Metabolic and Nutritional Aspects in Paediatric Patients with Klinefelter Syndrome: A Narrative Review
Abstract
:1. Klinefelter Syndrome: Clinical Characteristics and Diagnosis
2. Methods
3. Metabolic Aspects
3.1. Adiposity
3.2. Diabetes
3.3. Metabolic Syndrome
3.4. Cardiovascular Risk
3.5. Bone Metabolism
4. Nutritional Aspects
5. Hormonal Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, S.; Howell, S.; Wilson, R.; Tanda, T.; Ross, J.; Zeitler, P.; Tartaglia, N. Advances in the Interdisciplinary Care of Children with Klinefelter Syndrome. Adv. Pediatr. 2016, 63, 15–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gravholt, C.H.; Chang, S.; Wallentin, M.; Fedder, J.; Moore, P.; Skakkebæk, A. Klinefelter Syndrome: Integrating Genetics, Neuropsychology, and Endocrinology. Endocr. Rev. 2018, 39, 389–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calogero, A.E.; Giagulli, V.A.; Mongioì, L.M.; Triggiani, V.; Radicioni, A.F.; Jannini, E.A.; Pasquali, D.; Klinefelter ItaliaN Group (KING). Klinefelter Syndrome: Cardiovascular Abnormalities and Metabolic Disorders. J. Endocrinol. Investig. 2017, 40, 705–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.; Skakkebæk, A.; Højbjerg Gravholt, C. Klinefelter Syndrome and Medical Treatment: Hypogonadism and Beyond. Hromosones 2015, 14, 531–548. [Google Scholar] [CrossRef]
- Tartaglia, N.; Ayari, N.; Howell, S.; D’Epagnier, C.; Zeitler, P. 48,XXYY, 48,XXXY and 49,XXXXY Syndromes: Not Just Variants of Klinefelter Syndrome: 48,XXYY, 48,XXXY and 49,XXXXY Syndromes. Acta Paediatr. 2011, 100, 851–860. [Google Scholar] [CrossRef]
- Samplaski, M.K.; Lo, K.C.; Grober, E.D.; Millar, A.; Dimitromanolakis, A.; Jarvi, K.A. Phenotypic Differences in Mosaic Klinefelter Patients as Compared with Non-Mosaic Klinefelter Patients. Fertil. Steril. 2014, 101, 950–955. [Google Scholar] [CrossRef]
- Bojesen, A.; Groth, K.; Høst, C.; Skakkeb’k, A. The Role of Hypogonadism in Klinefelter Syndrome. Asian J. Androl. 2014, 16, 185. [Google Scholar] [CrossRef]
- Han, S.J.; Kim, K.-S.; Kim, W.; Kim, J.H.; Lee, Y.; Nam, J.S.; Seo, J.A.; Kim, B.K.; Lee, J.; Chung, J.O.; et al. Obesity and Hyperglycemia in Korean Men with Klinefelter Syndrome: The Korean Endocrine Society Registry. Endocrinol. Metab. 2016, 31, 598. [Google Scholar] [CrossRef]
- Nassau, D.E.; Best, J.C.; Cohen, J.; Gonzalez, D.C.; Alam, A.; Ramasamy, R. Androgenization in Klinefelter Syndrome: Clinical Spectrum from Infancy through Young Adulthood. J. Pediatr. Urol. 2021, 17, 346–352. [Google Scholar] [CrossRef]
- Bojesen, A.; Gravholt, C.H. Morbidity and Mortality in Klinefelter Syndrome (47,XXY). Acta Paediatr. 2011, 100, 807–813. [Google Scholar] [CrossRef]
- Bojesen, A.; Juul, S.; Birkebæk, N.H.; Gravholt, C.H. Morbidity in Klinefelter Syndrome: A Danish Register Study Based on Hospital Discharge Diagnoses. J. Clin. Endocrinol. Metab. 2006, 91, 1254–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, A.V.; Costanzo, P.R.; Peuchot, V.A.; Knoblovits, P. Testosterone Replacement Therapy and the Risk of Hypoglycemia. Case Rep. Endocrinol. 2019, 2019, 9616125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang-Feng, M.; Hong-Li, X.; Xue-Yan, W.; Min, N.; Shuang-Yu, L.; Hong-Ding, X.; Liang-Ming, L. Prevalence and Risk Factors of Diabetes in Patients with Klinefelter Syndrome: A Longitudinal Observational Study. Fertil. Steril. 2012, 98, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.T.; Denniss, A.R. An Introduction to Writing Narrative and Systematic Reviews—Tasks, Tips and Traps for Aspiring Authors. Heart Lung Circ. 2018, 27, 893–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gravholt, C.H.; Jensen, A.S.; Høst, C.; Bojesen, A. Body Composition, Metabolic Syndrome and Type 2 Diabetes in Klinefelter Syndrome: Klinefelter Syndrome and Metabolic Syndrome. Acta Paediatr. 2011, 100, 871–877. [Google Scholar] [CrossRef]
- Davis, S.M.; DeKlotz, S.; Nadeau, K.J.; Kelsey, M.M.; Zeitler, P.S.; Tartaglia, N.R. High Prevalence of Cardiometabolic Risk Features in Adolescents with 47, XXY/Klinefelter Syndrome. Am. J. Med. Genet. 2020, 184, 327–333. [Google Scholar] [CrossRef]
- Bardsley, M.Z.; Falkner, B.; Kowal, K.; Ross, J.L. Insulin Resistance and Metabolic Syndrome in Prepubertal Boys with Klinefelter Syndrome: Insulin Resistance in Boys with KS. Acta Paediatr. 2011, 100, 866–870. [Google Scholar] [CrossRef]
- Davis, S.; Lahlou, N.; Bardsley, M.; Temple, M.-C.; Kowal, K.; Pyle, L.; Zeitler, P.; Ross, J. Gonadal Function Is Associated with Cardiometabolic Health in Pre-Pubertal Boys with Klinefelter Syndrome. Andrology 2016, 4, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.M.; Cox-Martin, M.; Bardsley, M.; Kowal, K.; Zeitler, P.S.; Ross, J.L. Effects of Oxandrolone on Cardiometabolic Health in Boys with Klinefelter Syndrome: A Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2017, 102, 176–184. [Google Scholar] [CrossRef]
- Aksglaede, L.; Molgaard, C.; Skakkebaek, N.E.; Juul, A. Normal Bone Mineral Content but Unfavourable Muscle/Fat Ratio in Klinefelter Syndrome. Arch. Dis. Child. 2008, 93, 30–34. [Google Scholar] [CrossRef]
- Davis, S.M.; Reynolds, R.M.; Dabelea, D.M.; Zeitler, P.S.; Tartaglia, N.R. Testosterone Treatment in Infants With 47,XXY: Effects on Body Composition. J. Endocr. Soc. 2019, 3, 2276–2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samango-Sprouse, C.A.; Sadeghin, T.; Mitchell, F.L.; Dixon, T.; Stapleton, E.; Kingery, M.; Gropman, A.L. Positive Effects of Short Course Androgen Therapy on the Neurodevelopmental Outcome in Boys with 47,XXY Syndrome at 36 and 72 Months of Age. Am. J. Med. Genet. 2013, 161, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.L.; Kushner, H.; Kowal, K.; Bardsley, M.; Davis, S.; Reiss, A.L.; Tartaglia, N.; Roeltgen, D. Androgen Treatment Effects on Motor Function, Cognition, and Behavior in Boys with Klinefelter Syndrome. J. Pediatr. 2017, 185, 193–199.e4. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, N.; Fennoy, I.; Carel, J.-C.; Roger, M. Inhibin B and Anti-Müllerian Hormone, But Not Testosterone Levels, Are Normal in Infants with Nonmosaic Klinefelter Syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 1864–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, J.L.; Samango-Sprouse, C.; Lahlou, N.; Kowal, K.; Elder, F.F.; Zinn, A. Early Androgen Deficiency in Infants and Young Boys with 47, XXY Klinefelter Syndrome. Horm. Res. Paediatr. 2005, 64, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Lizarazo, A.H.; McLoughlin, M.; Vogiatzi, M.G. Endocrine Aspects of Klinefelter Syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Tsai, E.; Boyko, E.; Leonetti, D.; Fujimoto, W. Low Serum Testosterone Level as a Predictor of Increased Visceral Fat in Japanese-American Men. Int. J. Obes. 2000, 24, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Diez, J.; Iglesias, P. The Role of the Novel Adipocyte-Derived Hormone Adiponectin in Human Disease. Eur. J. Endocrinol. 2003, 148, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Bojesen, A.; Host, C.; Gravholt, C.H. Klinefelter’s Syndrome, Type 2 Diabetes and the Metabolic Syndrome: The Impact of Body Composition. Mol. Hum. Reprod. 2010, 16, 396–401. [Google Scholar] [CrossRef] [Green Version]
- Ratcliffe, S.G. The Sexual Development of Boys with the Chromosome Constitution 47,XXY (Klinefelter’s Syndrome). Clin. Endocrinol. Metab. 1982, 11, 703–716. [Google Scholar] [CrossRef]
- Bojesen, A.; Kristensen, K.; Birkebaek, N.H.; Fedder, J.; Mosekilde, L.; Bennett, P.; Laurberg, P.; Frystyk, J.; Flyvbjerg, A.; Christiansen, J.S.; et al. The Metabolic Syndrome Is Frequent in Klinefelter’s Syndrome and Is Associated With Abdominal Obesity and Hypogonadism. Diabetes Care 2006, 29, 1591–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zitzmann, M.; Depenbusch, M.; Gromoll, J.; Nieschlag, E. X-Chromosome Inactivation Patterns and Androgen Receptor Functionality Influence Phenotype and Social Characteristics as Well as Pharmacogenetics of Testosterone Therapy in Klinefelter Patients. J. Clin. Endocrinol. Metab. 2004, 89, 6208–6217. [Google Scholar] [CrossRef] [PubMed]
- Reue, K. Sex Differences in Obesity: X Chromosome Dosage as a Risk Factor for Increased Food Intake, Adiposity and Co-Morbidities. Physiol. Behav. 2017, 176, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yuan, T.; Song, S.; Chen, S.; Wang, L.; Fu, Y.; Dong, Y.; Tang, Y.; Zhao, W. Glucose Metabolic Disorder in Klinefelter Syndrome: A Retrospective Analysis in a Single Chinese Hospital and Literature Review. BMC Endocr. Disord. 2021, 21, 239. [Google Scholar] [CrossRef] [PubMed]
- Salzano, A.; D’Assante, R.; Heaney, L.M.; Monaco, F.; Rengo, G.; Valente, P.; Pasquali, D.; Bossone, E.; Gianfrilli, D.; Lenzi, A.; et al. Klinefelter Syndrome, Insulin Resistance, Metabolic Syndrome, and Diabetes: Review of Literature and Clinical Perspectives. Endocrine 2018, 61, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Yesilova, Z.; Oktenli, C.; Sanisoglu, S.Y.; Musabak, U.; Cakir, E.; Ozata, M.; Dagalp, K. Evaluation of Insulin Sensitivity in Patients with Klinefelter’s Syndrome: A Hyperinsulinemic Euglycemic Clamp Study. ENDO 2005, 27, 11–16. [Google Scholar] [CrossRef]
- Nishizawa, H.; Shimomura, I.; Kishida, K.; Maeda, N.; Kuriyama, H.; Nagaretani, H.; Matsuda, M.; Kondo, H.; Furuyama, N.; Kihara, S.; et al. Androgens Decrease Plasma Adiponectin, an Insulin-Sensitizing Adipocyte-Derived Protein. Diabetes 2002, 51, 2734–2741. [Google Scholar] [CrossRef] [Green Version]
- Lanfranco, F.; Zitzmann, M.; Simoni, M.; Nieschlag, E. Serum Adiponectin Levels in Hypogonadal Males: Influence of Testosterone Replacement Therapy. Clin. Endocrinol. 2004, 60, 500–507. [Google Scholar] [CrossRef]
- Corona, G.; Monami, M.; Rastrelli, G.; Aversa, A.; Tishova, Y.; Saad, F.; Lenzi, A.; Forti, G.; Mannucci, E.; Maggi, M. Testosterone and Metabolic Syndrome: A Meta-Analysis Study. J. Sex. Med. 2011, 8, 272–283. [Google Scholar] [CrossRef]
- Panimolle, F.; Tiberti, C.; Granato, S.; Semeraro, A.; Gianfrilli, D.; Anzuini, A.; Lenzi, A.; Radicioni, A. Screening of Endocrine Organ-Specific Humoral Autoimmunity in 47,XXY Klinefelter’s Syndrome Reveals a Significant Increase in Diabetes-Specific Immunoreactivity in Comparison with Healthy Control Men. Endocrine 2016, 52, 157–164. [Google Scholar] [CrossRef]
- O’Connor, M.J.; Snyder, E.A.; Hayes, F.J. Klinefelter Syndrome and Diabetes. Curr. Diab. Rep. 2019, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- de Ferranti, S.D.; Gauvreau, K.; Ludwig, D.S.; Neufeld, E.J.; Newburger, J.W.; Rifai, N. Prevalence of the Metabolic Syndrome in American Adolescents: Findings from the Third National Health and Nutrition Examination Survey. Circulation 2004, 110, 2494–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bojesen, A.; Gravholt, C.H. Klinefelter Syndrome in Clinical Practice. Nat. Rev. Urol. 2007, 4, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Spaziani, M.; Radicioni, A.F. Metabolic and Cardiovascular Risk Factors in Klinefelter Syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2020, 184, 334–343. [Google Scholar] [CrossRef]
- Sesti, F.; Pofi, R.; Pozza, C.; Minnetti, M.; Gianfrilli, D.; Kanakis, G.A. Cardiovascular Complications in Patients with Klinefelter’s Syndrome. Curr. Pharm. Des. 2020, 26, 5556–5563. [Google Scholar] [CrossRef]
- Di Mambro, A.; Ferlin, A.; De Toni, L.; Selice, R.; Caretta, N.; Foresta, C. Endothelial Progenitor Cells as a New Cardiovascular Risk Factor in Klinefelter’s Syndrome. Mol. Hum. Reprod. 2010, 16, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Katznelson, L. Therapeutic Role of Androgens in the Treatment of Osteoporosis in Men. Baillière’s Clin. Endocrinol. Metab. 1998, 12, 453–470. [Google Scholar] [CrossRef]
- Swerdlow, A.J.; Higgins, C.D.; Schoemaker, M.J.; Wright, A.F.; Jacobs, P.A. Mortality in Patients with Klinefelter Syndrome in Britain: A Cohort Study. J. Clin. Endocrinol. Metab. 2005, 90, 6516–6522. [Google Scholar] [CrossRef]
- Pizzocaro, A.; Vena, W.; Condorelli, R.; Radicioni, A.; Rastrelli, G.; Pasquali, D.; Selice, R.; Ferlin, A.; Foresta, C.; Jannini, E.A.; et al. Testosterone Treatment in Male Patients with Klinefelter Syndrome: A Systematic Review and Meta-Analysis. J. Endocrinol. Investig. 2020, 43, 1675–1687. [Google Scholar] [CrossRef]
- Bojesen, A.; Birkebæk, N.; Kristensen, K.; Heickendorff, L.; Mosekilde, L.; Christiansen, J.S.; Gravholt, C.H. Bone Mineral Density in Klinefelter Syndrome Is Reduced and Primarily Determined by Muscle Strength and Resorptive Markers, but Not Directly by Testosterone. Osteoporos. Int. 2011, 22, 1441–1450. [Google Scholar] [CrossRef]
- Jo, D.G.; Lee, H.S.; Joo, Y.M.; Seo, J.T. Effect of Testosterone Replacement Therapy on Bone Mineral Density in Patients with Klinefelter Syndrome. Yonsei Med. J. 2013, 54, 1331. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.T.; Lee, J.S.; Oh, T.H.; Joo, K.J. The Clinical Significance of Bone Mineral Density and Testosterone Levels in Korean Men with Non-Mosaic Klinefelter’s Syndrome. BJU Int. 2007, 99, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Aksglaede, L.; Andersson, A.-M.; Jørgensen, N.; Jensen, T.K.; Carlsen, E.; McLachlan, R.I.; Skakkebæk, N.E.; Petersen, J.H.; Juul, A. Primary Testicular Failure in Klinefelter’s Syndrome: The Use of Bivariate Luteinizing Hormone-Testosterone Reference Charts. Clin. Endocrinol. 2007, 66, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Kalak, R.; Lue, Y.; Jia, Y.; Erkkila, K.; Zhou, H.; Seibel, M.J.; Wang, C.; Swerdloff, R.S.; Dunstan, C.R. Genetic and Hormonal Control of Bone Volume, Architecture, and Remodeling in XXY Mice. J. Bone Miner. Res. 2010, 25, 2148–2154. [Google Scholar] [CrossRef] [Green Version]
- Ferlin, A.; Schipilliti, M.; Di Mambro, A.; Vinanzi, C.; Foresta, C. Osteoporosis in Klinefelter’s Syndrome. Mol. Hum. Reprod. 2010, 16, 402–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overvad, S.; Bay, K.; Bojesen, A.; Gravholt, C.H. Low INSL3 in Klinefelter Syndrome Is Related to Osteocalcin, Testosterone Treatment and Body Composition, as Well as Measures of the Hypothalamic-Pituitary-Gonadal Axis. Andrology 2014, 2, 421–427. [Google Scholar] [CrossRef]
- Di Cesare, M.; Sorić, M.; Bovet, P.; Miranda, J.J.; Bhutta, Z.; Stevens, G.A.; Laxmaiah, A.; Kengne, A.-P.; Bentham, J. The Epidemiological Burden of Obesity in Childhood: A Worldwide Epidemic Requiring Urgent Action. BMC Med. 2019, 17, 212. [Google Scholar] [CrossRef] [Green Version]
- Akseer, N.; Mehta, S.; Wigle, J.; Chera, R.; Brickman, Z.J.; Al-Gashm, S.; Sorichetti, B.; Vandermorris, A.; Hipgrave, D.B.; Schwalbe, N.; et al. Non-Communicable Diseases among Adolescents: Current Status, Determinants, Interventions and Policies. BMC Public Health 2020, 20, 1908. [Google Scholar] [CrossRef]
- Alabduljabbar, S.; Zaidan, S.A.; Lakshmanan, A.P.; Terranegra, A. Personalized Nutrition Approach in Pregnancy and Early Life to Tackle Childhood and Adult Non-Communicable Diseases. Life 2021, 11, 467. [Google Scholar] [CrossRef]
- Zitzmann, M.; Aksglaede, L.; Corona, G.; Isidori, A.M.; Juul, A.; T’Sjoen, G.; Kliesch, S.; D’Hauwers, K.; Toppari, J.; Słowikowska-Hilczer, J.; et al. European Academy of Andrology Guidelines on Klinefelter Syndrome Endorsing Organization: European Society of Endocrinology. Andrology 2021, 9, 145–167. [Google Scholar] [CrossRef]
- World Health Organization. Global Recommendations on Physical Activity for Health; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- World Health Organization. Guidelines on Physical Activity, Sedentary Behaviour and Sleep for Children under 5 Years of Age; WHO: Geneva, Swizerland, 2010. [Google Scholar]
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric Obesity-Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757. [Google Scholar] [CrossRef] [PubMed]
- Valerio, G.; Maffeis, C.; Saggese, G.; Ambruzzi, M.A.; Balsamo, A.; Bellone, S.; Bergamini, M.; Bernasconi, S.; Bona, G.; Calcaterra, V.; et al. Diagnosis, Treatment and Prevention of Pediatric Obesity: Consensus Position Statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital. J. Pediatr. 2018, 44, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert Pérez, E.; Mateu Olivares, V.; Martínez-Espinosa, R.M.; Molina Vila, M.D.; Reig García-Galbis, M. New Insights about How to Make an Intervention in Children and Adolescents with Metabolic Syndrome: Diet, Exercise vs. Changes in Body Composition. A Systematic Review of RCT. Nutrients 2018, 10, 878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savarino, G.; Corsello, A.; Corsello, G. Macronutrient Balance and Micronutrient Amounts through Growth and Development. Ital. J. Pediatr. 2021, 47, 109. [Google Scholar] [CrossRef]
- Cribb, V.L.; Northstone, K.; Hopkins, D.; Emmett, P.M. Sources of Vitamin D and Calcium in the Diets of Preschool Children in the UK and the Theoretical Effect of Food Fortification. J. Hum. Nutr. Diet. 2015, 28, 583–592. [Google Scholar] [CrossRef]
- Hu, T.-Y.; Chen, Y.C.; Lin, P.; Shih, C.-K.; Bai, C.-H.; Yuan, K.-C.; Lee, S.-Y.; Chang, J.-S. Testosterone-Associated Dietary Pattern Predicts Low Testosterone Levels and Hypogonadism. Nutrients 2018, 10, 1786. [Google Scholar] [CrossRef] [Green Version]
- Zamir, A.; Ben-Zeev, T.; Hoffman, J.R. Manipulation of Dietary Intake on Changes in Circulating Testosterone Concentrations. Nutrients 2021, 13, 3375. [Google Scholar] [CrossRef]
- Aksglaede, L.; Davis, S.M.; Ross, J.L.; Juul, A. Minipuberty in Klinefelter Syndrome: Current Status and Future Directions. Am. J. Med. Genet. 2020, 184, 320–326. [Google Scholar] [CrossRef]
- Fennoy, I. Testosterone and the Child (0–12 Years) with Klinefelter Syndrome (47XXY): A Review: Testosterone and Prepubertal Klinefelter Syndrome. Acta Paediatr. 2011, 100, 846–850. [Google Scholar] [CrossRef]
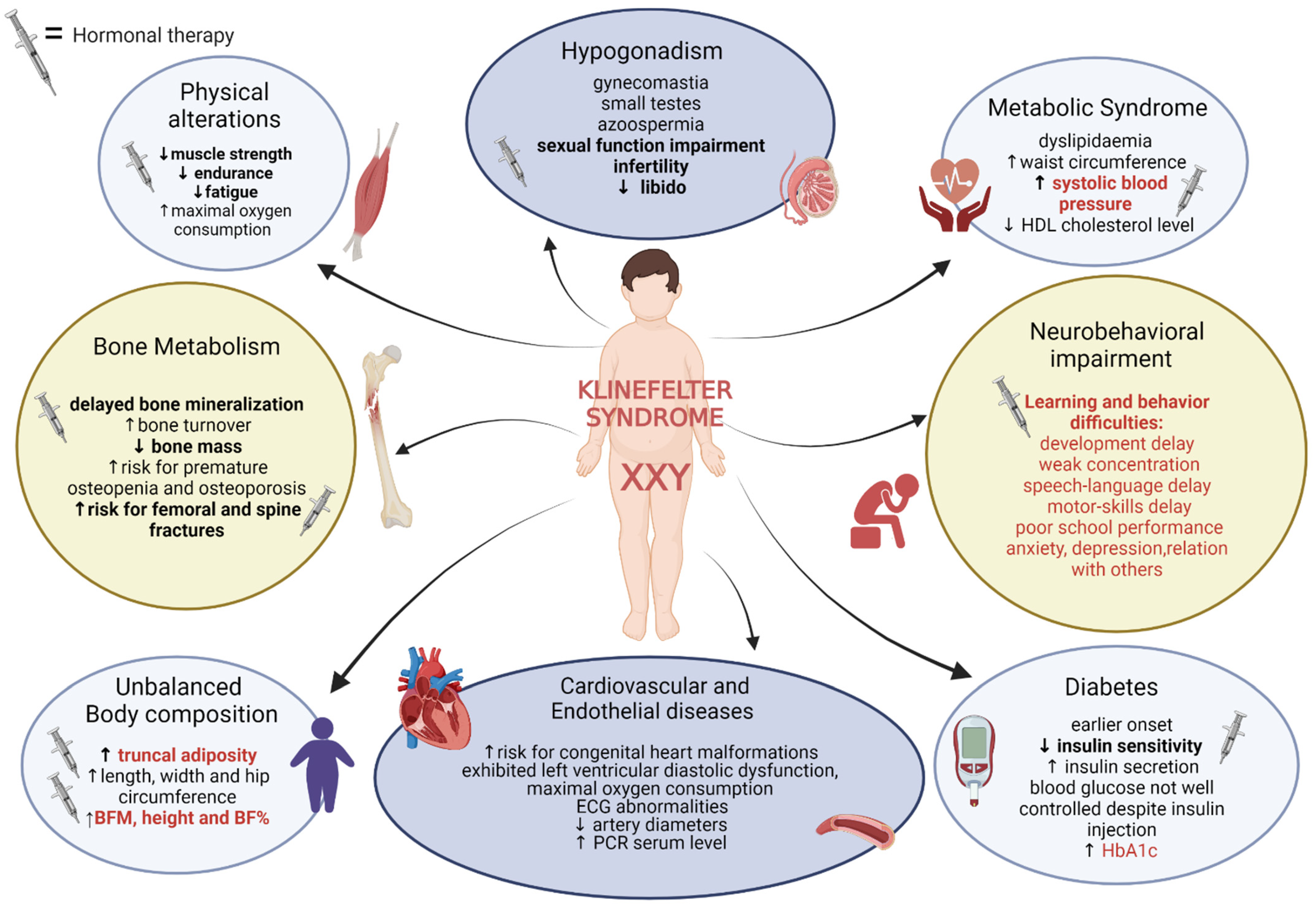
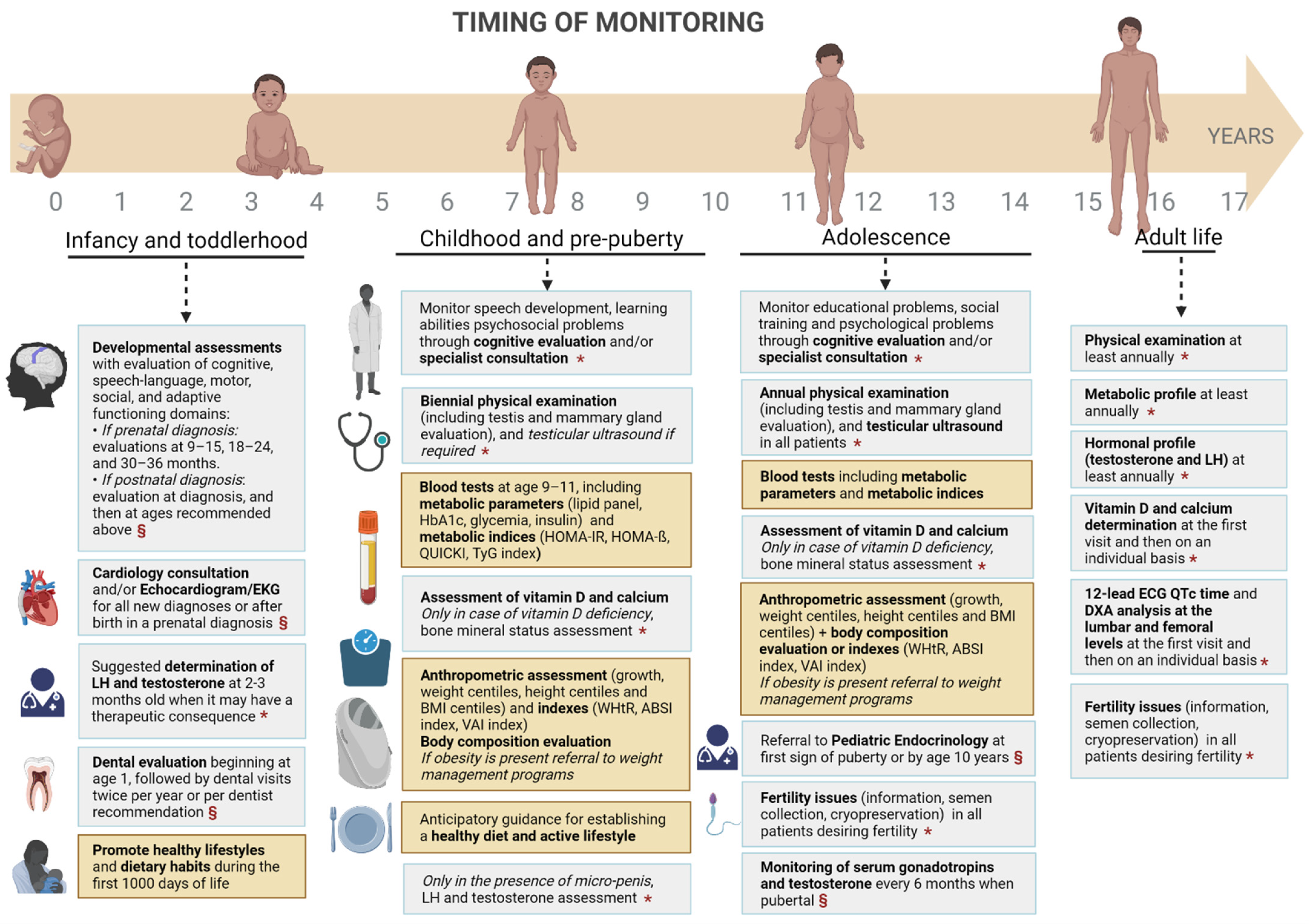
| Authors | Type of Study | Population | Intervention or Objective | Results |
|---|---|---|---|---|
| Diabetes | ||||
| Davis et al. 2020 [16] | Cross-sectional study | 50 KS adolescents age 10–18 years | evaluate cardio-metabolic risk in KS adolescents compared to 50 age- and BMI-matched healthy controls. Subgroup analysis performed in regard of TRT therapy |
|
| Bardsley et al. 2011 [17] | Observational prospective study | 89 prepubertal KS boys age 4–12 years | compare auxologic measures and truncal obesity in prepubertal boys with KS versus age-matched controls (n = 34) |
|
| Metabolic syndrome | ||||
| Bardsley et al. 2011 [17] | Observational prospective study | 89 prepubertal KS boys age 4–12 years | compare auxologic measures and truncal obesity in prepubertal boys with KS versus age-matched controls (n = 34) |
|
| Davis et al. 2016 [18] | Observational study | 93 pre-pubertal boys with KS age 4–12 years | assess the relationship of gonadal and cardiometabolic function in children with KS |
|
| Davis et al. 2017 [19] | Double-blind RCT | 79 pre-pubertal boys with KS age 4–12 years | children were randomized to receive oral oxandrolone (Ox) 0.06 mg/kg/d (n = 38) or placebo (n = 41) for 2 years. |
|
| Cardiovascular risk | ||||
| Davis et al. 2020 [16] | Cross-sectional study | 50 KS adolescents age 10–18 years | evaluate cardio-metabolic risk in KS adolescents compared to age- and BMI-matched healthy controls. Subgroup analysis performed in regard of TRT therapy |
|
| Bone metabolism | ||||
| Aksglaede et al. 2007 [20] | Retrospective cross-sectional study | 24 children with a median age of 11.0 years (range 4.3–18.6) | 18 untreated; 6 received oral testosterone undecanoate (40 mg twice daily increasing to 80 mg twice daily) for a median period of 1.3 years |
|
| Testosterone replacement therapy and adiposity | ||||
| Davis et al. 2017 [19] | Double-blind, placebo-controlled RCT | 93 boys age 4–12 years | Administration of oral oxandrolone (0.06 mg/kg/day) or placebo for 2 years |
|
| Davis et al. 2019 [21] | Prospective randomized trial | 20 infants, 6–15 weeks of age | Administration of 25 mg testosterone cypionate intramuscular monthly for three doses vs. no treatment |
|
| Testosterone replacement therapy and cognitive function | ||||
| Samango-Sprouse et al. 2013 [22] | Placebo-controlled RCT | 101 children 36–72 months of age | Administration of injections (25 mg each) of testosterone enanthate, or placebo. 1 injection/month for 3 months 34 treated, 67 untreated |
|
| Ross et al. 2017 [23] | Placebo-controlled RCT | 84 children age 4–12 years | Administration of oxandrolone (0.06 mg/kg daily) or placebo for 24 months 43 treated, 41 untreated |
|
| Hypogonadism and hormonal aspects | ||||
| Lahalou et al. 2004 [24] | Observative prospective study | 18 KS infants from birth to 3 years | Blood samples were collected from birth to 3 years of age and compared with those in 6 adolescents (14–18 years) with XXY karyotype and reference values established in 215 control infants |
|
| Ross et al. 2005 [25] | Observative prospective study | 22 infants and young boys with KS, age 1–23 months | Auxologic measurements, biologic indices of testicular function by blood samples and clinical assessment of muscle tone in KS infants were measured. |
|
| Future Research Gaps and Needs in Klinefelter Syndrome | ||
|---|---|---|
| Metabolic and Hormonal Aspects | Nutritional Aspects | TRT |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mameli, C.; Fiore, G.; Sangiorgio, A.; Agostinelli, M.; Zichichi, G.; Zuccotti, G.; Verduci, E. Metabolic and Nutritional Aspects in Paediatric Patients with Klinefelter Syndrome: A Narrative Review. Nutrients 2022, 14, 2107. https://doi.org/10.3390/nu14102107
Mameli C, Fiore G, Sangiorgio A, Agostinelli M, Zichichi G, Zuccotti G, Verduci E. Metabolic and Nutritional Aspects in Paediatric Patients with Klinefelter Syndrome: A Narrative Review. Nutrients. 2022; 14(10):2107. https://doi.org/10.3390/nu14102107
Chicago/Turabian StyleMameli, Chiara, Giulia Fiore, Arianna Sangiorgio, Marta Agostinelli, Giulia Zichichi, Gianvincenzo Zuccotti, and Elvira Verduci. 2022. "Metabolic and Nutritional Aspects in Paediatric Patients with Klinefelter Syndrome: A Narrative Review" Nutrients 14, no. 10: 2107. https://doi.org/10.3390/nu14102107
APA StyleMameli, C., Fiore, G., Sangiorgio, A., Agostinelli, M., Zichichi, G., Zuccotti, G., & Verduci, E. (2022). Metabolic and Nutritional Aspects in Paediatric Patients with Klinefelter Syndrome: A Narrative Review. Nutrients, 14(10), 2107. https://doi.org/10.3390/nu14102107









