Persistence of Anti SARS-CoV-2 Antibodies in Breast Milk from Infected and Vaccinated Women after In Vitro-Simulated Gastrointestinal Digestion
Abstract
:1. Introduction
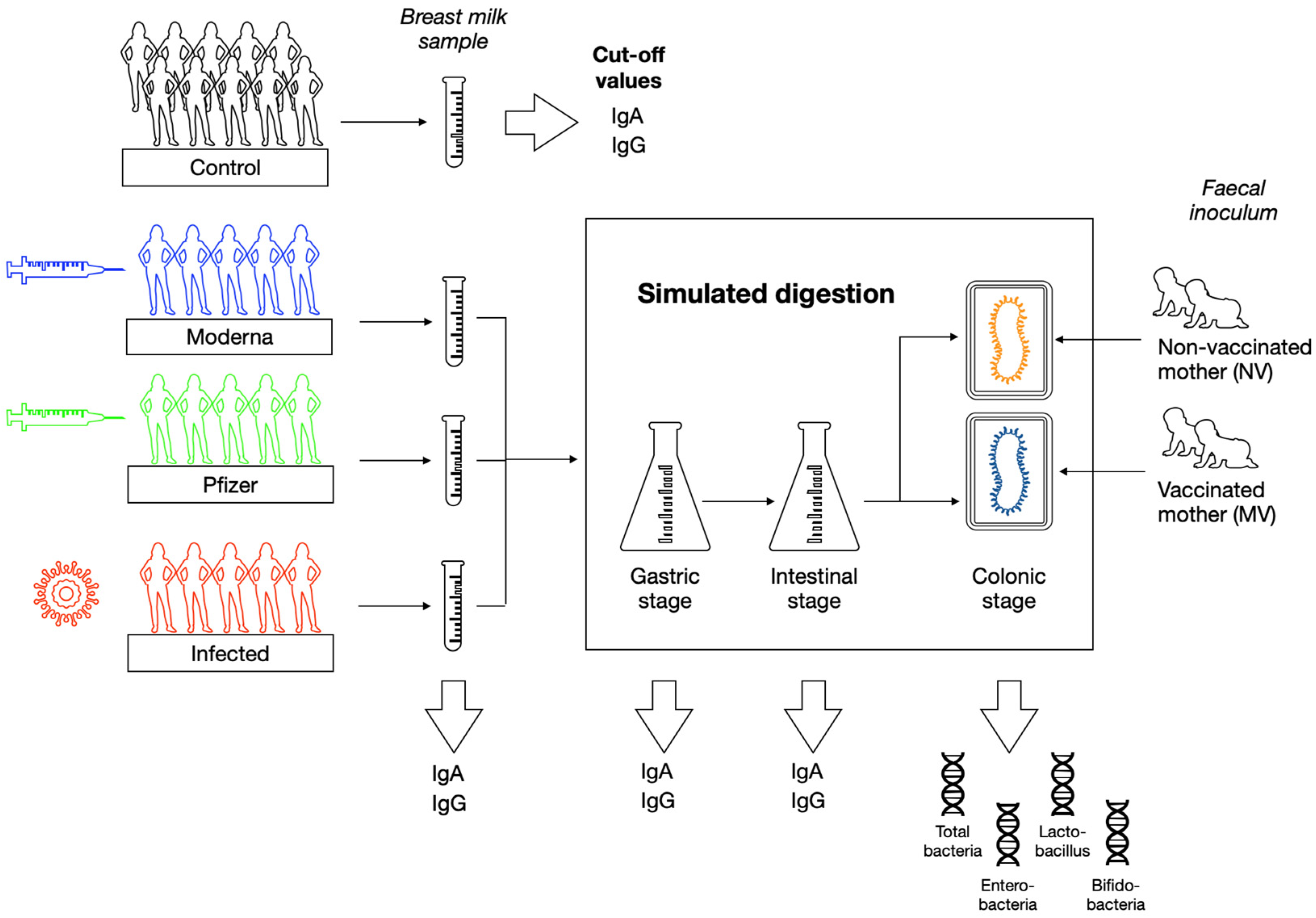
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Breast Milk Samples Collection and Processing
2.3. Quantification of Specific SARS-CoV-2-IgA and IgG Concentration in Breast Milk
2.4. In Vitro Infant Digestion
2.5. In Vitro Colonic Fermentation
2.6. Microbial Profile by Quantitative PCR Analysis (qPCR)
2.7. Statistical Analyses
3. Results
3.1. Subjects Characteristics
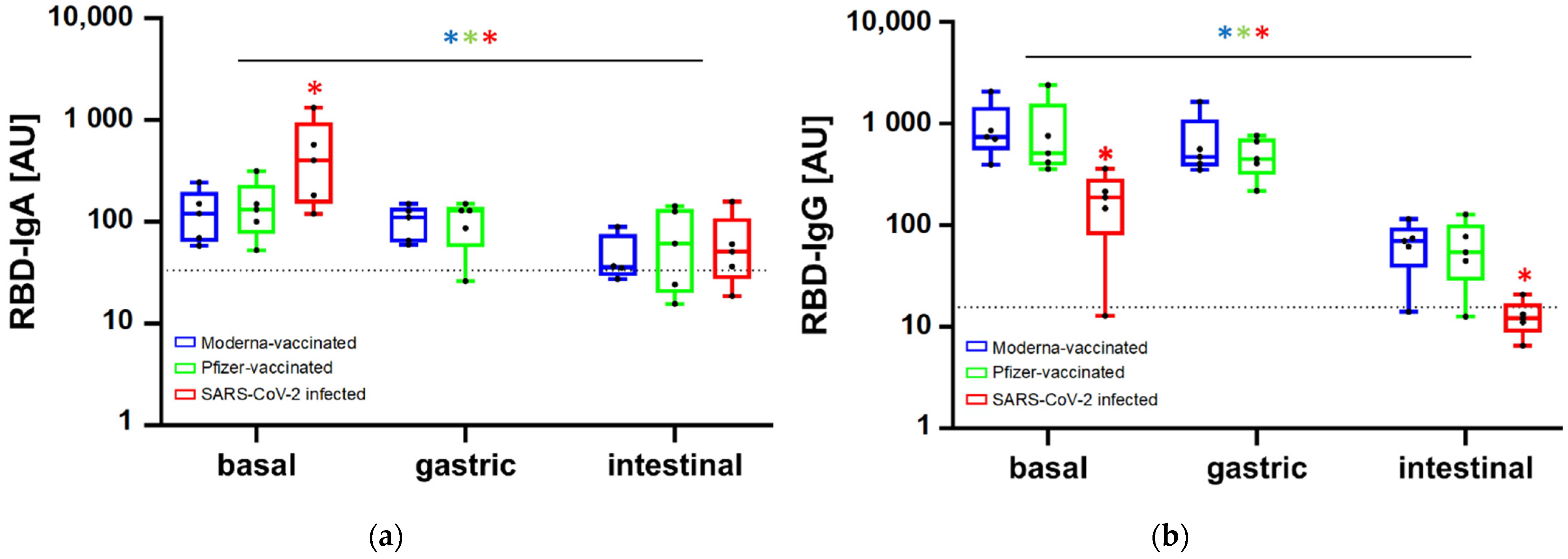
3.2. Persistence of Antibodies along In Vitro Gastrointestinal Digestion
3.3. Effect of Vaccination or Infection on the Colonic Microbiota Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bell, G.; Deshpande, M.; Hales, H.; Harding, D.; Pendlebury, G.; White, O. Multidisciplinary research priorities for the COVID-19 pandemic. Lancet Psychiatry 2020, 7, e37–e38. [Google Scholar] [CrossRef]
- European Commission. Safe COVID-19 Vaccines for Europeans. European Commission Report. Last Updated: 2 February 2022. Source: Vaccines Producers and ECDC Data. Available online: https://ec.europa.eu/info/live-work-travel-eu/coronavirus-response/safe-covid-19-vaccines-europeans_en (accessed on 15 March 2022).
- Bäuerl, C.; Randazzo, W.; Sánchez-Moragas, G.; Selma-Royo, M.; Garcia-Verdevio, E.; Martinez-Rodriguez, L. SARS-CoV-2 RNA and antibody detection in human milk from a prospective multicenter study in Spain. Arch. Dis. Child Fetal Neonatal. Ed. 2022, 107, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Rio-Aige, K.; Azagra-Boronat, I.; Castell, M.; Selma-Royo, M.; Collado, M.C.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. The Breast Milk Immunoglobulinome. Nutrients 2021, 13, 1810. [Google Scholar] [CrossRef]
- Perl, S.H.; Uzan-Yulzari, A.; Klainer, H.; Asiskovich, L.; Youngster, M.; Rinott, E.; Youngster, I. SARS-CoV-2-Specific Antibodies in Breast Milk After COVID-19 Vaccination of Breastfeeding Women. JAMA 2021, 325, 2013–2014. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E.; Shurin, G.V.; Yost, M.; Anderson, A.; Pinto, L.; Wells, A.; Shurin, M.R. Differential Antibody Response to mRNA COVID-19 Vaccines in Healthy Subjects. Microbiol. Spectr. 2021, 9, e00341-21. [Google Scholar] [CrossRef]
- Altawalah, H. Antibody Responses to Natural SARS-CoV-2 Infection or after COVID-19 Vaccination. Vaccines 2021, 9, 910. [Google Scholar] [CrossRef]
- Sah BN, P.; Lueangsakulthai, J.; Kim, B.J.; Hauser, B.R.; Woo, Y.; Olyaei, A.; Dallas, D.C. Partial degradation of recombinant antibody functional activity during infant gastrointestinal digestion: Implications for oral antibody supplementation. Front. Nutr. 2020, 7, 130. [Google Scholar]
- Lueangsakulthai, J.; Sah, B.N.P.; Scottoline, B.P.; Dallas, D.C. Survival of recombinant monoclonal antibodies (IgG, IgA and sIgA) versus naturally-occurring antibodies (IgG and sIgA/IgA) in an ex vivo infant digestion model. Nutrients 2020, 12, 621. [Google Scholar] [CrossRef] [Green Version]
- Rogier, E.W.; Frantz, A.L.; Bruno, M.E.; Wedlund, L.; Cohen, D.A.; Stromberg, A.J.; Kaetzel, C.S. Lessons from mother: Long-term impact of antibodies in breast milk on the gut microbiota and intestinal immune system of breastfed offspring. Gut Microbes 2014, 5, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Macías, E.; Selma-Royo, M.; Martínez-Costa, C.; Collado, M.C. Breastfeeding Practices Influence the Breast Milk Microbiota Depending on Pre-Gestational Maternal BMI and Weight Gain over Pregnancy. Nutrients 2021, 13, 1518. [Google Scholar] [CrossRef]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.; Chromikova, V.; McMahon, M.; Krammer, F. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, D.; Amanat, F.; Chromikova, V.; Jiang, K.; Strohmeier, S.; Arunkumar, G.A.; Krammer, F. SARS-CoV-2 seroconversion in humans: A detailed protocol for a serological assay, antigen production, and test setup. Curr. Protoc. Microbiol. 2020, 57, e100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ménard, O.; Bourlieu, C.; De Oliveira, S.C.; Dellarosa, N.; Laghi, L.; Carrière, F.; Deglaire, A. A first step towards a consensus static in vitro model for simulating full-term infant digestion. Food Chem. 2018, 240, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Rubio-del-Campo, A.; Alcántara, C.; Collado, M.C.; Rodríguez-Díaz, J.; Yebra, M.J. Human milk and mucosa-associated disaccharides impact on cultured infant fecal microbiota. Sci. Rep. 2020, 10, 11845. [Google Scholar] [CrossRef]
- Pieri, M.; Nicolaidou, V.; Paphiti, I.; Pipis, S.; Felekkis, K.; Papaneophytou, C. Survival of vaccine-induced human milk SARS-CoV-2 IgG and IgA immunoglobulins across simulated human infant gastrointestinal digestion. MedRxiv 2021. [Google Scholar] [CrossRef]
- Fox, A.; Norris, C.; Amanat, F.; Zolla-Pazner, S.; Powell, R.L. The vaccine-elicited immunoglobulin profile in milk after COVID-19 mRNA-based vaccination is IgG-dominant and lacks secretory antibodies. MedRxiv 2021. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Gorochov, G. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, 577. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; Underwood, M.; Beverly, R.; Nielsen, S.; Dallas, D. Comparison of Human Milk Immunoglobulin Survival during Gastric Digestion between Preterm and Term Infants. Nutrients 2018, 10, 631. [Google Scholar] [CrossRef] [Green Version]
- Jasion, V.; Burnett, B. Survival and digestibility of orally-administered immunoglobulin preparations containing IgG through the gastrointestinal tract in humans. Nutr. J. 2015, 14, 22. [Google Scholar] [CrossRef] [Green Version]
- Andreas, N.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Rosenberg-Friedman, M.; Kigel, A.; Bahar, Y.; Werbner, M.; Alter, J.; Yogev, Y.; Wine, Y. BNT162b2 mRNA vaccine elicited antibody response in blood and milk of breastfeeding women. Nat. Commun. 2021, 12, 6222. [Google Scholar] [CrossRef] [PubMed]
- Lackey, K.A.; Pace, R.M.; Williams, J.E.; Bode, L.; Donovan, S.M.; Järvinen, K.M.; McGuire, M.K. SARS-CoV-2 and human milk: What is the evidence? Matern Child Nutr. 2020, 16, e13032. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gao, J.; Yang, H.M.; Yuan, X.L.; Chen, T.X.; He, Z.J. The Role of the Lactadherin in Promoting Intestinal DCs Development In Vivo and Vitro. Clin. Dev. Immunol. 2010, 2010, 357541. [Google Scholar] [CrossRef]
- Habte, H.H.; Kotwal, G.J.; Lotz, Z.E.; Tyler, M.G.; Abrahams, M.; Rodriques, J.; Mall, A.S. Antiviral activity of purified human breast milk mucin. Neonatology 2007, 92, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Minami, J.; Odamaki, T.; Hashikura, N.; Abe, F.; Xiao, J.Z. Lysozyme in breast milk is a selection factor for bifidobacterial colonisation in the infant intestine. Benef. Microbes 2016, 7, 53–60. [Google Scholar] [CrossRef]
- Ang, L.; Arboleya, S.; Lihua, G.; Chuihui, Y.; Nan, Q.; Suarez, M.; Gueimonde, M. The establishment of the infant intestinal microbiome is not affected by rotavirus vaccination. Sci. Rep. 2014, 4, 7417. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, G.P.; Ladinsky, M.S.; Yu, K.B.; Sanders, J.G.; Yoo, B.B.; Chou, W.C.; Mazmanian, S. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 2018, 360, 795–800. [Google Scholar] [CrossRef] [Green Version]
- Rekima, A.; Bonnart, C.; Macchiaverni, P.; Metcalfe, J.; Tulic, M.K.; Halloin, N.; Verhasselt, V. A role for early oral exposure to house dust mite allergens through breast milk in IgE-mediated food allergy susceptibility. J. Allergy Clin. Immunol. 2020, 145, 1416–1429. [Google Scholar] [CrossRef] [Green Version]
- Senda, S.; Fujiyama, Y.; Ushijima, T.; Hodohara, K.; Bamba, T.; Hosoda, S.; Kobayashi, K. Clostridium ramosum, an IgA protease-producing species and its ecology in the human intestinal tract. Microbiol. Immunol. 1985, 29, 1019–1028. [Google Scholar] [CrossRef]
- Resman, F.; Manat, G.; Lindh, V.; Murphy, T.F.; Riesbeck, K. Differential distribution of IgA-protease genotypes in mucosal and invasive isolates of Haemophilus influenzae in Sweden. BMC Infect. Dis. 2018, 18, 592. [Google Scholar] [CrossRef]
- Mistry, D.; Stockley, R.A. IgA1 protease. Int. J. Biochem. Cell Biol. 2006, 38, 1244–1248. [Google Scholar] [CrossRef] [PubMed]

| Pfizer (n = 5) | Moderna (n = 5) | SARS-CoV-2 Infected (n = 5) | Controls (n = 10) | |
|---|---|---|---|---|
| Maternal age (years, median, 1st, 3rd Q) | 31 (29, 32) | 35 (34.2, 37.2) | 34 (31, 25) | 37 (34, 38) |
| Lactation time (months, median, 1st, 3rd Q) | 8 (7, 10) | 8.5 (6.5, 11) | 0.8 (0.5, 1.1) | 6 (3.5, 60.5) |
| Maternal use of antibiotics | 0/5 | 0/5 | 0/5 | 1/10 |
| Type of delivery | Vaginal 2/5 C-Section 3/5 | Vaginal 4/5 C-Section 1/5 | Vaginal 5/5 | Vaginal 8/10 C-Section 2/10 |
| Exclusive breastfeeding | 4/5 | 4/5 | 5/5 | 7/10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Lerma, J.; Bueno-Llamoga, P.; Bäuerl, C.; Cortés-Macias, E.; Selma-Royo, M.; Pérez-Cano, F.; Lerin, C.; Martínez-Costa, C.; Collado, M.C. Persistence of Anti SARS-CoV-2 Antibodies in Breast Milk from Infected and Vaccinated Women after In Vitro-Simulated Gastrointestinal Digestion. Nutrients 2022, 14, 2117. https://doi.org/10.3390/nu14102117
Calvo-Lerma J, Bueno-Llamoga P, Bäuerl C, Cortés-Macias E, Selma-Royo M, Pérez-Cano F, Lerin C, Martínez-Costa C, Collado MC. Persistence of Anti SARS-CoV-2 Antibodies in Breast Milk from Infected and Vaccinated Women after In Vitro-Simulated Gastrointestinal Digestion. Nutrients. 2022; 14(10):2117. https://doi.org/10.3390/nu14102117
Chicago/Turabian StyleCalvo-Lerma, Joaquim, Pierre Bueno-Llamoga, Christine Bäuerl, Erika Cortés-Macias, Marta Selma-Royo, Francisco Pérez-Cano, Carles Lerin, Cecilia Martínez-Costa, and Maria Carmen Collado. 2022. "Persistence of Anti SARS-CoV-2 Antibodies in Breast Milk from Infected and Vaccinated Women after In Vitro-Simulated Gastrointestinal Digestion" Nutrients 14, no. 10: 2117. https://doi.org/10.3390/nu14102117
APA StyleCalvo-Lerma, J., Bueno-Llamoga, P., Bäuerl, C., Cortés-Macias, E., Selma-Royo, M., Pérez-Cano, F., Lerin, C., Martínez-Costa, C., & Collado, M. C. (2022). Persistence of Anti SARS-CoV-2 Antibodies in Breast Milk from Infected and Vaccinated Women after In Vitro-Simulated Gastrointestinal Digestion. Nutrients, 14(10), 2117. https://doi.org/10.3390/nu14102117







