The Cost-Effectiveness of Supplemental Carnosine in Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model
2.2. Model Population
2.3. Mortality Risk
2.4. The Efficacy of Supplemental Carnosine in People with Type 2 Diabetes
2.5. Utilities
2.6. Costs
2.7. Outcomes
2.8. Scenario and Sensitivity Analyses
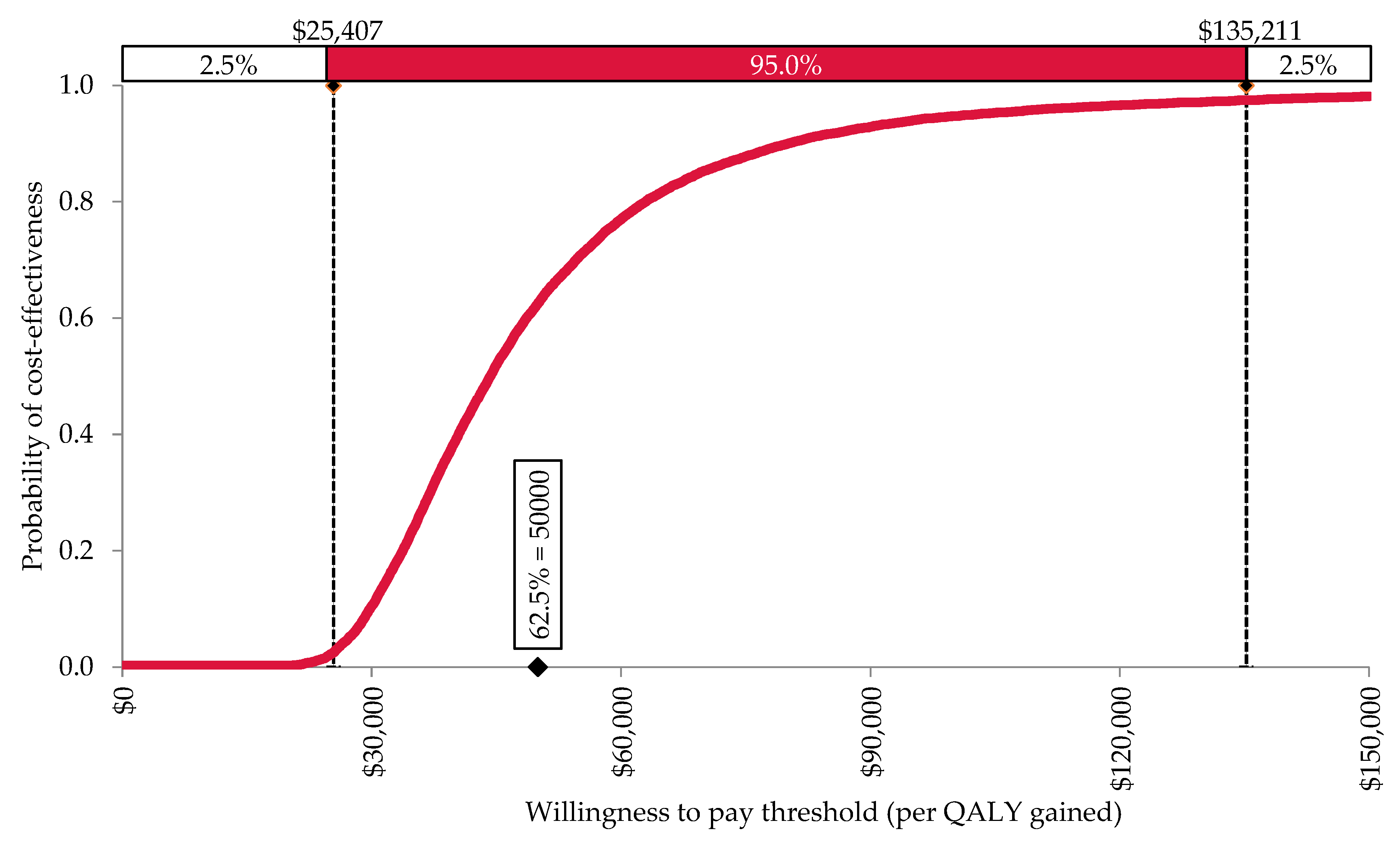
3. Results
3.1. Base Case
3.2. Scenario Analyses
3.3. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDF. IDF Diabetes Atlas 2021—10th ed. Available online: https://diabetesatlas.org (accessed on 5 October 2021).
- AIHW. Diabetes. In Vol Cat.no.CVD 82; Australian Institute of Health and Welfare (AIHW): Canberra, Australia, 2020. [Google Scholar]
- Zhang, P.; Zhang, X.; Brown, J.; Vistisen, D.; Sicree, R.; Shaw, J.; Nichols, G. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Aldini, G.; Orioli, M.; Rossoni, G.; Savi, F.; Braidotti, P.; Vistoli, G.; Yeum, K.J.; Negrisoli, G.; Carini, M. The carbonyl scavenger carnosine ameliorates dyslipidaemia and renal function in Zucker obese rats. J. Cell. Mol. Med. 2010, 15, 1339–1354. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Hsu, C.-C.; Lin, M.-H.; Liu, K.-S.; Yin, M.-C. Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur. J. Pharmacol. 2005, 513, 145–150. [Google Scholar] [CrossRef]
- De Courten, B.; Jakubova, M.; De Courten, M.P.; Kukurova, I.J.; Vallova, S.; Krumpolec, P.; Valkovič, L.; Kurdiova, T.; Garzon, D.; Barbaresi, S.; et al. Effects of carnosine supplementation on glucose metabolism: Pilot clinical trial. Obesity 2016, 24, 1027–1034. [Google Scholar] [CrossRef]
- Houjeghani, S.; Kheirouri, S.; Faraji, E.; Jafarabadi, M.A. l-Carnosine supplementation attenuated fasting glucose, triglycerides, advanced glycation end products, and tumor necrosis factor– α levels in patients with type 2 diabetes: A double-blind placebo-controlled randomized clinical trial. Nutr. Res. 2018, 49, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Menon, K.; Marquina, C.; Liew, D.; Mousa, A.; De Courten, B. Histidine-containing dipeptides reduce central obesity and improve glycaemic outcomes: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2020, 21, e12975. [Google Scholar] [CrossRef]
- Schön, M.; Mousa, A.; Berk, M.; Chia, W.L.; Ukropec, J.; Majid, A.; Ukropcová, B.; De Courten, B. The potential of carnosine in brain-related disorders: A comprehensive review of current evidence. Nutrients 2019, 11, 1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baye, E.; Ukropcova, B.; Ukropec, J.; Hipkiss, A.; Aldini, G.; De Courten, B. Physiological and therapeutic effects of carnosine on cardiometabolic risk and disease. Amino Acids 2016, 48, 1131–1149. [Google Scholar] [CrossRef]
- Menon, K.; de Courten, B.; Liew, D.; Ademi, Z.; Owen, A.J.; Magliano, D.J.; Zomer, E. Productivity benefits of preventing type 2 diabetes in Australia: A 10-year analysis. Diabetes Care 2021, 44, 715–721. [Google Scholar] [CrossRef]
- Lilford, R.J.; Pauker, S.G.; Braunholtz, A.D.; Chard, J. Getting research findings into practice: Decision analysis and the implementation of research findings. BMJ 1998, 317, 405–409. [Google Scholar] [CrossRef]
- Diabetes Australia. National Diabetes Services Scheme. Available online: https://www.ndss.com.au/about-the-ndss/ (accessed on 5 October 2021).
- ABS. Population Projections, Australia, 2017 (Base)—2066, Cat No 3222.0; Australian Bureau of Statistics (ABS): Canberra, Australia, 2018.
- ABS. Deaths, Australia, 2014, Cat No 3302.0; Australian Bureau of Statistics (ABS): Canberra, Australia, 2014.
- Stratton, I.M.; Adler, I.A.; Neil, H.A.W.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef] [Green Version]
- McCaffrey, N.; Kaambwa, B.; Currow, D.C.; Ratcliffe, J. Health-related quality of life measured using the EQ-5D–5L: South Australian population norms. Heal. Qual. Life Outcomes 2016, 14, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Brown, M.B.; Bilik, D.; Ackermann, R.T.; Li, R.; Herman, W.H. Health utility scores for people with type 2 diabetes in U.S. managed care health plans: Results from Translating Research into Action for Diabetes (TRIAD). Diabetes Care 2012, 35, 2250–2256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.M.Y.; Colagiuri, R.; Magliano, D.; Cameron, A.; Shaw, J.; Zimmet, P.; Colagiuri, S. The cost of diabetes in adults in Australia. Diabetes Res. Clin. Pract. 2013, 99, 385–390. [Google Scholar] [CrossRef]
- AIHW. Health Expenditure Australia 2017–18; Australian Bureau of Statistics (ABS): Canberra, Australia, 2019.
- iHerb. 2020. Available online: Au.iherb.com (accessed on 5 October 2021).
- Henry, D.A.; Hill, S.R.; Harris, A. Drug prices and value for money. JAMA 2005, 294, 2630–2632. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gum, D.; Merlin, T. Comparing the ICERs in medicine reimbursement submissions to NICE and PBAC—Does the presence of an explicit threshold affect the ICER proposed? Value Health 2018, 21, 938–943. [Google Scholar] [CrossRef] [Green Version]
- Dubois, R.W. Cost–effectiveness thresholds in the USA: Are they coming? Are they already here? J. Comp. Eff. Res. 2016, 5, 9–12. [Google Scholar] [CrossRef] [Green Version]
- World Bank. Available online: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=AU (accessed on 21 July 2020).
- Attema, A.E.; Brouwer, W.; Claxton, K. Discounting in economic evaluations. PharmacoEconomics 2018, 36, 745–758. [Google Scholar] [CrossRef] [Green Version]
- Briggs, A.; Sculpher, M.; Claxton, K. Decision Modelling for Health Economic Evaluation; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- González-Rodríguez, A.; Santamaría, B.; Mas-Gutierrez, J.A.; Rada, P.; Millán, E.F.; Pardo, V.; Álvarez, C.; Cuadrado, A.; Ros, M.; Serrano, M.; et al. Resveratrol treatment restores peripheral insulin sensitivity in diabetic mice in a sirt1-independent manner. Mol. Nutr. Food Res. 2015, 59, 1431–1442. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, F.; Ge, X.; Yan, T.; Chen, X.; Shi, X.; Zhai, Q. SIRT1 Improves Insulin Sensitivity under Insulin-Resistant Conditions by Repressing PTP1B. Cell Metab. 2007, 6, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Zhou, R.; Wang, B.; Mi, M.-T. Effect of resveratrol on glucose control and insulin sensitivity: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2014, 99, 1510–1519. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Wu, C.; Qiu, S.; Yuan, X.; Li, L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: Systematic review and meta-analysis. Nutr. Metab. 2017, 14, 60. [Google Scholar] [CrossRef]
- Hausenblas, H.A.; Schoulda, J.A.; Smoliga, J.M. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus-systematic review and meta-analysis. Mol. Nutr. Food Res. 2014, 59, 147–159. [Google Scholar] [CrossRef]
- Zhang, T.; He, Q.; Liu, Y.; Chen, Z.; Hu, H. Efficacy and safety of resveratrol supplements on blood lipid and blood glucose control in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Evid. Based Complement. Altern. Med. 2021, 2021, 5644171. [Google Scholar] [CrossRef] [PubMed]
- Abdelhaleem, I.A.; Brakat, A.M.; Adayel, H.M.; Asla, M.M.; Rizk, M.A.; Aboalfetoh, A.Y. The effects of resveratrol on glycemic control and cardiometabolic parameters in patients with T2DM: A systematic review and meta-analysis. Med. Clín. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Zeraattalab-Motlagh, S.; Jayedi, A.; Shab-Bidar, S. The effects of resveratrol supplementation in patients with type 2 diabetes, metabolic syndrome, and nonalcoholic fatty liver disease: An umbrella review of meta-analyses of randomized controlled trials. Am. J. Clin. Nutr. 2021, 114, 1675–1685. [Google Scholar] [CrossRef]
- Taylor, C.; Jan, S. Economic evaluation of medicines. Aust. Prescr. 2017, 40, 76–78. [Google Scholar] [CrossRef] [Green Version]
- Roche Products Pty Limited. Access to Oncology Medicines in Australia Roche Response to Medicines Australia Oncology Industry Taskforce Report. 2013. Available online: https://medicinesaustralia.com.au/wp-con-tent/uploads/sites/52/2013/07/131021_OIT_Roche_response_FINAL_.pdf (accessed on 1 August 2020).
- Edney, L.C.; Afzali, H.H.A.; Cheng, T.C.; Karnon, J. Estimating the reference incremental cost-effectiveness ratio for the Australian health system. Pharmacoeconomics 2017, 36, 239–252. [Google Scholar] [CrossRef]
- Sherifali, D.; Nerenberg, K.; Pullenayegum, E.; Cheng, J.E.; Gerstein, H.C. The effect of oral antidiabetic agents on A1C levels: A systematic review and meta-analysis. Diabetes Care 2010, 33, 1859–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbarbary, N.S.; Ismail, E.A.R.; El-Naggar, A.R.; Hamouda, M.H.; El-Hamamsy, M. The effect of 12 weeks carnosine supplementation on renal functional integrity and oxidative stress in pediatric patients with diabetic nephropathy: A randomized placebo-controlled trial. Pediatr. Diabetes 2017, 19, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Magliano, D.J.; Rancière, F.; Harding, J.L.; Nanayakkara, N.; Shaw, J.E.; Carstensen, B. Impact of age at diagnosis and duration of type 2 diabetes on mortality in Australia 1997–2011. Diabetologia 2018, 61, 1055–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Parameter | Base Case | Lower Limit 95% CI | Upper Limit 95% CI | Distribution for PSA | Source |
|---|---|---|---|---|---|
| Utilities | |||||
| No type 2 diabetes | see Table S1 | Beta | McCaffrey et al. [18] | ||
| With type 2 diabetes | see Table S1 | Beta | Zhang et al. [19] | ||
| Disease costs | |||||
| No type 2 diabetes | $1932 | $1795 | $2071 | Gamma | Lee et al. [20] |
| With type 2 diabetes | $4190 | $3268 | $5110 | Gamma | Lee et al. [20] |
| Treatment costs | |||||
| Annual carnosine cost * | $423.40 | $211.70 | $635.10 | Fixed | iHerb [22] |
| Carnosine treatment effect | |||||
| Reduction in HbA1c (%) | 0.76 | 0.24 | 1.29 | Normal | Menon et al. [9] |
| HbA1c and all-cause mortality | |||||
| Reduction in HbA1c and all-cause mortality (%) | 14.00 | 9.00 | 19.00 | Normal | Stratton et al. [17] |
| Parameter | Standard Care Only | Standard Care + Carnosine | Difference |
|---|---|---|---|
| Clinical parameters | |||
| Total years of life lived | 172,083,114 | 172,227,026 | 143,913 |
| Total QALYs | 155,139,783 | 155,255,645 | 115,862 |
| Costs parameters | |||
| Disease costs | $355,690,912,502 | $356,293,906,561 | $602,994,059 |
| Treatment costs | $0 | $4,410,289,017 | $4,410,289,017 |
| Total healthcare costs | $355,690,912,502 | $360,704,195,577 | $5,013,283,075 |
| Incremental cost-effectiveness ratios | |||
| Costs per YoLS | $34,836 | ||
| Costs per QALY | $43,270 | ||
| Base Case Values | Scenario/ Variation | Upper and Lower Bound Values | ICER | ||
|---|---|---|---|---|---|
| Cost per YoLS | Cost per QALY | ||||
| Base Case * | $34,836 | $43,270 | |||
| Scenario analysis | |||||
| Discounting | 5% | 3% | $33,946 | $42,165 | |
| 0% | $32,679 | $40,592 | |||
| Time horizon | 10 years | 5 years | $58,595 | $72,764 | |
| Effect of carnosine on HbA1c | 0.76% (8.3 mmol/mol) | 0.6% (6.6 mmol/mol) | $43,019 | $53,434 | |
| Resveratrol versus standard care | $117,612 | $146,089 | |||
| Carnosine versus resveratrol | Dominant | Dominant | |||
| Sensitivity analysis | |||||
| Utility (no diabetes population) † | see Table S1 | 95% CI | see Table S1 | $34,836 | $43,270 |
| $34,836 | $43,270 | ||||
| Utility (diabetes population) | see Table S1 | 95% CI | see Table S1 | $34,836 | $43,752 |
| $34,836 | $42,796 | ||||
| Healthcare costs (no diabetes population) † | $1932 | 95% CI | Lower bound: $1795 | $34,836 | $43,270 |
| Upper bound: $2071 | $34,836 | $43,270 | |||
| Healthcare costs (diabetes population) | $4190 | 95% CI | Lower bound: $3268 | $33,914 | $42,124 |
| Upper bound: $5110 | $35,756 | $44,412 | |||
| Carnosine cost | $423.40 | ±50% | Lower bound: $211.70 | $19,513 | $24,237 |
| Upper bound: $635.10 | $50,158 | $62,302 | |||
| Effect of carnosine on HbA1c | 0.76% | 95% CI | Lower bound: 0.24% | $101,323 | $125,858 |
| Upper bound: 1.29% | $22,228 | $27,609 | |||
| Mortality reduction for every 1% reduction in HbA1c | 14% | 95% CI | Lower bound: 9.00% | $51,884 | $64,446 |
| Upper bound: 19.00% | $26,760 | $33,239 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menon, K.; de Courten, B.; Magliano, D.J.; Ademi, Z.; Liew, D.; Zomer, E. The Cost-Effectiveness of Supplemental Carnosine in Type 2 Diabetes. Nutrients 2022, 14, 215. https://doi.org/10.3390/nu14010215
Menon K, de Courten B, Magliano DJ, Ademi Z, Liew D, Zomer E. The Cost-Effectiveness of Supplemental Carnosine in Type 2 Diabetes. Nutrients. 2022; 14(1):215. https://doi.org/10.3390/nu14010215
Chicago/Turabian StyleMenon, Kirthi, Barbora de Courten, Dianna J. Magliano, Zanfina Ademi, Danny Liew, and Ella Zomer. 2022. "The Cost-Effectiveness of Supplemental Carnosine in Type 2 Diabetes" Nutrients 14, no. 1: 215. https://doi.org/10.3390/nu14010215
APA StyleMenon, K., de Courten, B., Magliano, D. J., Ademi, Z., Liew, D., & Zomer, E. (2022). The Cost-Effectiveness of Supplemental Carnosine in Type 2 Diabetes. Nutrients, 14(1), 215. https://doi.org/10.3390/nu14010215







