal Bacteria and the Activity of Fecal Enzymes. Nutrients 2022, 14, 2158. https://doi.org/10.3390/nu14102158
Włodarczyk M, Śliżewska K, Barczyńska R, Kapuśniak J. Effects of Resistant Dextrin from Potato Starch on the Growth Dynamics of Selected Co-Cultured Strains of Gastrointestinal Bacteria and the Activity of Fecal Enzymes. Nutrients. 2022; 14(10):2158. https://doi.org/10.3390/nu14102158
Chicago/Turabian StyleWłodarczyk, Michał, Katarzyna Śliżewska, Renata Barczyńska, and Janusz Kapuśniak. 2022. "Effects of Resistant Dextrin from Potato Starch on the Growth Dynamics of Selected Co-Cultured Strains of Gastrointestinal Bacteria and the Activity of Fecal Enzymes" Nutrients 14, no. 10: 2158. https://doi.org/10.3390/nu14102158
APA StyleWłodarczyk, M., Śliżewska, K., Barczyńska, R., & Kapuśniak, J. (2022). Effects of Resistant Dextrin from Potato Starch on the Growth Dynamics of Selected Co-Cultured Strains of Gastrointestinal Bacteria and the Activity of Fecal Enzymes. Nutrients, 14(10), 2158. https://doi.org/10.3390/nu14102158









