HPLC/MSn Profiling and Healing Activity of a Muco-Adhesive Formula of Salvadora persica against Acetic Acid-Induced Oral Ulcer in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Animals
2.3. Chemicals
2.4. Preparation of S. persica Ethyl Acetate Fraction (SPEAF)
2.5. High-Resolution HPLC-ESI-QTOF-MS-MS Analysis
Tentative Identification of Metabolites
2.6. Preparation of Plain and Ethyl Acetate Fraction Muco-Adhesive Formulae
2.7. Cytotoxicity Assay
2.8. Acute Oral Toxicity Study
2.9. Experimental Design
2.10. Histopathological Examination
2.11. Immunohistochemical Staining
2.12. Biochemical Analysis
2.13. RT-qPCR for Collagen Type I Alpha 1 (Col1A1) and Angiopoietin-1 (Ang-1)
2.14. Statistical Analysis
3. Results
3.1. Identification of SPEAF
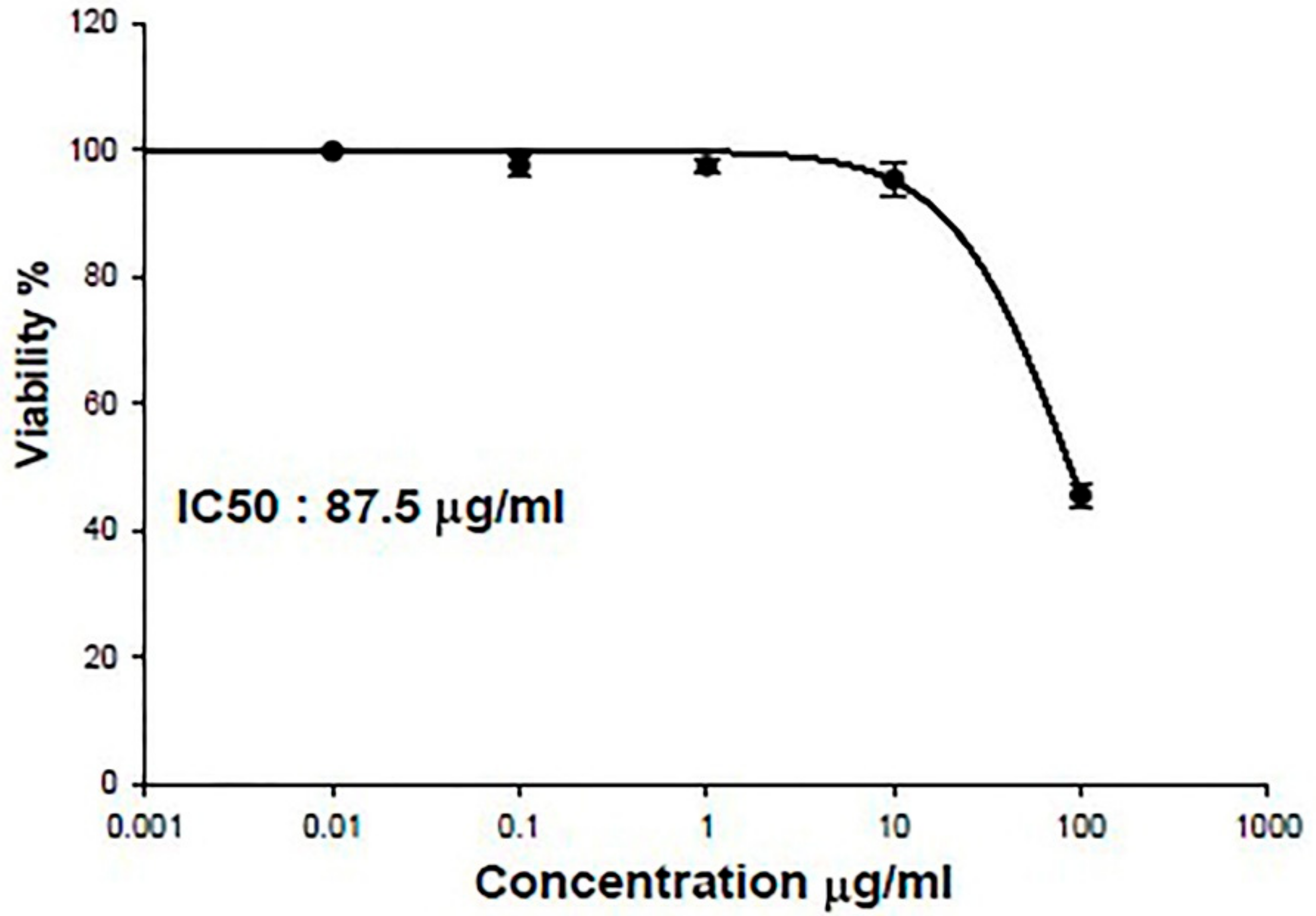
3.2. IC50 of SPEAF on OEC
3.3. Acute Oral Toxicity Study
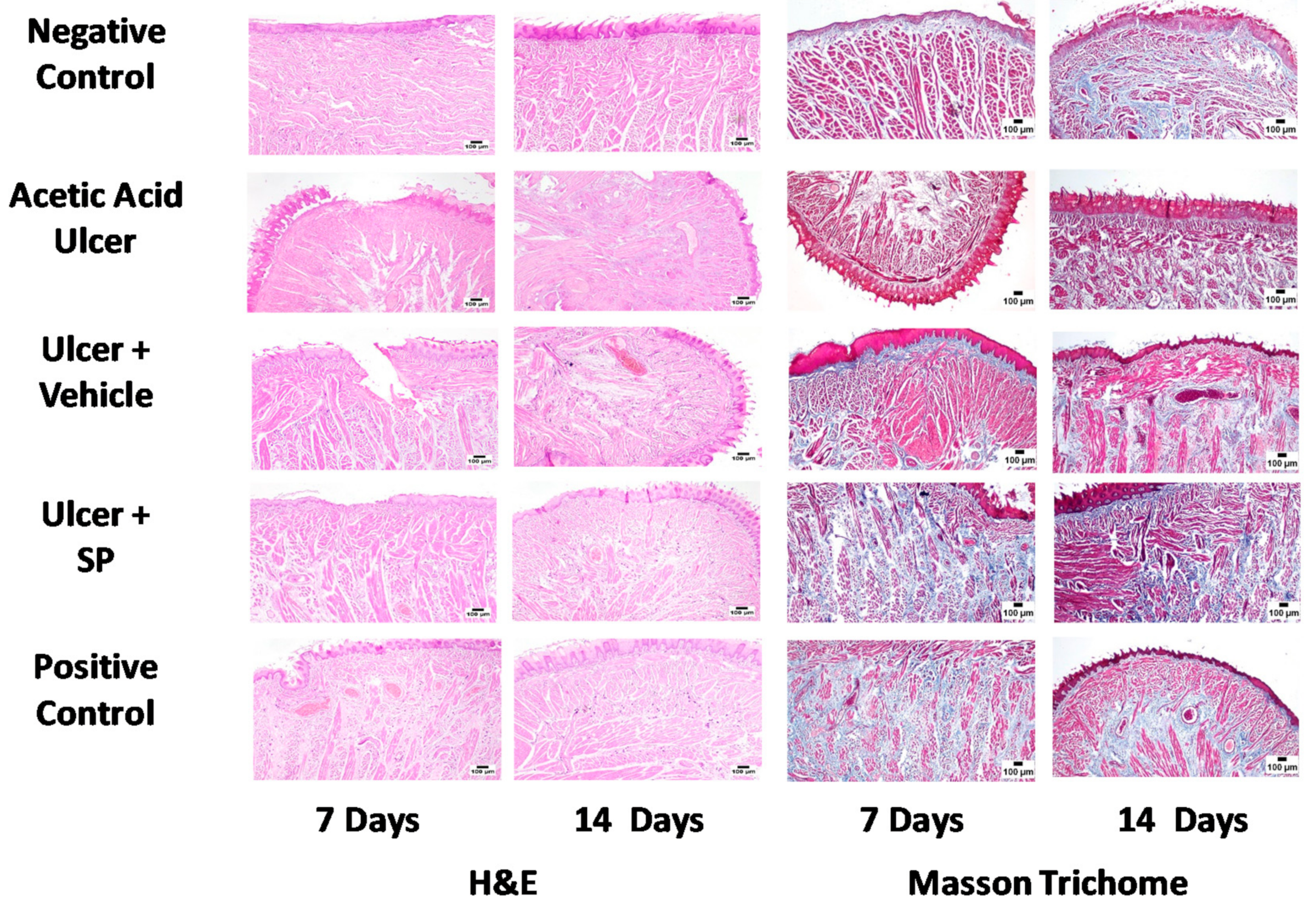
3.4. Histopathological Examination
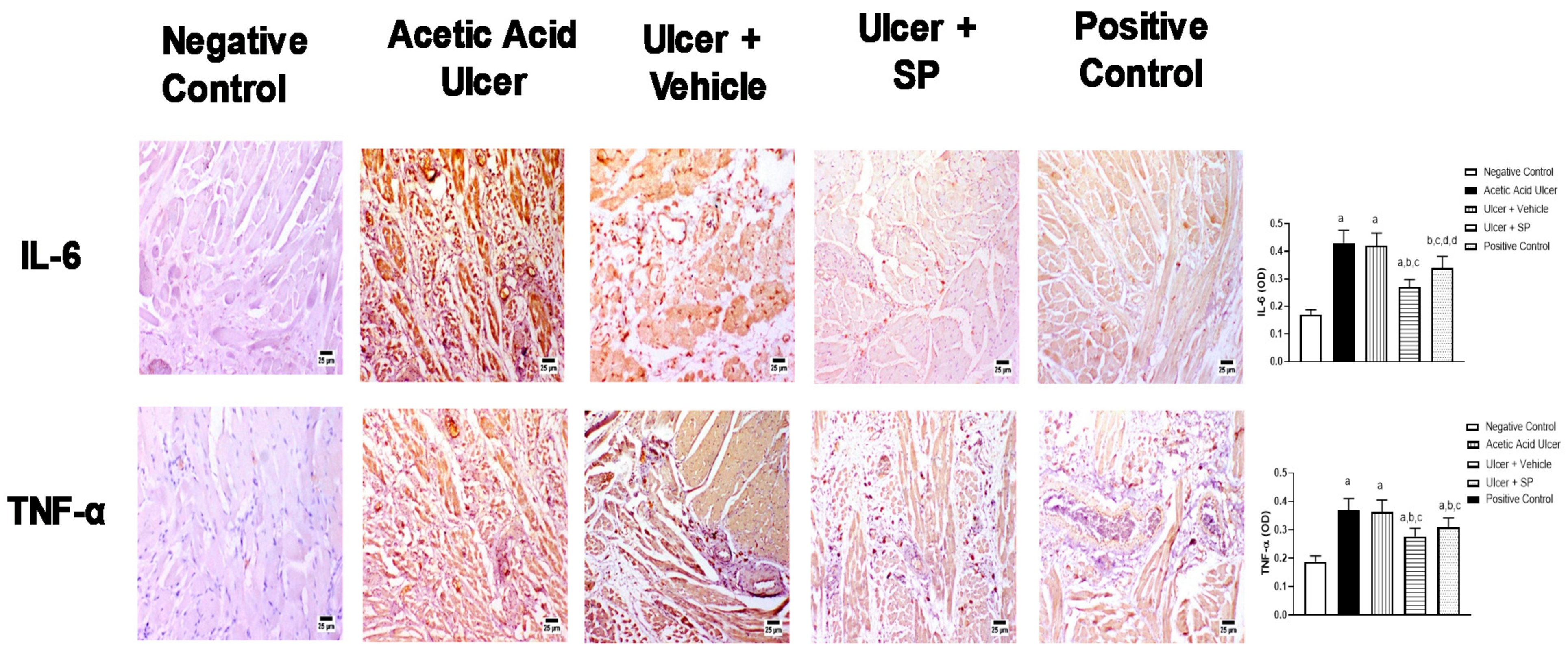
3.5. Immunohistochemical Assessment of the Expression of Inflammation Markers
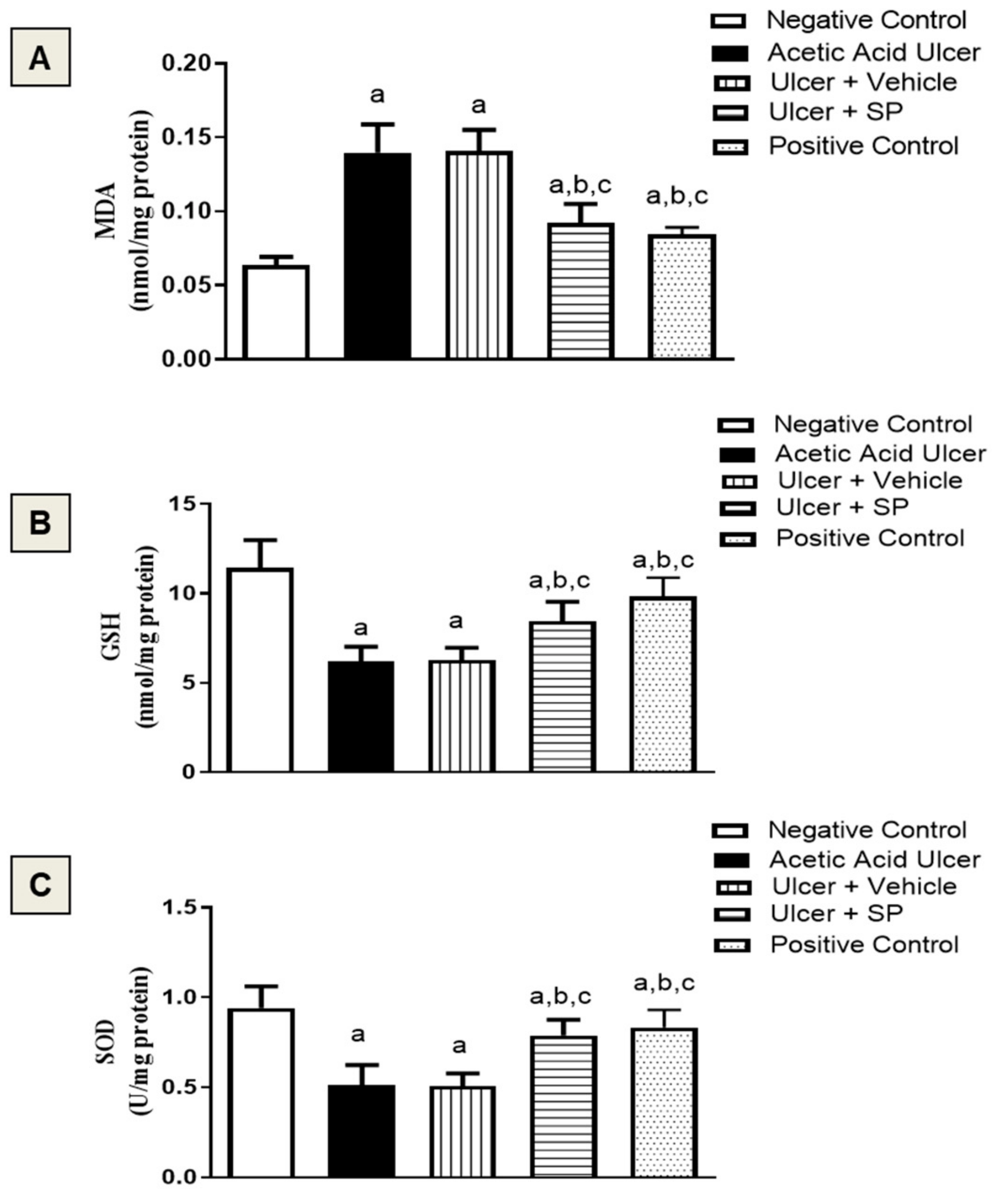
3.6. Assessment of Oxidative Status
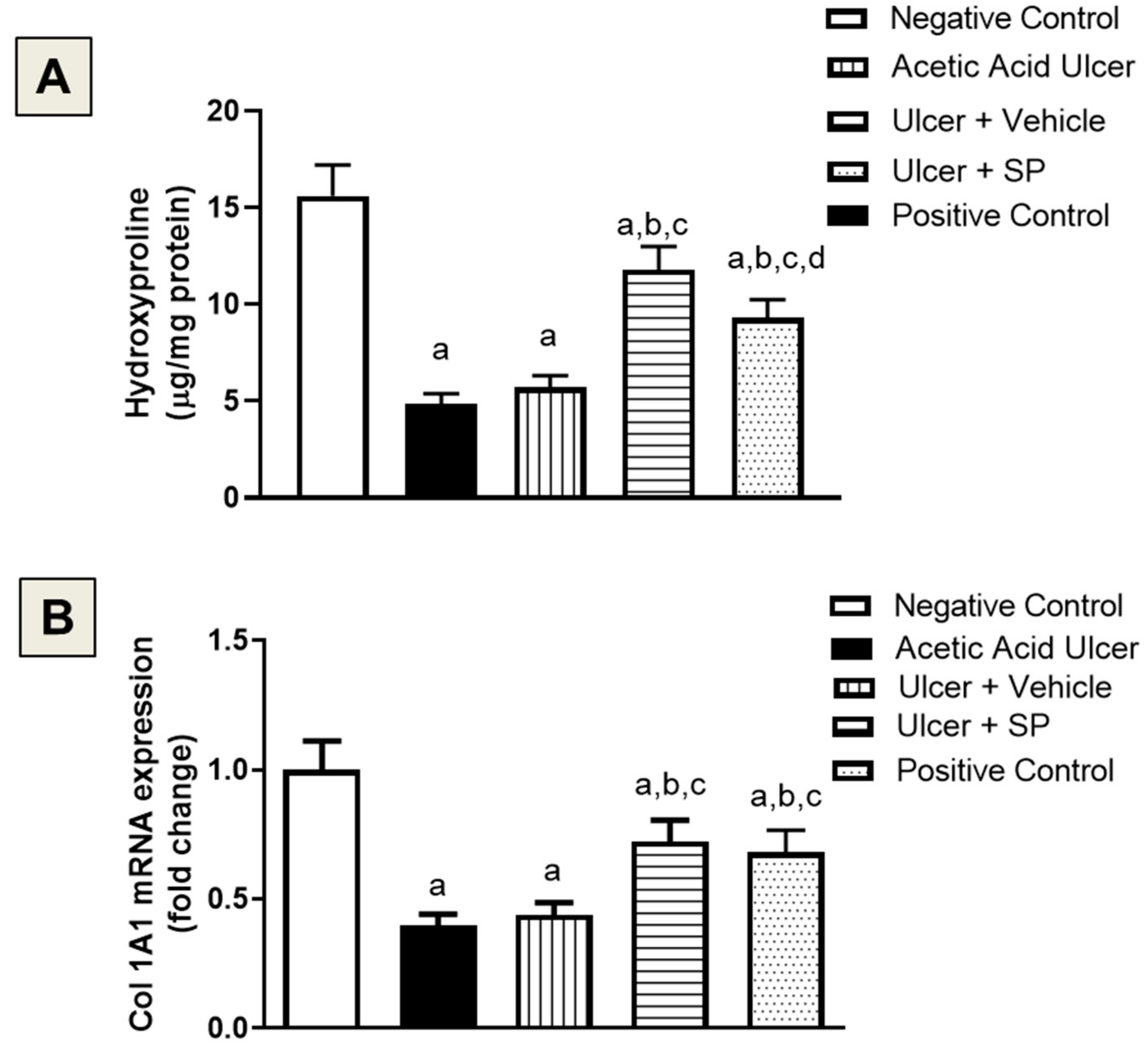
3.7. Assessment of Collagen Content
3.8. Assessment of Ang-1 mRNA Expression
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burgess, J.A.; Johnson, B.D.; Sommers, E. Pharmacological management of recurrent oral mucosal ulceration. Drugs 1990, 39, 54–65. [Google Scholar] [CrossRef]
- Chen, P.; Yao, H.; Su, W.; He, Y.; Cheng, K.; Wang, Y.; Peng, W.; Li, P. Sleep deprivation worsened oral ulcers and delayed healing process in an experimental rat model. Life Sci. 2019, 232, 116594. [Google Scholar] [CrossRef]
- Field, E.A.; Allan, R.B. Review article: Oral ulceration–aetiopathogenesis, clinical diagnosis and management in the gastrointestinal clinic. Aliment. Pharm. Ther. 2003, 18, 949–962. [Google Scholar] [CrossRef]
- McGettigan, P.; Ferner, R.E. Painful perianal ulcers with nicorandil. BMJ 2020, 370, m3351. [Google Scholar] [CrossRef]
- Critchlow, D. Part 3: Impact of systemic conditions and medications on oral health. Br. J. Community Nurs. 2017, 22, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Adara, A.; Onalana, O.; Aktasb, H.; Ertugrulc, S.; Cakana, F. A very rare complication of sublingual captopril. J. Exp. Clin. Med. 2019, 36, 91–93. [Google Scholar] [CrossRef]
- Hasan, A.A.; Ciancio, S. Association between ingestion of nonsteroidal anti-inflammatory drugs and the emergence of aphthous-like ulcers. J. Int. Acad. Periodontol. 2009, 11, 155–159. [Google Scholar] [PubMed]
- Shetty, K. Thalidomide in the concurrent management of recurrent aphthous ulcerations and Kaposi sarcoma in HIV patients with severe immunosuppression. Oral Oncol. Extra 2006, 42, 26–31. [Google Scholar] [CrossRef][Green Version]
- Adler, S.; Baumgartner, I.; Villiger, P.M. Behcet’s disease: Successful treatment with infliximab in 7 patients with severe vascular manifestations. A retrospective analysis. Arthritis Care Res. 2012, 64, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Yazarlu, O.; Iranshahi, M.; Kashani, H.R.K.; Reshadat, S.; Habtemariam, S.; Iranshahy, M.; Hasanpour, M. Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharm. Res. 2021, 174, 105841. [Google Scholar] [CrossRef]
- Gomes, M.S.; Lins, R.D.A.U.; Langassner, S.M.Z.; da Silveira, E.J.D.; de Carvalho, T.G.; de Sousa Lopes, M.L.D.; de Souza Araujo, L.; de Medeiros, C.A.C.X.; de Carvalho Leitao, R.F.; Guerra, G.C.B.; et al. Anti-inflammatory and antioxidant activity of hydroethanolic extract of Spondias mombin leaf in an oral mucositis experimental model. Arch. Oral Biol. 2020, 111, 104664. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Jin, C.; Xin, M.; He, J. Effect of Aloe vera polysaccharides on immunity and antioxidant activities in oral ulcer animal models. Carbohydr. Polym. 2009, 75, 307–311. [Google Scholar] [CrossRef]
- Sakarcan, A.; Sehirli, O.; Velioglu-Ovunc, A.; Ercan, F.; Erkanl, G.; Gedik, N.; Sener, G. Ginkgo biloba extract improves oxidative organ damage in a rat model of thermal trauma. J. Burn Care Rehabil. 2005, 26, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Tatke, P. Antioxidant, antimicrobial and wound healing activity of Salvadora persica twig extracts. J. Complement. Med. Altern. Healthc. 2018, 7, 555720. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Tungcharoen, P.; Sudsai, T.; Karalai, C.; Ponglimanont, C.; Yodsaoue, O. Antiinflammatory and Wound Healing Effects of Caesalpinia sappan L. Phytother. Res. 2015, 29, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.L.; Truong, C.T.; Nguyen, B.C.Q.; Vo, T.V.; Dao, T.T.; Nguyen, V.D.; Trinh, D.T.; Huynh, H.K.; Bui, C.B. Anti-inflammatory and wound healing activities of calophyllolide isolated from Calophyllum inophyllum Linn. PLoS ONE 2017, 12, e0185674. [Google Scholar] [CrossRef]
- Chen, Z.C.; Wu, S.S.; Su, W.Y.; Lin, Y.C.; Lee, Y.H.; Wu, W.H.; Chen, C.H.; Wen, Z.H. Anti-inflammatory and burn injury wound healing properties of the shell of Haliotis diversicolor. BMC Complement. Altern. Med. 2016, 16, 487. [Google Scholar] [CrossRef]
- Deveci, M.; Eski, M.; Sengezer, M.; Kisa, U. Effects of cerium nitrate bathing and prompt burn wound excision on IL-6 and TNF-a levels in burned rats. Burns 1999, 26, 41–45. [Google Scholar] [CrossRef]
- Upadhyay, N.K.; Kumar, R.; Siddiqui, M.S.; Gupta, A. Mechanism of wound-healing activity of Hippophae rhamnoides L. leaf extract in experimental burns. Evid. Based Complement. Altern. Med. 2011, 2011, 659705. [Google Scholar] [CrossRef]
- Farag, M.; Abdel-Mageed, W.M.; El Gamal, A.A.; Basudan, O.A. Salvadora persica L.: Toothbrush tree with health benefits and industrial applications—An updated evidence-based review. Saudi Pharm. J. 2021, 29, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Nordin, A.; Bin Saim, A.; Ramli, R.; Abdul Hamid, A.; Mohd Nasri, N.W.; Bt Hj Idrus, R. Miswak and oral health: An evidence-based review. Saudi J. Biol. Sci. 2020, 27, 1801–1810. [Google Scholar] [CrossRef]
- Farag, M.; Shakour, Z.T.; Lubken, T.; Frolov, A.; Wessjohann, L.A.; Mahrous, E. Unraveling the metabolome composition and its implication for Salvadora persica L. use as dental brush via a multiplex approach of NMR and LC-MS metabolomics. J. Pharm. Biomed. Anal. 2021, 193, 113727. [Google Scholar] [CrossRef]
- Ahangar, P.; Mills, S.J.; Smith, L.E.; Gronthos, S.; Cowin, A.J. Human gingival fibroblast secretome accelerates wound healing through anti-inflammatory and pro-angiogenic mechanisms. NPJ Regen. Med. 2020, 5, 24. [Google Scholar] [CrossRef]
- Ossama, M.; Lamie, C.; Tarek, M.; Wagdy, H.A.; Attia, D.A.; Elmazar, M.M. Management of recurrent aphthous ulcers exploiting polymer-based Muco-adhesive sponges: In-vitro and in-vivo evaluation. Drug Deliv. 2021, 28, 87–99. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, W.; Lei, Y.; Wu, T.; Zhang, S.; Guo, Y.; Liu, Y.; Chen, D.; Yuan, Q.; Wang, Y. Effect of gelatin sponge with colloid silver on bone healing in infected cranial defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70 Pt 1, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Swilam, N.; Nematallah, K.A. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci. Rep. 2020, 10, 14851. [Google Scholar] [CrossRef] [PubMed]
- Duhrkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Bocker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Dührkop, K.; Shen, H.; Meusel, M.; Rousu, J.; Böcker, S. Searching molecular structure databases with tandem mass spectra using CSI:FingerID. Proc. Natl. Acad. Sci. USA 2015, 112, 12580–12585. [Google Scholar] [CrossRef]
- Duhrkop, K.; Nothias, L.F.; Fleischauer, M.; Reher, R.; Ludwig, M.; Hoffmann, M.A.; Petras, D.; Gerwick, W.H.; Rousu, J.; Dorrestein, P.C.; et al. Systematic classification of unknown metabolites using high-resolution fragmentation mass spectra. Nat. Biotechnol. 2021, 39, 462–471. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.; Bokesch, H.; Kenney, S.; Boyd, M. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 423: Acute Oral Toxicity—Acute Toxic Class Method; OECD Publishing: Paris, France, 2002. [Google Scholar] [CrossRef]
- Majumder, R.; Adhikari, L.; Dhara, M.; Sahu, J. Evaluation of anti-inflammatory, analgesic and TNF-alpha inhibition (upon RAW 264.7 cell line) followed by the selection of extract (leaf and stem) with respect to potency to introduce anti-oral-ulcer model obtained from Olax psittacorum (Lam.) Vahl in addition to GC-MS illustration. J. Ethnopharmacol. 2020, 263, 113146. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Fahmy, S.; Choucry, M.A.; Wahdan, M.O.; Elsebai, M.F. Metabolites profiling reveals for antimicrobial compositional differences and action mechanism in the toothbrushing stick “miswak” Salvadora persica. J. Pharm. Biomed. Anal. 2017, 133, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Naqvi, B.; Watson, I.A. Possibility of converting indigenous Salvadora persica L. seed oil into biodiesel in Pakistan. Int. J. Green Energy 2018, 15, 427–435. [Google Scholar] [CrossRef]
- Khalil, A.T. Benzylamides from Salvadora persica. Arch. Pharm. Res. 2006, 29, 952–956. [Google Scholar] [CrossRef]
- Noumi, E.; Hajlaoui, H.; Trabelsi, N.; Ksouri, R.; Bakhrouf, A.; Snoussi, M. Antioxidant activities and RP-HPLC identification of polyphenols in the acetone extract of Salvadora persica. Afr. J. Pharm. Pharmacol. 2011, 5, 966–971. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Al Sahli, A.A.; Alaraidh, I.A.; Al-Homaidan, A.A.; Mostafa, E.M.; El-Gaaly, G.A. Assessment of antioxidant activities in roots of Miswak (Salvadora persica) plants grown at two different locations in Saudi Arabia. Saudi J. Biol. Sci. 2015, 22, 168–175. [Google Scholar] [CrossRef]
- Ohtani, K.; Kasai, R.; Yamasaki, K.; Tanaka, O.; Kamel, M.S.; Assaf, M.H.; El-Shanawani, M.A.; Ali, A.A. Lignan glycosides from stems of Salvadora persica. Phytochemistry 1992, 31, 2469–2471. [Google Scholar] [CrossRef]
- Kumari, A.; Parida, A.K. Metabolite profiling of the leaf extract reveals the antioxidant and nutraceuticals potential of the halophyte Salvadora persica. RSC Adv. 2016, 6, 51629–51641. [Google Scholar] [CrossRef]
- Porter, S.R.; Leao, J.C. Review article: Oral ulcers and its relevance to systemic disorders. Aliment. Pharm. Ther. 2005, 21, 295–306. [Google Scholar] [CrossRef]
- Altenburg, A.; El-Haj, N.; Micheli, C.; Puttkammer, M.; Abdel-Naser, M.B.; Zouboulis, C.C. The treatment of chronic recurrent oral aphthous ulcers. Dtsch. Arztebl. Int. 2014, 111, 665–673. [Google Scholar] [CrossRef]
- Fisher, D.A. Adverse effects of topical corticosteroid use. West. J. Med. 1995, 162, 123–126. [Google Scholar]
- De la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2019. An analysis of FDA drug approvals from the perspective of molecules. Molecules 2020, 25, 745. [Google Scholar] [CrossRef] [PubMed]
- Jassoma, E.; Baeesa, L.; Sabbagh, H. The antiplaque/anticariogenic efficacy of Salvadora persica (Miswak) mouthrinse in comparison to that of chlorhexidine: A systematic review and meta-analysis. BMC Oral Health 2019, 19, 64. [Google Scholar] [CrossRef]
- Mekhemar, M.; Geib, M.; Kumar, M.; Hassan, Y.; Dorfer, C. Salvadora persica: Nature’s gift for periodontal health. Antioxidants 2021, 10, 712. [Google Scholar] [CrossRef]
- Fatima, N.; Iqbal, W.; Yaqeen, S.S. Evaluation of wound healing effects between Salvadora persica ointment and Solcoseryl jelly in animal model. Pak. J. Pharm. Sci. 2015, 28, 1777–1780. [Google Scholar] [PubMed]
- Faruk, E.M.; Nafea, O.E.; Fouad, H.; Ebrahim, U.F.A.; Hasan, R.A.A. Possible healing effects of Salvadora persica extract (MISWAK) and laser therapy in a rabbit model of a caustic-induced tongue ulcers: Histological, immunohistochemical and biochemical study. J. Mol. Histol. 2020, 51, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Brandi, J.; Cheri, S.; Manfredi, M.; Di Carlo, C.; Vita Vanella, V.; Federici, F.; Bombiero, E.; Bazaj, A.; Rizzi, E.; Manna, L.; et al. Exploring the wound healing, anti-inflammatory, anti-pathogenic and proteomic effects of lactic acid bacteria on keratinocytes. Sci. Rep. 2020, 10, 11572. [Google Scholar] [CrossRef]
- Lebda, M.A.; El-Far, A.H.; Noreldin, A.E.; Elewa, Y.H.A.; Al Jaouni, S.K.; Mousa, S.A. Protective effects of Miswak (Salvadora persica) against experimentally induced gastric ulcers in rats. Oxidative Med. Cell. Longev. 2018, 2018, 6703296. [Google Scholar] [CrossRef]
- Ibrahim, A.Y.; El-Gengahi, S.E.; Motawea, H.M.; Sleem, A.M. Anti-Inflammatory activity of Salvadora persica L. against carrageenan induced paw oedema in rat relevant to inflammatory cytokinese. Not. Sci. Biol. 2011, 3, 22–28. [Google Scholar] [CrossRef]
- Niazi, F.H.; Noushad, M.; Tanvir, S.B.; Ali, S.; Al-Khalifa, K.S.; Qamar, Z.; Al-Sheikh, R. Antimicrobial efficacy of indocyanine green-mediated photodynamic therapy compared with Salvadora persica gel application in the treatment of moderate and deep pockets in periodontitis. Photodiagn. Photodyn. Ther. 2020, 29, 101665. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.O.; Valenzuela, C.A.; Baker, E.J.; Miles, E.A.; Rosa Neto, J.C.; Calder, P.C. Palmitoleic acid has stronger anti-Inflammatory potential in human endothelial cells compared to oleic and palmitic acids. Mol. Nutr. Food Res. 2018, 62, e1800322. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Cavia Mdel, M.; Alonso-Torre, S. Role of oleic acid in immune system; mechanism of action; a review. Nutr. Hosp. 2012, 27, 978–990. [Google Scholar] [CrossRef]
- Keche, A.P.; Kamble, V.M. Synthesis and anti-inflammatory and antimicrobial activities of some novel 2-methylquinazolin-4(3H)-one derivatives bearing urea, thiourea and sulphonamide functionalities. Arab. J. Chem. 2019, 12, 1522–1531. [Google Scholar] [CrossRef]
- Sofrata, A.; Santangelo, E.M.; Azeem, M.; Borg-Karlson, A.K.; Gustafsson, A.; Putsep, K. Benzyl isothiocyanate, a major component from the roots of Salvadora persica is highly active against Gram-negative bacteria. PLoS ONE 2011, 6, e23045. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, N.; Takabayashi, S.; Osawa, T.; Nakamura, Y. Benzyl isothiocyanate inhibits excessive superoxide generation in inflammatory leukocytes: Implication for prevention against inflammation-related carcinogenesis. Carcinogenesis 2004, 25, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Kesarwala, A.H.; Krishna, M.C.; Mitchell, J.B. Oxidative stress in oral diseases. Oral Dis. 2016, 22, 9–18. [Google Scholar] [CrossRef]
- Ibrahim, I.I.; Moussa, A.A.; Chen, Z.; Zhang, J.; Cao, W.G.; Yu, C. Bioactive phenolic components and antioxidant activities of water-based extracts and flavonoid-rich fractions from Salvadora persica L. leaves. Nat. Prod. Res. 2021. [Google Scholar] [CrossRef]
- Abd El-Naby, A.S.; El Asely, A.M.; Amin, A.A.; Samir, F.; El-Ashram, A.; Dawood, M.A.O. Miswak (Salvadora persica) modulated the growth performance, antioxidative response, and histopathological damage induced by zinc toxicity in Nile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. Int. 2020, 27, 31918–31932. [Google Scholar] [CrossRef]
- Lebda, M.A.; El-Hawarry, W.N.; Shourbela, R.M.; El-Far, A.H.; Shewita, R.S.; Mousa, S.A. Miswak (Salvadora persica) dietary supplementation improves antioxidant status and nonspecific immunity in Nile tilapia (Oreochromis niloticus). Fish. Shellfish Immunol. 2019, 88, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Kharouf, N.; Haikel, Y.; Ball, V. Polyphenols in dental applications. Bioengineering 2020, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Elagbar, Z.A.; Naik, R.R.; Shakya, A.K.; Bardaweel, S.K. Fatty acids analysis, antioxidant and biological activity of fixed oil of Annona muricata L. seeds. J. Chem. 2016, 2016, 6948098. [Google Scholar] [CrossRef]
- Farag, M.; Abdel-Mageed, W.M.; Basudan, O.; El-Gamal, A. Persicaline, a new antioxidant sulphur-containing imidazoline alkaloid from Salvadora persica roots. Molecules 2018, 23, 483. [Google Scholar] [CrossRef]
- Comino-Sanz, I.M.; Lopez-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The role of antioxidants on wound healing: A review of the current evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef] [PubMed]
- Schinella, G.R.; Tournier, H.A.; Prieto, J.M.; De Buschiazzo, P.M.; Rıos, J.L. Antioxidant activity of anti-inflammatory plant extracts. Life Sci. 2002, 70, 1023–1033. [Google Scholar] [CrossRef]
- Narayanan, A.S.; Page, R.C.; Swanso, J. Collagen synthesis by human fibroblasts. Regulation by transforming growth factor-fl in the presence of other inflammatory mediators. Biochem. J. 1989, 260, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.G.; van Kuppevelt, T.H.; Daamen, W.F.; Kuijpers-Jagtman, A.M.; Von den Hoff, J.W. Tissue reactions to collagen scaffolds in the oral mucosa and skin of rats: Environmental and mechanical factors. Arch. Oral Biol. 2008, 53, 376–387. [Google Scholar] [CrossRef]
- Khunkar, S.; Hariri, I.; Alsayed, E.; Linjawi, A.; Khunkar, S.; Islam, S.; Bakhsh, T.A.; Nakashima, S. Inhibitory effect of Salvadora persica extract (Miswak) on collagen degradation in demineralized dentin: In vitro study. J. Dent. Sci. 2021, 16, 208–213. [Google Scholar] [CrossRef]
- Arslan, Y.E.; Kantarcıoğlu, İ. Salvadora persica extract-laden jellyfish collagen hybrid constructs for periodontal tissue regeneration. J. Turk. Chem. Soc. 2019, 6, 51–62. [Google Scholar] [CrossRef][Green Version]
- Balto, H.A.; Halawany, H.S.; Jacob, V.; Abraham, N.B. The efficacy of Salvadora persica extracts in preserving the viability of human foreskin fibroblasts. Saudi Dent. J. 2015, 27, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Markowicz, M.; Pallua, N.; Noah, E.M.; Steffens, G. The effect of cross-linking of collagen matrices on their angiogenic capability. Biomaterials 2008, 29, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Cucci, L.M.; Satriano, C.; Marzo, T.; La Mendola, D. Angiogenin and copper crossing in wound healing. Int. J. Mol. Sci. 2021, 22, 10704. [Google Scholar] [CrossRef]
- Pan, D.; Gong, X.; Wang, X.; Li, M. Role of active components of medicinal food in the regulation of angiogenesis. Front. Pharm. 2020, 11, 594050. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, S.; Li, L.; Hu, F.; Weng, N.; Fan, X.; Kuang, S. Total favonoids in Caragana (TFC) promotes angiogenesis and enhances cerebral perfusion in a rat model of ischemic stroke. Front. Neurosci. 2018, 12, 635. [Google Scholar] [CrossRef]

| # | Retention Time | Compound | Area% | MS1 (−ve) | MS1 (+ve) | MS2 | Molecular Formula | Error |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.02 | Gallic acid | 1.5 | 169.01417 | 125 | C7H6O5 | 6.04 | |
| 2 | 10.6 | Glutaric acid | 0.43 | 131.042 | --- | C5H8O4 | −1.53 | |
| 3 | 11.16 | Deoxy ellagic acid | 0.67 | 287.0191 | 241, 181, 151 | C14H8O7 | −2.08 | |
| 4 | 13.97 | Hydroxy stachrydine | tr | 158.0822 | 141, 131, 115 | C7H13O3N | −0.6 | |
| 5 | 16.17 | Sugar derivative | 0.43 | 267.1081 | 221, 153 | C10H20O8 | −0.41 | |
| 6 | 21.2 | Methoxy ellagic acid | 0.3 | 315.01459 | 241, 181, 151 | C15H8O8 | −0.16 | |
| 7 | 22.38 | Benzamide | 1.13 | 122.0599 | 105 | C7H7NO | −1.15 | |
| 8 | 23.95 | Salicylic acid | 0.73 | 137.0231 | --- | C7H6O3 | −1.28 | |
| 9 | 24.69 | Diethyl malate | 0.7 | 189.0764 | 145, 100 | C8H14O5 | −0.46 | |
| 10 | 25.4 | Sulfated hexosyl phenolic derivative | 0.22 | 395.0676 | 315, 241, 153 | C14H20O11S | 2.22 | |
| 11 | 27.067 | Methylbenzamide | 0.32 | 136.0747 | --- | C8H9NO | −7.27 | |
| 12 | 27.41 | Unknown | 1.43 | 194.0809 | 164, 134 | C10H13NO3 | −1.31 | |
| 13 | 28.111 | Benzyl urea | 2.52 | 151.0857 | --- | C8H10N2O | 5.89 | |
| 14 | 31.31 | O-benzyl hexosyl sulfate | 0.42 | 349.0585 | 269, 241, 193 | C13H18O9S | −3.94 | |
| 15 | 32.83 | Unknown | 0.35 | 521.2331 (2M − H) | 260 | C11H19NO6 | −2.97 | |
| 16 | 34.32 | Phenolic acid derivative | 0.14 | 281.13909 | 151 | C15H22O5 | −1.27 | |
| 17 | 34.36 | Di-O-methyl ellagic acid | 0.98 | 329.032 | 315, 241, 181, 151 | C16H10O8 | 5.19 | |
| 18 | 35.902 | Benzyl isothiocyanate | 8.7 | 150.0374 | --- | C8H7NS | −5.95 | |
| 19 | 37.29 | Coumaric acid | tr | 163.03897 | 119 | C9H8O3 | −1.54 | |
| 20 | 38.88 | Acetyl Phenyl alanine | tr | 413.16868 (2M − H) | 415.1810 (2M + H) | 206, 188 | C11H13NO3 | −7.58 |
| 21 | 47.645 | Unknown | 1.53 | 123.0431 | --- | C5H4N3O | 3.15 | |
| 22 | 56.8 | Ferulic acid | 0.39 | 193.0492 | 179, 149 | C10H10O4 | −1.74 | |
| 23 | 57.55 | Methoxy flavanone hexosyl rhamnoside | 0.58 | 609.18129 | 463, 301 | C28H34O15 | −1.98 | |
| 24 | 59.78 | Caffeic acid conjugate | 0.13 | 387.0352 | 341, 193 | C18H12O10 | −3.41 | |
| 25 | 60.399 | N-benzyl-N′ hydroxy benzyl urea | 0.09 | 257.1269 | 241, 198, 181, 163 | C8H10N2O2 | −6.03 | |
| 26 | 66.42 | Caffeic acid conjugate | 0.07 | 377.18179 | 341, 161 | C17H30O9 | 1.67 | |
| 27 | 67.61 | Syringin | 0.15 | 371.1344 | 209 | C17H24O9 | 0.51 | |
| 28 | 70.88 | N-benzyl benzamide | 0.15 | 212.1057 | --- | C14H13NO | −6.08 | |
| 29 | 71.663 | N-benzyl 2-phenyl acetamide | 0.2 | 226.1215 | --- | C15H15NO | −5.04 | |
| 30 | 71.7 | N,N′ dibenzyl urea | 7.73 | 241.1326 | 181, 163, 108 | C15H18N2O | −3.9 | |
| 31 | 74.819 | Unknown | 0.06 | 353.1957 | --- | C19H28O6 | −1.94 | |
| 32 | 75.37 | Sulfur compound derivative | 0.61 | 281.0402 | 186 | C12H12N2O2S2 | −7.8 | |
| 33 | 92.482 | Hydroxy tetradecanoic acid | 3.94 | 487.4005 (2M − H) | 243 | C14H28O3 | 1.31 | |
| 34 | 102.79 | Hydroxy hexadecanoic acid | 0.69 | 543.4565 (2M − H) | 271.2266 | C16H32O3 | −0.63 | |
| 35 | 106.09 | Linolenic acid | 1.49 | 555.4408 (2M − H) | 557.4496 (2M + H) | 277 | C18H30O2 | −2.01 |
| 36 | 107.327 | Myristic acid | 0.45 | 455.4111 (2M − H) | 227 | C14H28O2 | 1.19 | |
| 37 | 108.559 | Hydroxy octadecenoic acid | 0.99 | 595.4890 (2M − H) | 297 | C18H34O3 | −8.92 | |
| 38 | 109.176 | Hexadecenoic acid | 0.95 | 507.4416 (2M − H) | 509.4512 (2M + H) | 253 | C16H30O2 | −0.64 |
| 39 | 109.35 | Unknown | 3.09 | 339.2299 | 253, 113 | C23H32O2 | −3.05 | |
| 40 | 111.332 | Arachidic acid | 0.12 | 313.2727 | 285, 267 | C20H42O2 | 4.52 | |
| 41 | 111.527 | Linoleic acid | 2.43 | 559.4781 (2M − H) | 561.4821 (2M + H) | 279 | C18H32O2 | −5.52 |
| 42 | 111.552 | Fatty acid amide derivative | 0.45 | 635.5489 (2M + H) | 318 | C21H35NO | −1.89 | |
| 43 | 113.309 | Fatty acid amide derivative | 0.88 | 687.5803 (2M + H) | 344 | C23H37NO | −4.5 | |
| 44 | 113.598 | Heptadecenoic acid | 0.29 | 535.4730 (2M − H) | 537.4845 (2M + H) | 267 | C17H32O2 | −0.36 |
| 45 | 115.622 | Hydroxy octadecanoic acid | 0.54 | 599.5241 (2M − H) | 299 | C18H36O3 | −2.59 | |
| 46 | 116.188 | Palmitic acid | 4.98 | 511.4714 (2M − H) | 513.4861 (2M + H) | 255 | C16H32O2 | −2.42 |
| 47 | 116.23 | Cholesterol derivative | tr | 663.4529 | 607, 551 | C39H58N4O5 | 5.74 | |
| 48 | 117.307 | Oleic acid | 4.71 | 563.5026 (2M − H) | 565.5175 (2M + H) | 281 | C18H34O2 | −2.38 |
| 49 | 118.394 | N-benzylpalmitamide | 0.06 | 691.6144 (2M + H) | 346 | C23H39NO | −0.42 | |
| 50 | 120.34 | N-benzyl octadecenamide | 1.79 | 743.6433 (2M + H) | 372 | C25H41NO | −0.05 | |
| 51 | 121.418 | Nonadecenoic acid | 0.26 | 591.5330 (2M − H) | 295 | C19H36O2 | −3.79 | |
| 52 | 122.592 | N-benzyl heptadecanamide | 0.1 | 719.6405 (2M + H) | 360 | C24H41NO | 3.63 | |
| 53 | 123.163 | Hydroxy eicosanoic acid | 0.73 | 655.5859 (2M − H) | 327 | C20H40O3 | −2.7 | |
| 54 | 123.505 | Stearic acid | 0.31 | 567.5334 (2M − H) | 283 | C18H36O2 | −3.24 | |
| 55 | 123.666 | Diisooctyl phthalate | 1.2 | 391.2832 | 167, 149 | C24H38O4 | −5.58 | |
| 56 | 125.314 | 13-Docosenamide | 1.44 | 338.3416 | 321 | C22H43NO | −0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoub, N.; Badr, N.; Al-Ghamdi, S.S.; Alsanosi, S.; Alzahrani, A.R.; Abdel-Naim, A.B.; Nematallah, K.A.; Swilam, N. HPLC/MSn Profiling and Healing Activity of a Muco-Adhesive Formula of Salvadora persica against Acetic Acid-Induced Oral Ulcer in Rats. Nutrients 2022, 14, 28. https://doi.org/10.3390/nu14010028
Ayoub N, Badr N, Al-Ghamdi SS, Alsanosi S, Alzahrani AR, Abdel-Naim AB, Nematallah KA, Swilam N. HPLC/MSn Profiling and Healing Activity of a Muco-Adhesive Formula of Salvadora persica against Acetic Acid-Induced Oral Ulcer in Rats. Nutrients. 2022; 14(1):28. https://doi.org/10.3390/nu14010028
Chicago/Turabian StyleAyoub, Nahla, Nadia Badr, Saeed S. Al-Ghamdi, Safaa Alsanosi, Abdullah R. Alzahrani, Ashraf B. Abdel-Naim, Khaled A. Nematallah, and Noha Swilam. 2022. "HPLC/MSn Profiling and Healing Activity of a Muco-Adhesive Formula of Salvadora persica against Acetic Acid-Induced Oral Ulcer in Rats" Nutrients 14, no. 1: 28. https://doi.org/10.3390/nu14010028
APA StyleAyoub, N., Badr, N., Al-Ghamdi, S. S., Alsanosi, S., Alzahrani, A. R., Abdel-Naim, A. B., Nematallah, K. A., & Swilam, N. (2022). HPLC/MSn Profiling and Healing Activity of a Muco-Adhesive Formula of Salvadora persica against Acetic Acid-Induced Oral Ulcer in Rats. Nutrients, 14(1), 28. https://doi.org/10.3390/nu14010028






