Low Serum Vitamin D in COVID-19 Patients Is Not Related to Inflammatory Markers and Patients’ Outcomes—A Single-Center Experience and a Brief Review of the Literature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Clinical Evaluation for Disease Severity
- Mild infection: patients who had any of the various signs and symptoms of COVID-19, but without shortness of breath, dyspnea, or abnormal chest imaging.
- Moderate infection: patients with evidence of lower respiratory disease during clinical assessment or imaging and with an oxygen saturation (SpO2) ≥ 94% on room air.
- Severe infection: SpO2 < 94% on room air, a respiratory rate > 30 breaths/min, or lung infiltrates > 50%.
2.5. Sampling Procedure and Data Collection
2.6. Statistical Analysis
3. Results
3.1. Characterization of the Patients with COVID-19
3.2. Comparative Analysis of the Vitamin D Serum Levels for COVID-19 Patients and the Control Group
3.3. Characterization of the COVID-19 Patients in Relation to Vitamin D Status and Inflammatory Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bivona, G.; Agnello, L.; Ciaccio, M. The immunological implication of the new vitamin D metabolism. Cent. J. Immunol. 2018, 43, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.L.; Moran, H.R. Vitamin D: Vitamin or Hormone? Nurs. Clin. N. Am. 2021, 56, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Demer, L.L.; Hsu, J.J.; Tintut, Y. Steroid Hormone Vitamin D. Circ. Res. 2018, 122, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science 1983, 221, 1181–1183. [Google Scholar] [CrossRef]
- Fiske, C.T.; Blackman, A.; Maruri, F.; Rebeiro, P.F.; Huaman, M.; Kator, J.; Algood, H.M.S.; Sterling, T.R. Increased vitamin D receptor expression from macrophages after stimulation with M. tuberculosis among persons who have recovered from extrapulmonary tuberculosis. BMC Infect. Dis. 2019, 19, 366. [Google Scholar] [CrossRef]
- Barragan, M.; Good, M.; Kolls, J.K. Regulation of Dendritic Cell Function by Vitamin D. Nutrients 2015, 7, 8127–8151. [Google Scholar] [CrossRef]
- Kongsbak, M.; Levring, T.B.; Geisler, C.; von Essen, M.R. The vitamin D receptor and T cell function. Front. Immunol. 2013, 4, 148. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [Green Version]
- Carlberg, C. Vitamin D: A Micronutrient Regulating Genes. Curr. Pharm. Des. 2019, 25, 1740–1746. [Google Scholar] [CrossRef]
- Trombetta, A.C.; Paolino, S.; Cutolo, M. Vitamin D, Inflammation and Immunity: Review of Literature and Considerations on Recent Translational and Clinical Research Developments. Open Rheumatol. J. 2018, 12, 201–213. [Google Scholar] [CrossRef]
- Siddiqui, M.; Manansala, J.S.; Abdulrahman, H.A.; Nasrallah, G.K.; Smatti, M.K.; Younes, N.; Althani, A.A.; Yassine, H.M. Immune modulatory effects of vitamin d on viral infections. Nutrients 2020, 12, 2879. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.S.; Zhang, C.; Cheng, B.H.; Lee, C.H. Mechanisms of action of vitamin D as supplemental therapy for Pneumocystis pneumonia. Antimicrob. Agents Chemother. 2017, 61, e01226-17. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.K.; Weinberg, A. Ramping Up Antimicrobial Peptides against Severe Acute Respiratory Syndrome Coronavirus-2. Front. Mol. Biosci. 2021, 8, 620806. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin d supplementation could reduce risk of influenza and covid-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagnini, C.; Picchianti-Diamanti, A.; Bruzzese, V.; Lorenzetti, R.; Luchetti, M.M.; Martin, L.S.M.; Pica, R.; Scolieri, P.; Scribano, M.L.; Zampaletta, C.; et al. Vitamin d signaling in gastro-rheumatology: From immuno-modulation to potential clinical applications. Int. J. Mol. Sci. 2021, 22, 2456. [Google Scholar] [CrossRef]
- Sloka, S.; Silva, C.; Wang, J.; Yong, V.W. Predominance of Th2 polarization by Vitamin D through a STAT6-dependent mechanism. J. Neuroinflammation 2011, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Fisher, S.A.; Rahimzadeh, M.; Brierley, C.; Gration, B.; Doree, C.; Kimber, C.E.; Cajide, A.P.; Lamikanra, A.A.; Roberts, D.J. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: A systematic review. PLoS ONE 2019, 14, e0222313. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, A.; Mohammadi, V.; Aghababaee, S.K.; Golzarand, M.; Clark, C.C.T.; Babajafari, S. Association of Vitamin D Status with SARS-CoV-2 Infection or COVID-19 Severity: A Systematic Review and Meta-analysis. Adv. Nutr. 2021, 12, 1636–1658. [Google Scholar] [CrossRef]
- Saponaro, F.; Franzini, M.; Okoye, C.; Antognoli, R.; Campi, B.; Scalese, M.; Neri, T.; Carrozzi, L.; Monzani, F.; Zucchi, R.; et al. Is There a Crucial Link Between Vitamin D Status and Inflammatory Response in Patients With COVID-19? Front. Immunol. 2022, 12, 745713. [Google Scholar] [CrossRef]
- Campi, I.; Gennari, L.; Merlotti, D.; Mingiano, C.; Frosali, A.; Giovanelli, L.; Torlasco, C.; Pengo, M.F.; Heilbron, F.; Soranna, D.; et al. Vitamin D and COVID-19 severity and related mortality: A prospective study in Italy. BMC Infect. Dis. 2021, 21, 566. [Google Scholar] [CrossRef] [PubMed]
- Merzon, E.; Tworowski, D.; Gorohovski, A.; Vinker, S.; Cohen, A.G.; Green, I.; Frenkel-Morgenstern, M. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: An Israeli population-based study. FEBS J. 2020, 287, 3693–3702. [Google Scholar] [CrossRef] [PubMed]
- Jevalikar, G.; Mithal, A.; Singh, A.; Sharma, R.; Farooqui, K.J.; Mahendru, S.; Dewan, A.; Budhiraja, S. Lack of association of baseline 25-hydroxyvitamin D levels with disease severity and mortality in Indian patients hospitalized for COVID-19. Sci. Rep. 2021, 11, 6258. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Pagliuca, A.; D’Ascanio, M.; Innammorato, M.; De Vitis, C.; Mancini, R.; Giovagnoli, S.; Facchiano, F.; Sposato, B.; Anibaldi, P.; et al. Circulating Vitamin D levels status and clinical prognostic indices in COVID-19 patients. Respir Res. 2021, 22, 76. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Chaurasia, R.; Sengar, N.S.; Singh, M.; Mahor, S.; Narain, S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci. Rep. 2020, 10, 20191. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.L.; Nan, D.; Fernandez-Ayala, M.; García-Unzueta, M.; Hernández-Hernández, M.A.; López-Hoyos, M.; Muñoz-Cacho, P.; Olmos, J.M.; Gutiérrez-Cuadra, M.; Ruiz-Cubillán, J.J.; et al. Vitamin D Status in Hospitalized Patients with SARS-CoV-2 Infection. J. Clin. Endocrinol. Metab. 2021, 106, e1343–e1353. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Di Lecce, V.; Quaranta, V.N.; Zito, A.; Buonamico, E.; Capozza, E.; Palumbo, A.; Di Gioia, G.; Valerio, V.N.; Resta, O. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J. Endocrinol. Invest. 2021, 44, 765–771. [Google Scholar] [CrossRef]
- Chirita-Emandi, A.; Socolov, D.; Haivas, C.; Calapiş, A.; Gheorghiu, C.; Puiu, M. Vitamin D Status: A Different Story in the Very Young versus the Very Old Romanian Patients. PLoS ONE 2015, 10, e0128010. [Google Scholar]
- Niculescu, D.A.; Capatina, C.A.M.; Dusceac, R.; Caragheorgheopol, A.; Ghemigian, A.; Poiana, C. Seasonal variation of serum vitamin D levels in Romania. Arch. Osteoporos. 2017, 12, 113. [Google Scholar] [CrossRef]
- Zugravu, C.-A.; Soptica, F.; Tarcea, M.; Cucu, A. Pertinence of Vitamin D Supplementation In The Adult Romanian Population. Farmacia 2016, 64, 467–472. [Google Scholar]
- Risk for COVID-19 Infection, Hospitalization, and Death by Age Group|CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html (accessed on 13 March 2022).
- WHO—Emergency Situation Reports. Available online: https://www.who.int/emergencies/situation-reports (accessed on 13 March 2022).
- ROCHE-eLabDoc. Available online: https://pim-eservices.roche.com/eLD/web/gb/en/documents (accessed on 13 March 2022).
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, S.; Fischer, I.; Naderi, S.; Jouibari, M.F.; Abdolreza, S.; Karimialavijeh, E.; Aslzadeh, S.; Mashayekhi, M.; Zojaji, M.; Kahlert, U.D.; et al. Systemic Inflammatory Index Is a Novel Predictor of Intubation Requirement and Mortality after SARS-CoV-2 Infection. Pathogens 2021, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Dyussenbayev, A. Age Periods of Human Life. Adv. Soc. Sci. Res. J. 2017, 4, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Kongsbak-Wismann, M.; Al-Jaberi, F.A.H.; Schmidt, J.D.; Ghanizada, M.; Hansen, C.B.; Lopez, D.V.; Woetmann, A.; Ødum, N.; Bonefeld, C.M.; Stryhn, A.; et al. Normal T and B Cell Responses Against SARS-CoV-2 in a Family With a Non-Functional Vitamin D Receptor: A Case Report. Front. Immunol. 2021, 12, 758154. [Google Scholar] [CrossRef]
- Dror, A.A.; Morozov, N.; Daoud, A.; Namir, Y.; Yakir, O.; Shachar, Y.; Lifshitz, M.; Segal, E.; Fisher, L.; Mizrachi, M.; et al. Pre-infection 25-hydroxyvitamin D3 levels and association with severity of COVID-19 illness. PLoS ONE 2022, 17, e0263069. [Google Scholar] [CrossRef]
- Gallelli, L.; Chiara Mannino, G.; Luciani, F.; de Sire, A.; Mancuso, E.; Gangemi, P.; Cosco, L.; Monea, G.; Averta, C.; Minchella, P.; et al. Vitamin D Serum Levels in Subjects Tested for SARS-CoV-2: What Are the Differences among Acute, Healed, and Negative COVID-19 Patients? A Multicenter Real-Practice Study Vitamin D Serum Levels in Subjects. Nutrients 2021, 13, 3932. [Google Scholar] [CrossRef]
- De Smet, D.; De Smet, K.; Herroelen, P.; Gryspeerdt, S.; Martens, G.A. Serum 25(OH)D Level on Hospital Admission Associated With COVID-19 Stage and Mortality. Am. J. Clin. Pathol. 2021, 155, 381–388. [Google Scholar] [CrossRef]
- White, J.H. Emerging Roles of Vitamin D-Induced Antimicrobial Peptides in Antiviral Innate Immunity. Nutrients 2022, 14, 284. [Google Scholar] [CrossRef]
- Bishop, E.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus 2021, 5, e10405. [Google Scholar] [CrossRef]
- Fabri, M.; Stenger, S.; Shin, D.M.; Yuk, J.M.; Liu, P.T.; Realegeno, S.; Lee, H.M.; Krutzik, S.R.; Schenk, M.; Sieling, P.A.; et al. Vitamin D Is Required for IFN-γ–Mediated Antimicrobial Activity of Human Macrophages. Sci. Transl. Med. 2011, 3, 104ra102. [Google Scholar] [CrossRef] [Green Version]
- Coveney, C.; Tellier, M.; Lu, F.; Maleki-Toyserkani, S.; Jones, R.; Bart, V.M.T.; Jones, R.; Coveney, C.; Lu, F.; Tellier, M.; et al. Innate immunology in COVID-19—A living review. Part I: Viral entry, sensing and evasion. Oxf. Open Immunol. 2020, 1, iqaa004. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.R.S.; Alrubayyi, A.; Pring, E.; Bart, V.M.T.; Jones, R.; Coveney, C.; Lu, F.; Tellier, M.; Maleki-Toyserkani, S.; Richter, F.C.; et al. Innate immunology in COVID-19—A living review. Part II: Dysregulated inflammation drives immunopathology. Oxf. Open Immunol. 2020, 1, iqaa005. [Google Scholar] [CrossRef] [PubMed]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.; et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef]
- Mardani, R.; Alamdary, A.; Mousavi Nasab, S.D.; Gholami, R.; Ahmadi, N.; Gholami, A. Association of vitamin D with the modulation of the disease severity in COVID-19. Virus Res. 2020, 289, 198148. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, I.N. Vitamin D and COVID-19: How much vitamin d does a man need? Nutrients 2021, 13, 4311. [Google Scholar] [CrossRef] [PubMed]
- Alkhafaji, D.; Al Argan, R.; Albaker, W.; Al Elq, A.; Al-Hariri, M.; Alsaid, A.; Alwaheed, A.; Alqatari, S.; Alzaki, A.; Alwarthan, S.; et al. The Impact of Vitamin D Level on the Severity and Outcome of Hospitalized Patients with COVID-19 Disease. Int. J. Gen. Med. 2022, 15, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, A.; Najafi, N.; Aarabi, M.; Tayebi, A.; Nikaeen, R.; Izadyar, H.; Salar, Z.; Delavarian, L.; Vaseghi, N.; Daftarian, Z.; et al. Lack of association between vitamin D insufficiency and clinical outcomes of patients with COVID-19 infection. BMC Infect. Dis. 2021, 21, 450. [Google Scholar] [CrossRef]
- Butler-Laporte, G.; Nakanishi, T.; Mooser, V.; Morrison, D.R.; Abdullah, T.; Adeleye, O.; Mamlouk, N.; Kimchi, N.; Afrasiabi, Z.; Rezk, N.; et al. Vitamin D and COVID-19 susceptibility and severity in the COVID-19 host genetics initiative: A Mendelian randomization study. PLoS Med. 2021, 18, e1003605. [Google Scholar] [CrossRef]
- Hastie, C.E.; Pell, J.P.; Sattar, N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur. J. Nutr. 2021, 60, 545–548. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, J.; Ye, K. Extensive Mendelian randomization study identifies potential causal risk factors for severe COVID-19. Commun. Med. 2021, 1, 59. [Google Scholar] [CrossRef]
- Liu, D.; Tian, Q.Y.; Zhang, J.; Hou, H.F.; Li, Y.; Wang, W.; Meng, Q.; Wang, Y.X. Association between 25 Hydroxyvitamin D Concentrations and the Risk of COVID-19: A mendelian randomization study. Biomed. Environ. Sci. 2021, 9, 750–754. [Google Scholar]
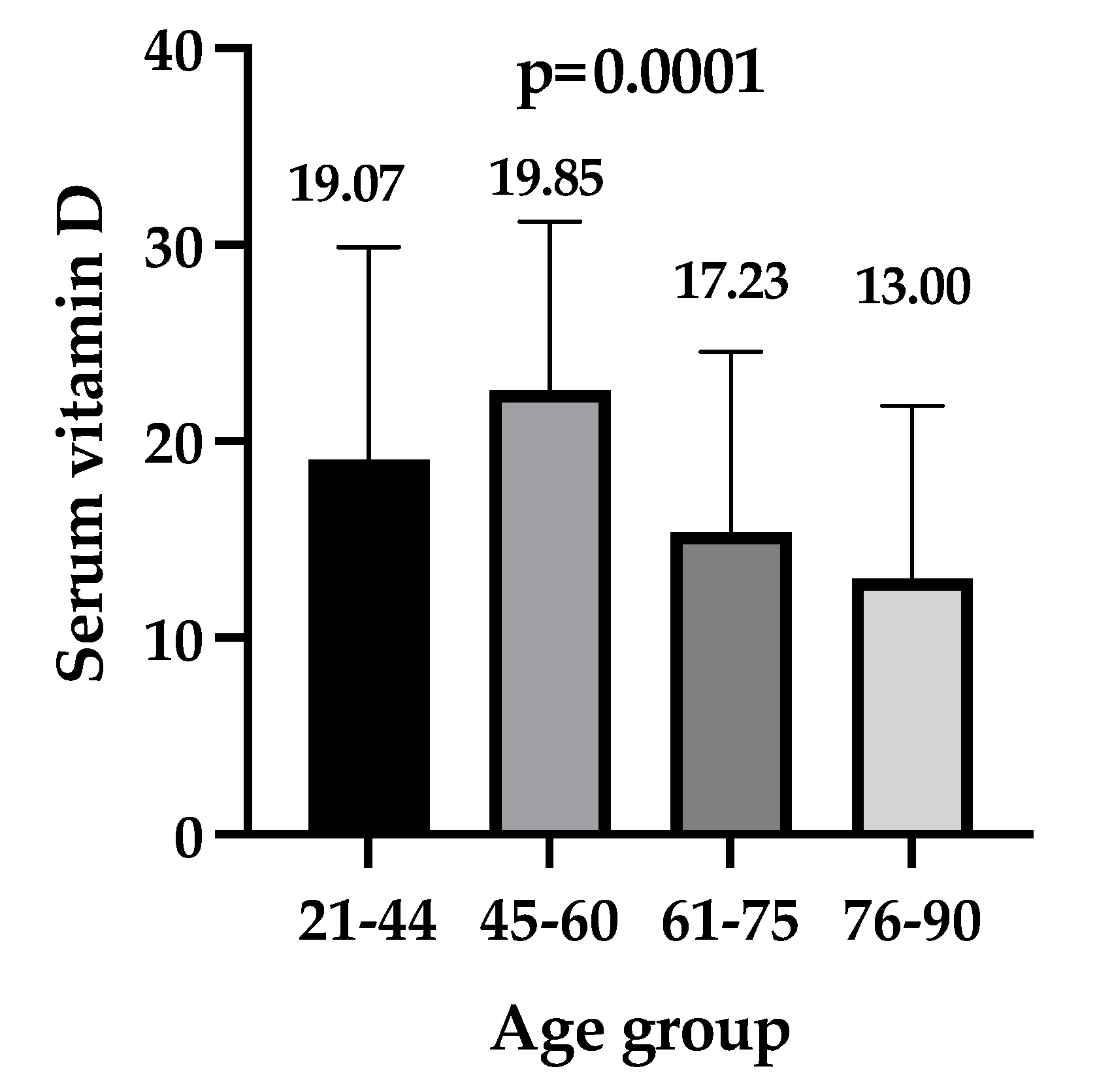
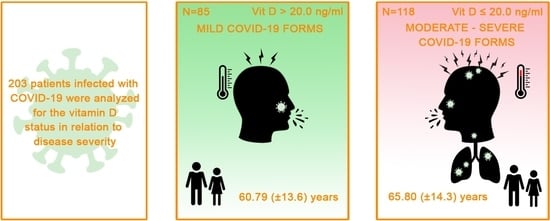
| Age (Years) | n = 203 (%) |
|---|---|
| Mean (SD) | 63.7 (14.2) |
| Age group | |
| 21–44 years | 21 (10.3%) |
| 45–60 years | 60 (29.6%) |
| 61–75 years | 70 (34.5%) |
| 76–90 years | 52 (25.6%) |
| Gender n (%) | |
| Male | 118 (58.1%) |
| Female | 85 (41.9%) |
| Disease severity among cases n (%) | |
| Mild | 14 (6.9%) |
| Moderate | 86 (42.4%) |
| Severe | 103 (50.7%) |
| ICU | 39 (18.75%) |
| Death | 25 (12.3%) |
| Comorbidities n (%) | |
| Without comorbidities | 32 (15.7%) |
| Hypertension | 127 (62.6%) |
| Diabetes mellitus (DM) | 35 (16.8%) |
| Dyslipidemia | 64 (31.5%) |
| Obesity | 76 (37.4%) |
| Asthma | 7 (3.4%) |
| COPD | 6 (3.0%) |
| Chronic renal disease | 16 (7.9%) |
| Chronic hepatopathy | 2 (1.0%) |
| Age Groups | Control Group (n = 1830) | Patients Group (n = 203) | p-Value |
|---|---|---|---|
| 21–44 years | 24.90 (19.62–31.71) | 19.07 (16.77–29.51) | 0.110 |
| 45–60 years | 28.65 (21.53–36.78) | 22.59 (14.81–31.22) | <0.0001 |
| 61–75 years | 30.96 (22.61–39.71) | 15.37 (9.89–24.22) | <0.0001 |
| 76–90 years | 30.26 (20.76–39.08) | 13.00 (8.83–21.55) | <0.0001 |
| Parameters | Vit D ≤ 20.0 ng/mL n = 118 | Vit D > 20.0 ng/mL n = 85 | p Value |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years), mean (SD) | 65.8 ( ±14.3) | 60.79 (±13.6) | 0.01 * |
| Male, n (%) | 62 (52.5) | 56 (65.9) | 0.057 ** |
| Hypertension, n (%) | 79 (66.9) | 48 (56.5) | 0.13 ** |
| Diabetes, n (%) | 17 (14.4) | 7 (8.2) | 0.18 ** |
| Cardiovascular Diseases n (%) | 26 (22.0) | 19 (22.4) | 0.95 ** |
| COPD, n (%) | 5 (4.2) | 1 (1.2) | 0.20 ** |
| Clinical data | |||
| O2 Saturation, mean (SD) | 91.89 (5.44) | 90.86 (6.12) | 0.22 * |
| Ventricular allure, mean (SD) | 84.34 (15.9) | 89.58 (14.9) | 0.018 * |
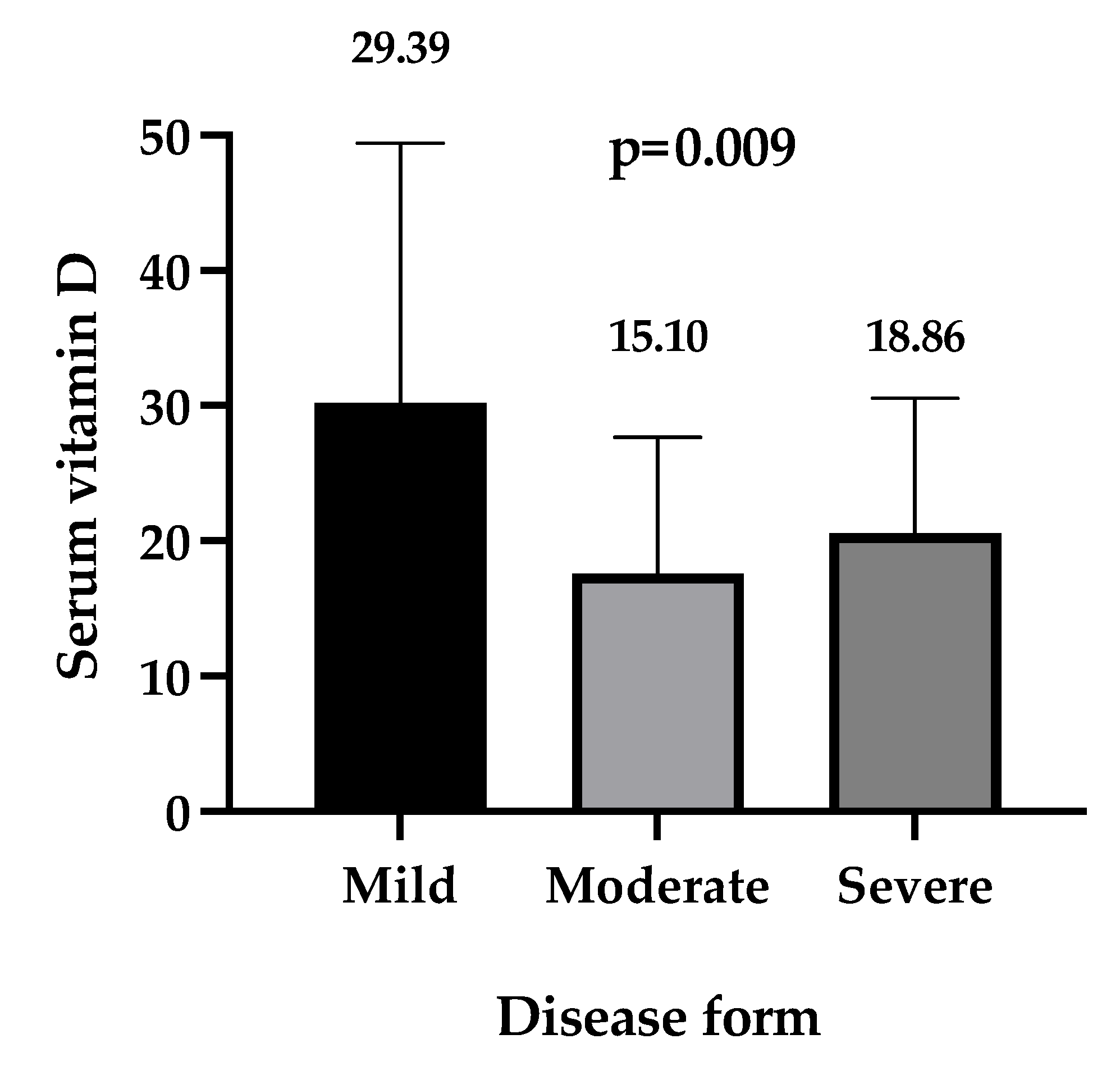
| Disease form | |||
| Mild, n (%) | 6 (5.1) | 8 (9.4) | 0.16 ** |
| Moderate, n (%) | 56 (47.5) | 30 (35.3) | |
| Severe, n (%) | 56 (47.5) | 47 (55.3) | |
| Disease outcome | |||
| ICU admission, n (%) | 21 (17.8) | 15 (17.6) | 0.97 ** |
| Death, n (%) | 15 (12.7) | 10 (11.8) | 0.84 ** |
| Length of hospitalization (days), median (IQR) | 13 (10–16) | 13 (10.5–15.2) | 0.83 *** |
| Laboratory parameters | |||
| Vitamin D ng/mL, median (IQR) | 11.85 (8.95–16.38) | 28.20 (24.10–34.80) | <0.0001 *** |
| Leucocytes ×103/µL, median (min.–max.) | 6975.5 (1462–46,100) | 7931 (2434–54,600) | 0.15 *** |
| Lymphocytes ×103/µL, median (min.–max.) | 1225 (200–9967) | 1300 (380–9674) | 0.43 *** |
| Neutrophils ×103/µL, median (min.–max.) | 5227 (3597.7–7842.2) | 5150 (1080–51,000) | 0.53 *** |
| NLR | 4.32 (0.28–55.15) | 3.88 (0.34–72.70) | 0.90 *** |
| PLR | 156.7 (16.37–876.50) | 176.9 (11.20–683.70) | 0.62 *** |
| SII median (min.–max.) | 675.63 (36.74–96,677.79) | 868.00 (40.91–8716.36) | 0.35 *** |
| CRP (mg/L), median (min.–max.) | 43.06 (0.12–254.0) | 33.09 (0.12–320.0) | 0.47 *** |
| Ferritin (ng/mL), median (min.–max.) | 605.0 (90.0–3647.9) | 656.0 (95–4555.6) | 0.57 *** |
| Fibrinogen (mg/dL), median (min.–max.) | 446.5 (102.0–900.0) | 487.0 (128.7–900.0) | 0.56 *** |
| Parameter | Survivors n = 178 | Non-Survivors n = 25 | p-Value |
|---|---|---|---|
| Age mean (SD) | 63.0 (14.4) | 70 (10.6) | 0.026 * |
| Male, n (%) | 106 (89.8%) | 12 (10.2%) | 0.06 ** |
| Laboratory parameters | |||
| Vitamin D ng/mL, median (IQR) | 17.85 (9.87–28.08) | 17.08 (11.19–26.58) | 0.838 *** |
| Vitamin D insuficiency, n (%) | 103 (57.8%) | 15 (60%) | 0.93 ** |
| Vitamin D suficiency | 75 (42.1%) | 10 (40%) | |
| Leucocytes ×103/µL, median (min.–max.) | 6710 (1462–46,100) | 11,150 (3240–54,600) | 0.0003 *** |
| Lymphocytes ×103/µL, median (min.–max.) | 1270 (282–9967) | 1091 (200–7730) | 0.099 *** |
| Neutrophils ×103/µL, median (min.–max.) | 5128 9370–36,700) | 9506 (1080–51,000) | 0.0006 *** |
| NLR median (min.–max.) | 3.75 (0.29–28.9) | 6.57 (0.47–72.71) | 0.003 *** |
| PLR median (min.–max.) | 170.0 (11.2–683.6) | 165.9 (21.7–876.5) | 0.376 *** |
| SII median (min.–max.) | 727.4 (36.7–8716.4) | 1399 (154.0–9667.8) | 0.015 *** |
| CRP (mg/L), median (min.–max.) | 43.1 (0.12–320) | 20.9 (0.12–254.0) | 0.563 *** |
| Ferritin (ng/mL), median (min.–max.) | 604.5 (90–4555) | 950 (243–3647) | 0.005 *** |
| Fibrinogen (mg/dL), median (min.–max.) | 455 (122–900) | 441 (102–900) | 0.863 *** |
| B | S.E. | Wald | p-Value | Exp(B) | 95% CI for Exp(B) | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age | 0.039 | 0.015 | 5.455 | 0.015 | 1.046 | 1.008 | 1.094 |
| Sex | 0.513 | 0.406 | 1.997 | 0.196 | 1.572 | 0.554 | 3.608 |
| Diabetes mellitus | −0.072 | 0.662 | 0.017 | 0.801 | 0.822 | 0.267 | 3.406 |
| Hypertension | 0.257 | 0.441 | 0.393 | 0.575 | 1.406 | 0.761 | 3.041 |
| CCD | 0.342 | 0.458 | 0.705 | 0.412 | 1.363 | 0.501 | 3.463 |
| COPD | 0.137 | 1.109 | 0.014 | 0.905 | 1.242 | 0.152 | 9.554 |
| NLR | 0.106 | 0.026 | 12.345 | 0.0001 | 1.311 | 1.052 | 1.273 |
| PLR | 0.002 | 0.001 | 4.065 | 0.034 | 1.002 | 1.000 | 1.005 |
| Ferritin | 0.000 | 0.000 | 3.100 | 0.044 | 1.000 | 1.000 | 1.001 |
| CRP | 0.001 | 0.003 | 0.077 | 0.751 | 1.001 | 0.595 | 1.007 |
| SII | 0.000 | 0.000 | 13.090 | 0.0001 | 1.000 | 1.000 | 1.001 |
| Leucocytes | 0.000 | 0.000 | 8.423 | 0.003 | 1.000 | 1.000 | 1.000 |
| Vitamin D | −0.010 | 0.018 | 0.180 | 0.396 | 0.490 | 0.955 | 1.027 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huțanu, A.; Georgescu, A.M.; Voidăzan, S.; Andrejkovits, A.V.; Negrea, V.; Dobreanu, M. Low Serum Vitamin D in COVID-19 Patients Is Not Related to Inflammatory Markers and Patients’ Outcomes—A Single-Center Experience and a Brief Review of the Literature. Nutrients 2022, 14, 1998. https://doi.org/10.3390/nu14101998
Huțanu A, Georgescu AM, Voidăzan S, Andrejkovits AV, Negrea V, Dobreanu M. Low Serum Vitamin D in COVID-19 Patients Is Not Related to Inflammatory Markers and Patients’ Outcomes—A Single-Center Experience and a Brief Review of the Literature. Nutrients. 2022; 14(10):1998. https://doi.org/10.3390/nu14101998
Chicago/Turabian StyleHuțanu, Adina, Anca Meda Georgescu, Septimiu Voidăzan, Akos Vince Andrejkovits, Valentina Negrea, and Minodora Dobreanu. 2022. "Low Serum Vitamin D in COVID-19 Patients Is Not Related to Inflammatory Markers and Patients’ Outcomes—A Single-Center Experience and a Brief Review of the Literature" Nutrients 14, no. 10: 1998. https://doi.org/10.3390/nu14101998
APA StyleHuțanu, A., Georgescu, A. M., Voidăzan, S., Andrejkovits, A. V., Negrea, V., & Dobreanu, M. (2022). Low Serum Vitamin D in COVID-19 Patients Is Not Related to Inflammatory Markers and Patients’ Outcomes—A Single-Center Experience and a Brief Review of the Literature. Nutrients, 14(10), 1998. https://doi.org/10.3390/nu14101998