Essentiality of Trace Elements in Pregnancy, Fertility, and Gynecologic Cancers—A State-of-the-Art Review
Abstract
:1. Introduction
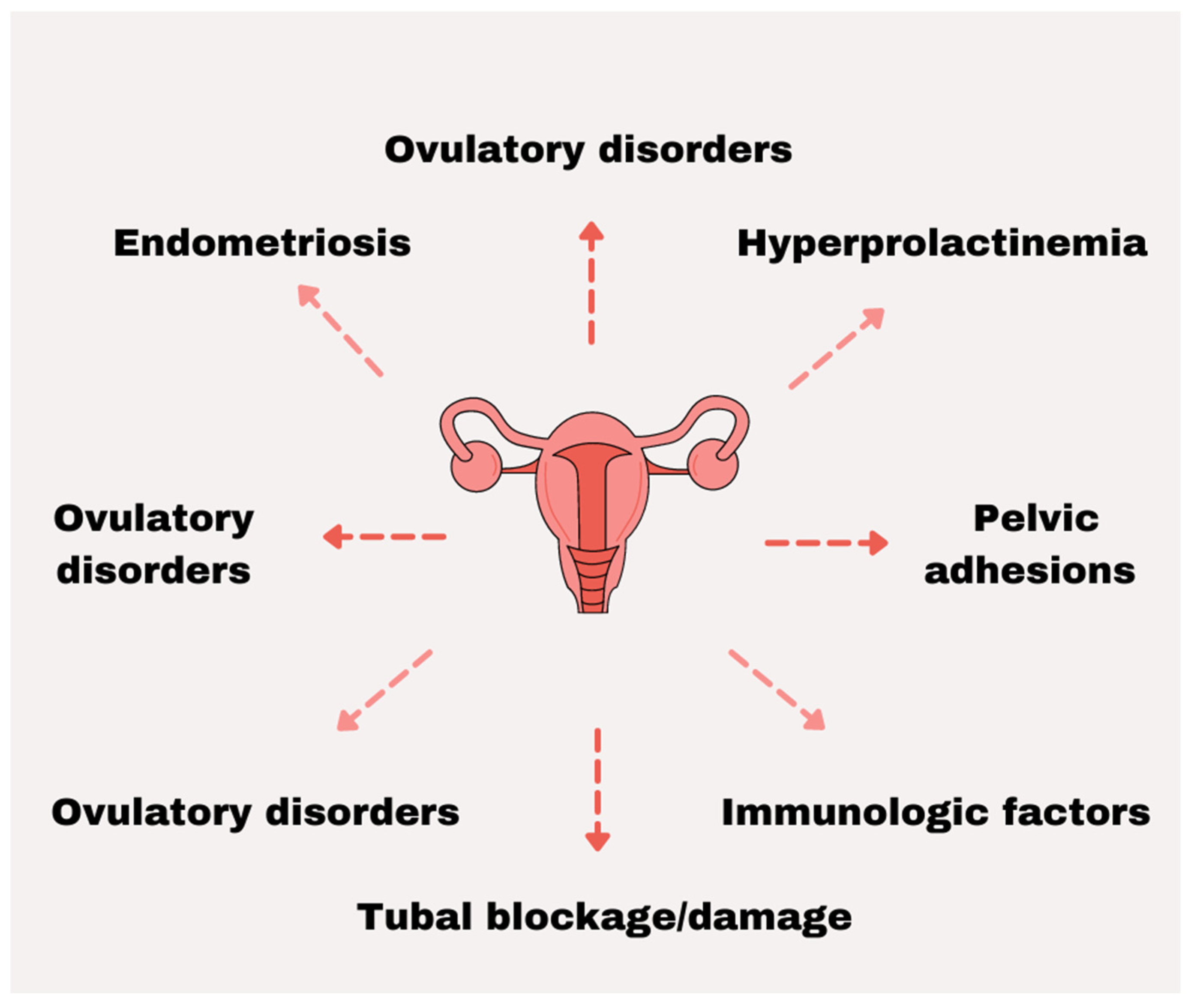
2. Female Infertility
3. Most Prevalent Cancers of the Female Reproductive System
3.1. Cervical Cancer
3.2. Ovarian Cancer
3.3. Corpus Uteri Cancer
3.4. Vaginal Cancer
3.5. Vulvar Cancer
4. Trace Elements and Female Reproductive Organs
4.1. Iron
Pregnancy/Fertility
4.2. Zinc
4.3. Copper
4.4. Fluoride
4.5. Selenium
4.6. Chromium
4.7. Iodine
4.8. Manganese
4.9. Aluminum
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vollenhoven, B.; Hunt, S. Ovarian ageing and the impact on female fertility. F1000Research 2018, 7, 1835. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and The Practice Committee of the American Society for Reproductive Medicine. Female age-related fertility decline. Committee Opinion No. 589. Fertil. Steril. 2014, 101, 633–634. [Google Scholar] [CrossRef]
- Baird, D.T.; Collins, J.; Egozcue, J.; Evers, L.H.; Gianaroli, L.; Leridon, H.; Sunde, A.; Templeton, A.; Van Steirteghem, A.; Cohen, J.; et al. Fertility and ageing. Hum. Reprod. Update 2005, 11, 261–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faddy, M.J.; Gosden, R.G.; Gougeon, A.; Richardson, S.J.; Nelson, J.F. Accelerated disappearance of ovarian follicles in mid-life: Implications for forecasting menopause. Hum. Reprod. 1992, 7, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Block, E. Quantitative morphological investigations of the follicular system in women; variations at different ages. Acta Anat. 1952, 14, 108–123. [Google Scholar] [CrossRef]
- Balasch, J.; Gratacos, E. Delayed childbearing: Effects on fertility and the outcome of pregnancy. Curr. Opin. Obstet. Gynecol. 2012, 24, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Brazal, S.; Rodriguez, A.; Prat, A.; Vassena, R. Knowledge of age-related fertility decline in women: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 230, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Ahmed, S.M.; El-Gammal, Z.; Shouman, S.; Ahmed, A.; Mansour, R.; El-Badri, N. Oocyte Aging: The Role of Cellular and Environmental Factors and Impact on Female Fertility. Adv. Exp. Med. Biol. 2020, 1247, 109–123. [Google Scholar] [CrossRef]
- Jungheim, E.S.; Moley, K.H. Current knowledge of obesity’s effects in the pre- and periconceptional periods and avenues for future research. Am. J. Obstet. Gynecol. 2010, 203, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Pedro, J.; Brandão, T.; Schmidt, L.; Costa, M.E.; Martins, M.V. What do people know about fertility? A systematic review on fertility awareness and its associated factors. Ups. J. Med. Sci. 2018, 123, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.A.M.; Killick, S.R. Negative lifestyle is associated with a significant reduction in fecundity. Fertil. Steril. 2004, 81, 384–392. [Google Scholar] [CrossRef]
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer in Women: Burden and Trends. Cancer Epidem. Biomar. 2017, 26, 444–457. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Gultekin, M.; Ak, S.; Ayhan, A.; Strojna, A.; Pletnev, A.; Fagotti, A.; Perrone, A.M.; Erzeneoglu, B.E.; Temiz, B.E.; Lemley, B.; et al. Perspectives, fears and expectations of patients with gynaecological cancers during the COVID-19 pandemic: A Pan-European study of the European Network of Gynaecological Cancer Advocacy Groups (ENGAGe). Cancer Med. 2021, 10, 208–219. [Google Scholar] [CrossRef]
- Wada, O. What are trace elements? Their deficiency and excess states. J. Jpn. Med. Assoc. 2004, 47, 351–358. [Google Scholar]
- Schomburg, L. Selenium Deficiency Due to Diet, Pregnancy, Severe Illness, or COVID-19-A Preventable Trigger for Autoimmune Disease. Int. J. Mol. Sci. 2021, 22, 8532. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R.; Negrouk, V.; Perera, M.; Llabre, M.M.; Ricordi, C.; Rongioletti, M.C.A.; Mendez, A.J. Serum copper profile in patients with type 1 diabetes in comparison to other metals. J. Trace Elem. Med. Biol. 2019, 56, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Tako, E. Dietary Trace Minerals. Nutrients 2019, 11, 2823. [Google Scholar] [CrossRef] [Green Version]
- Jurowski, K.; Szewczyk, B.; Nowak, G.; Piekoszewski, W. Biological consequences of zinc deficiency in the pathomechanisms of selected diseases. J. Biol. Inorg. Chem. 2014, 19, 1069–1079. [Google Scholar] [CrossRef] [Green Version]
- Candilish, D. Minerals. J. Am. Coll. Nutr. 2000, 17, 286–310. [Google Scholar]
- Weitzel, L.-R.B.; Mayles, W.J.; Sandoval, P.A.; Wischmeyer, P.E. Effects of pharmaconutrients on cellular dysfunction and the microcirculation in critical illness. Curr. Opin. Anesthesiol. 2009, 22, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Mirnamniha, M.; Faroughi, F.; Tahmasbpour, E.; Ebrahimi, P.; Beigi Harchegani, A. An overview on role of some trace elements in human reproductive health, sperm function and fertilization process. Rev. Environ. Health 2019, 34, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Grieger, J.A.; Grzeskowiak, L.E.; Wilson, R.L.; Bianco-Miotto, T.; Leemaqz, S.Y.; Jankovic-Karasoulos, T.; Perkins, A.V.; Norman, R.J.; Dekker, G.A.; Roberts, C.T. Maternal Selenium, Copper and Zinc Concentrations in Early Pregnancy, and the Association with Fertility. Nutrients 2019, 11, 1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, B.H.; Vanderlelie, J.J.; Perkins, A.V. Essentiality of Trace Element Micronutrition in Human Pregnancy: A Systematic Review. J. Preg. Child Health 2015, 2, 157. [Google Scholar] [CrossRef] [Green Version]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Tuschl, K.; Mills, P.B.; Clayton, P.T. Manganese and the brain. Int. Rev. Neurobiol. 2013, 110, 277–312. [Google Scholar] [CrossRef]
- Noli, F.; Tsamos, P. Concentration of heavy metals and trace elements in soils, waters and vegetables and assessment of health risk in the vicinity of a lignite-fired power plant. Sci. Total Environ. 2016, 563–564, 377–385. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]
- ATSDR. Chromium (Cr) Toxicity: What Are the Physiologic Effects of Chromium Exposure? Environmental Medicine ATSDR. 2021. Available online: https://www.atsdr.cdc.gov/csem/chromium/physiologic_effects_of_chromium_exposure.html (accessed on 24 November 2021).
- Davies, J.M. Lung cancer mortality among workers making lead chromate and zinc chromate pigments at three English factories. Br. J. Ind. Med. 1984, 41, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Yang, H. Ovarian cancer risk according to circulating zinc and copper concentrations: A meta-analysis and Mendelian randomization study. Clin. Nutr. 2021, 40, 2464–2468. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wang, Z.; Huang, C.; Fang, X.; Chen, D. Serum Selenium Levels and Cervical Cancer: Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2017, 179, 195–202. [Google Scholar] [CrossRef]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Hum. Reprod. 2017, 32, 1786–1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Med. 2012, 9, e1001356. [Google Scholar] [CrossRef] [Green Version]
- Starc, A.; Trampus, M.; Pavan Jukic, D.; Rotim, C.; Jukic, T.; Polona Mivsek, A. Infertility and Sexual Dysfunctions: A Systematic Literature Review. Acta Clin. Croat. 2019, 58, 508–515. [Google Scholar] [CrossRef]
- Hanson, B.; Johnstone, E.; Dorais, J.; Silver, B.; Peterson, C.M.; Hotaling, J. Female infertility, infertility-associated diagnoses, and comorbidities: A review. J. Assist. Reprod. Genet. 2017, 34, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Deroux, A.; Dumestre-Perard, C.; Dunand-Faure, C.; Bouillet, L.; Hoffmann, P. Female Infertility and Serum Auto-antibodies: A Systematic Review. Clin. Rev. Allergy Immunol. 2017, 53, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.J. Physiological Aspects of Female Fertility: Role of the Environment, Modern Lifestyle, and Genetics. Physiol. Rev. 2016, 96, 873–909. [Google Scholar] [CrossRef] [PubMed]
- Tersigni, C.; Castellani, R.; de Waure, C.; Fattorossi, A.; De Spirito, M.; Gasbarrini, A.; Scambia, G.; Di Simone, N. Celiac disease and reproductive disorders: Meta-analysis of epidemiologic associations and potential pathogenic mechanisms. Hum. Reprod. Update 2014, 20, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Carp, H.J.; Selmi, C.; Shoenfeld, Y. The autoimmune bases of infertility and pregnancy loss. J. Autoimmun. 2012, 38, J266–J274. [Google Scholar] [CrossRef] [PubMed]
- Vissenberg, R.; Manders, V.D.; Mastenbroek, S.; Fliers, E.; Afink, G.B.; Ris-Stalpers, C.; Goddijn, M.; Bisschop, P.H. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum. Reprod. Update 2015, 21, 378–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashiri, A.; Halper, K.I.; Orvieto, R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod. Biol. Endocrinol. 2018, 16, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasubramaniam, S.D.; Balakrishnan, V.; Oon, C.E.; Kaur, G. Key Molecular Events in Cervical Cancer Development. Medicina 2019, 55, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human papillomavirus and cervical cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef]
- Manini, I.; Montomoli, E. Epidemiology and prevention of Human Papillomavirus. Ann. Ig. 2018, 30, 28–32. [Google Scholar] [CrossRef]
- Fowler, J.R.; Maani, E.V.; Jack, B.W. Cervical Cancer. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Roura, E.; Castellsagué, X.; Pawlita, M.; Travier, N.; Waterboer, T.; Margall, N.; Bosch, F.X.; de Sanjosé, S.; Dillner, J.; Gram, I.T.; et al. Smoking as a major risk factor for cervical cancer and pre-cancer: Results from the EPIC cohort. Int. J. Cancer 2014, 135, 453–466. [Google Scholar] [CrossRef]
- Sultana, F.; English, D.R.; Simpson, J.A.; Brotherton, J.M.; Drennan, K.; Mullins, R.; Heley, S.; Wrede, C.D.; Saville, M.; Gertig, D.M. Rationale and design of the iPap trial: A randomized controlled trial of home-based HPV self-sampling for improving participation in cervical screening by never- and under-screened women in Australia. BMC Cancer 2014, 14, 207. [Google Scholar] [CrossRef] [Green Version]
- Bos, A.B.; Rebolj, M.; Habbema, J.D.F.; van Ballegooijen, M. Nonattendance is still the main limitation for the effectiveness of screening for cervical cancer in the Netherlands. Int. J. Cancer 2006, 119, 2372–2375. [Google Scholar] [CrossRef]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Drolet, M.; Benard, E.; Boily, M.C.; Ali, H.; Baandrup, L.; Bauer, H.; Beddows, S.; Brisson, J.; Brotherton, J.M.; Cummings, T.; et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect. Dis. 2015, 15, 565–580. [Google Scholar] [CrossRef] [Green Version]
- Slatnik, C.L.; Duff, E. Ovarian cancer: Ensuring early diagnosis. Nurse Pract. 2015, 40, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, P.M.; Jordan, S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obs. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef] [Green Version]
- National Institutes of Health; National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Female Breast Cancer; National Cancer Institute, NIH: Bethesda, MD, USA, 2020. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 20 November 2021).
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Tschernichovsky, R.; Goodman, A. Risk-Reducing Strategies for Ovarian Cancer in BRCA Mutation Carriers: A Balancing Act. Oncologist 2017, 22, 450–459. [Google Scholar] [CrossRef] [Green Version]
- Amant, F.; Mirza, M.R.; Koskas, M.; Creutzberg, C.L. Cancer of the corpus uteri. Int. J. Gynaecol. Obs. 2018, 143 (Suppl. 2), 37–50. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Cancer Today. Cancer Fact Sheets: Corpus uteri. WHO. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/24-Corpus-uteri-fact-sheet.pdf (accessed on 3 August 2021).
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Zwahlen, M.; Kitchener, H.C.; Egger, M.; Renehan, A.G. Body mass index, hormone replacement therapy, and endometrial cancer risk: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2010, 19, 3119–3130. [Google Scholar] [CrossRef] [Green Version]
- Sorosky, J.I. Endometrial cancer. Obs. Gynecol. 2012, 120, 383–397. [Google Scholar] [CrossRef]
- Braun, M.M.; Overbeek-Wager, E.A.; Grumbo, R.J. Diagnosis and Management of Endometrial Cancer. Am. Fam. Phys. 2016, 93, 468–474. [Google Scholar]
- The American College of Obstetricians and Gynecologists. Practice Bulletin No. 149: Endometrial cancer. Obs. Gynecol. 2015, 125, 1006–1026. [Google Scholar] [CrossRef]
- Lee, S.C.; Kaunitz, A.M.; Sanchez-Ramos, L.; Rhatigan, R.M. The oncogenic potential of endometrial polyps: A systematic review and meta-analysis. Obs. Gynecol. 2010, 116, 1197–1205. [Google Scholar] [CrossRef]
- Saso, S.; Chatterjee, J.; Georgiou, E.; Ditri, A.M.; Smith, J.R.; Ghaem-Maghami, S. Endometrial cancer. BMJ 2011, 343, d3954. [Google Scholar] [CrossRef] [Green Version]
- Prat, J.; Gallardo, A.; Cuatrecasas, M.; Catasus, L. Endometrial carcinoma: Pathology and genetics. Pathology 2007, 39, 72–87. [Google Scholar] [CrossRef]
- Buchanan, E.M.; Weinstein, L.C.; Hillson, C. Endometrial cancer. Am. Fam. Phys. 2009, 80, 1075–1080. [Google Scholar]
- Fung-Kee-Fung, M.; Dodge, J.; Elit, L.; Lukka, H.; Chambers, A.; Oliver, T. Follow-up after primary therapy for endometrial cancer: A systematic review. Gynecol. Oncol. 2006, 101, 520–529. [Google Scholar] [CrossRef]
- Adhikari, P.; Vietje, P.; Mount, S. Premalignant and malignant lesions of the vagina. Diagn. Histopathol. 2017, 23, 28–34. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Cancer Today. Cancer Fact Sheets: Vagina. WHO. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/22-Vagina-fact-sheet.pdf (accessed on 24 September 2021).
- Beller, U.; Benedet, J.L.; Creasman, W.T.; Ngan, H.Y.; Quinn, M.A.; Maisonneuve, P.; Pecorelli, S.; Odicino, F.; Heintz, A.P. Carcinoma of the vagina. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obs. 2006, 95 (Suppl. 1), S29–S42. [Google Scholar] [CrossRef]
- Adams, T.S.; Cuello, M.A. Cancer of the vagina. Int. J. Gynaecol. Obs. 2018, 143 (Suppl. 2), 14–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merino, M.J. Vaginal cancer: The role of infectious and environmental factors. Am. J. Obs. Gynecol. 1991, 165, 1255–1262. [Google Scholar] [CrossRef]
- Lamos, C.; Mihaljevic, C.; Aulmann, S.; Bruckner, T.; Domschke, C.; Wallwiener, M.; Paringer, C.; Fluhr, H.; Schott, S.; Dinkic, C.; et al. Detection of Human Papillomavirus Infection in Patients with Vaginal Intraepithelial Neoplasia. PLoS ONE 2016, 11, e0167386. [Google Scholar] [CrossRef]
- Daling, J.R.; Madeleine, M.M.; Schwartz, S.M.; Shera, K.A.; Carter, J.J.; McKnight, B.; Porter, P.L.; Galloway, D.A.; McDougall, J.K.; Tamimi, H. A population-based study of squamous cell vaginal cancer: HPV and cofactors. Gynecol. Oncol. 2002, 84, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, V.; Bellati, F.; Fischetti, M.; Plotti, F.; Perniola, G.; Panici, P.B. Vaginal cancer. Crit. Rev. Oncol. Hematol. 2012, 81, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Alfonzo, E.; Holmberg, E.; Sparén, P.; Milsom, I.; Strander, B. Risk of vaginal cancer among hysterectomised women with cervical intraepithelial neoplasia: A population-based national cohort study. BJOG 2020, 127, 448–454. [Google Scholar] [CrossRef]
- Tidy, J. The risk of vaginal cancer is associated with a history of cervical neoplasia. BJOG 2020, 127, 455. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.T.; Campbell, S.; Nygard, M. Long-term incidence trends of HPV-related cancers, and cases preventable by HPV vaccination: A registry-based study in Norway. BMJ Open 2018, 8, e019005. [Google Scholar] [CrossRef] [Green Version]
- Videlefsky, A.; Grossl, N.; Denniston, M.; Sehgal, R.; Lane, J.M.; Goodenough, G. Routine vaginal cuff smear testing in post-hysterectomy patients with benign uterine conditions: When is it indicated? J. Am. Board Fam. Pract. 2000, 13, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Akhtar-Danesh, N.; Elit, L.; Lytwyn, A. Trends in incidence and survival of women with invasive vulvar cancer in the United States and Canada: A population-based study. Gynecol. Oncol. 2014, 134, 314–318. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Hampl, M.; Deckers-Figiel, S.; Hampl, J.A.; Rein, D.; Bender, H.G. New aspects of vulvar cancer: Changes in localization and age of onset. Gynecol. Oncol. 2008, 109, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, D.; Gomez-Martinez, R.A. Vulvar Cancer. Obs. Gynecol. Clin. N. Am. 2019, 46, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Zweizig, S.; Korets, S.; Cain, J.M. Key concepts in management of vulvar cancer. Best Pract. Res. Clin. Obs. Gynaecol. 2014, 28, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Bodurka, D.C. Neoplastic disease of the vulva: Lichen sclerosus, intraepithelial neoplasia, Paget disease, and carcinoma. Compr. Gynecol. 2017, 685–703. [Google Scholar]
- Tan, A.; Bieber, A.K.; Stein, J.A.; Pomeranz, M.K. Diagnosis and management of vulvar cancer: A review. J. Am. Acad. Derm. 2019, 81, 1387–1396. [Google Scholar] [CrossRef]
- Francis, J.A.; Eiriksson, L.; Dean, E.; Sebastianelli, A.; Bahoric, B.; Salvador, S. No. 370-Management of Squamous Cell Cancer of the Vulva. J. Obs. Gynaecol. Can. 2019, 41, 89–101. [Google Scholar] [CrossRef]
- Merlo, S. Modern treatment of vulvar cancer. Radiol. Oncol. 2020, 54, 371–376. [Google Scholar] [CrossRef]
- Smith, J.S.; Green, J.; Berrington de Gonzalez, A.; Appleby, P.; Peto, J.; Plummer, M.; Franceschi, S.; Beral, V. Cervical cancer and use of hormonal contraceptives: A systematic review. Lancet 2003, 361, 1159–1167. [Google Scholar] [CrossRef]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef] [Green Version]
- Permuth-Wey, J.; Sellers, T.A. Epidemiology of Ovarian Cancer. In Cancer Epidemiology. Methods in Molecular Biology; Verma, M., Ed.; Humana Press: Totowa, NJ, USA, 2009; Volume 472. [Google Scholar] [CrossRef] [Green Version]
- Ryan, N.A.J.; Morris, J.; Green, K.; Lalloo, F.; Woodward, E.R.; Hill, J.; Crosbie, E.J.; Evans, D.G. Association of Mismatch Repair Mutation With Age at Cancer Onset in Lynch Syndrome: Implications for Stratified Surveillance Strategies. JAMA Oncol. 2017, 3, 1702–1706. [Google Scholar] [CrossRef]
- Halec, G.; Alemany, L.; Quiros, B.; Clavero, O.; Höfler, D.; Alejo, M.; Quint, W.; Pawlita, M.; Bosch, F.X.; de Sanjose, S. Biological relevance of human papillomaviruses in vulvar cancer. Mod. Pathol. 2017, 30, 549–562. [Google Scholar] [CrossRef] [Green Version]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Sigel, A.; Sigel, H.; Sigel, R.K. Interrelations between Essential Metal Ions and Human Diseases; Springer: Berlin, Germany, 2013; ISBN 978-94-007-7500-8. [Google Scholar]
- Gana, W.; De Luca, A.; Debacq, C.; Poitau, F.; Poupin, P.; Aidoud, A.; Fougère, B. Analysis of the Impact of Selected Vitamins Deficiencies on the Risk of Disability in Older People. Nutrients 2021, 13, 3163. [Google Scholar] [CrossRef]
- Severance, S.; Hamza, I. Trafficking of heme and porphyrins in metazoa. Chem. Rev. 2009, 109, 4596–4616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, I.J.; Chen, C.; Paw, B.H.; Hamza, I. Iron and porphyrin trafficking in heme biogenesis. J. Biol. Chem. 2010, 285, 26753–26759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trace Elements. In Diet and Health: Implications for Reducing Chronic Disease Risk; National Academies Press: Washington, DC, USA, 1989; Volume 14.
- Lynch, S.R.; Cook, J.D. Interaction of vitamin C and iron. Ann. N. Y. Acad. Sci. 1980, 355, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.L. Iron deficiency anemia: A common and curable disease. Cold Spring Harb. Perspect. Med. 2013, 3, a011866. [Google Scholar] [CrossRef] [Green Version]
- Fleming, M.D. The genetics of inherited sideroblastic anemias. Semin. Hematol. 2002, 39, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Mangaonkar, A.A.; Patnaik, M.M. Treatment of Acquired Sideroblastic Anemias. Hematol. Oncol. Clin. N. Am. 2020, 34, 401–420. [Google Scholar] [CrossRef]
- Camaschella, C. Hereditary sideroblastic anemias: Pathophysiology, diagnosis, and treatment. Semin. Hematol. 2009, 46, 371–377. [Google Scholar] [CrossRef] [PubMed]
- National Organization for Rare Disorders. NORD Guide to Rare Diseases; Lippincott Williams & Wilkins: London, UK, 2003; pp. 373–374. [Google Scholar]
- Atala, A. Tissue engineering of reproductive tissues and organs. Fertil. Steril. 2012, 98, 21–29. [Google Scholar] [CrossRef]
- Esen, U.I. Iron deficiency anaemia in pregnancy: The role of parenteral iron. J. Obs. Gynaecol. 2017, 37, 15–18. [Google Scholar] [CrossRef]
- Drexler, C.; Macher, S.; Lindenau, I.; Holter, M.; Moritz, M.; Stojakovic, T.; Pieber, T.R.; Schlenke, P.; Amrein, K. High-dose intravenous versus oral iron in blood donors with iron deficiency: The IronWoMan randomized, controlled clinical trial. Clin. Nutr. 2020, 39, 737–745. [Google Scholar] [CrossRef] [Green Version]
- Govindappagari, S.; Burwick, R.M. Treatment of Iron Deficiency Anemia in Pregnancy with Intravenous versus Oral Iron: Systematic Review and Meta-Analysis. Am. J. Perinatol. 2019, 36, 366–376. [Google Scholar] [CrossRef]
- Rogozinska, E.; Daru, J.; Nicolaides, M.; Amezcua-Prieto, C.; Robinson, S.; Wang, R.; Godolphin, P.J.; Saborido, C.M.; Zamora, J.; Khan, K.S.; et al. Iron preparations for women of reproductive age with iron deficiency anaemia in pregnancy (FRIDA): A systematic review and network meta-analysis. Lancet Haematol. 2021, 8, e503–e512. [Google Scholar] [CrossRef]
- Kochhar, P.K.; Kaundal, A.; Ghosh, P. Intravenous iron sucrose versus oral iron in treatment of iron deficiency anemia in pregnancy: A randomized clinical trial. J. Obs. Gynaecol. Res. 2013, 39, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Froessler, B.; Gajic, T.; Dekker, G.; Hodyl, N.A. Treatment of iron deficiency and iron deficiency anemia with intravenous ferric carboxymaltose in pregnancy. Arch. Gynecol. Obs. 2018, 298, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rushton, D.H.; Ramsay, I.D.; Gilkes, J.J.; Norris, M.J. Ferritin and fertility. Lancet 1991, 337, 1554. [Google Scholar] [CrossRef]
- Maccarinelli, F.; Regoni, M.; Carmona, F.; Poli, M.; Meyron-Holtz, E.G.; Arosio, P. Mitochondrial ferritin deficiency reduces male fertility in mice. Reprod. Fertil. Dev. 2017, 29, 2005–2010. [Google Scholar] [CrossRef] [Green Version]
- Georgsen, M.; Krog, M.C.; Korsholm, A.S.; Hvidman, H.W.; Kolte, A.M.; Rigas, A.S.; Ullum, H.; Ziebe, S.; Andersen, A.N.; Nielsen, H.S.; et al. Serum ferritin level is inversely related to number of previous pregnancy losses in women with recurrent pregnancy loss. Fertil. Steril. 2021, 115, 389–396. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Iron intake and risk of ovulatory infertility. Obs. Gynecol. 2006, 108, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Cousins, R.J. A role of zinc in the regulation of gene expression. Proc. Nutr. Soc. 1998, 57, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Hambidge, M.; Krebs, N.F. Interrelationships of key variables of human zinc homeostasis: Relevance to dietary zinc requirements. Annu. Rev. Nutr. 2001, 21, 429–452. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc homeostasis in humans. J. Nutr. 2000, 130, 1360S–1366S. [Google Scholar] [CrossRef] [Green Version]
- Akdas, S.; Yazihan, N. Cord blood zinc status effects on pregnancy outcomes and its relation with maternal serum zinc levels: A systematic review and meta-analysis. World J. Pediatr. 2020, 16, 366–376. [Google Scholar] [CrossRef]
- Sinha, R. National Seminar on Importance of Zinc in Human Health. Indian Pediatr. 2004, 41, 1213–1217. [Google Scholar]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Zinc Review. J. Nutr. 2015, 146, 858S–885S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, K.O.; Zavaleta, N.; Caulfield, L.E.; Wen, J.; Abrams, S.A. Prenatal iron supplements impair zinc absorption in pregnant Peruvian women. J. Nutr. 2000, 130, 2251–2255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hambidge, K.M.; Miller, L.V.; Mazariegos, M.; Westcott, J.; Solomons, N.W.; Raboy, V.; Kemp, J.F.; Das, A.; Goco, N.; Hartwell, T.; et al. Upregulation of Zinc Absorption Matches Increases in Physiologic Requirements for Zinc in Women Consuming High- or Moderate-Phytate Diets during Late Pregnancy and Early Lactation. J. Nutr. 2017, 147, 1079–1085. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Walravens, P.A. Disorders of mineral metabolism. Clin. Gastroenterol. 1982, 11, 87–117. [Google Scholar] [CrossRef]
- Garner, T.B.; Hester, J.M.; Carothers, A.; Diaz, F.J. Role of zinc in female reproduction. Biol. Reprod. 2021, 104, 976–994. [Google Scholar] [CrossRef]
- Velie, E.M.; Block, G.; Shaw, G.M.; Samuels, S.J.; Schaffer, D.M.; Kulldorff, M. Maternal supplemental and dietary zinc intake and the occurrence of neural tube defects in California. Am. J. Epidemiol. 1999, 150, 605–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruder, E.H.; Hartman, T.J.; Blumberg, J.; Goldman, M.B. Oxidative stress and antioxidants: Exposure and impact on female fertility. Hum. Reprod. Update 2008, 14, 345–357. [Google Scholar] [CrossRef] [Green Version]
- Forges, T.; Monnier-Barbarino, P.; Alberto, J.M.; Gueant-Rodriguez, R.M.; Daval, J.L.; Gueant, J.L. Impact of folate and homocysteine metabolism on human reproductive health. Hum. Reprod. Update 2007, 13, 225–238. [Google Scholar] [CrossRef]
- Hu, Q.; Duncan, F.E.; Nowakowski, A.B.; Antipova, O.A.; Woodruff, T.K.; O’Halloran, T.V.; Wolfner, M.F. Zinc Dynamics during Drosophila Oocyte Maturation and Egg Activation. iScience 2020, 23, 101275. [Google Scholar] [CrossRef]
- Wilson, R.L.; Bianco-Miotto, T.; Leemaqz, S.Y.; Grzeskowiak, L.E.; Dekker, G.A.; Roberts, C.T. Early pregnancy maternal trace mineral status and the association with adverse pregnancy outcome in a cohort of Australian women. J. Trace Elem. Med. Biol. 2018, 46, 103–109. [Google Scholar] [CrossRef]
- Casado-Espada, N.M.; de Alarcón, R.; de la Iglesia-Larrad, J.I.; Bote-Bonaechea, B.; Montejo, Á.L. Hormonal Contraceptives, Female Sexual Dysfunction, and Managing Strategies: A Review. J. Clin. Med. 2019, 8, 908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enzlin, P.; Weyers, S.; Janssens, D.; Poppe, W.; Eelen, C.; Pazmany, E.; Elaut, E.; Amy, J.J. Sexual functioning in women using levonorgestrel-releasing intrauterine systems as compared to copper intrauterine devices. J. Sex. Med. 2012, 9, 1065–1073. [Google Scholar] [CrossRef]
- Thomas, H.N.; Thurston, R.C. A biopsychosocial approach to women’s sexual function and dysfunction at midlife: A narrative review. Maturitas 2016, 87, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Casey, P.M.; MacLaughlin, K.L.; Faubion, S.S. Impact of Contraception on Female Sexual Function. J. Womens Health 2017, 26, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, J.P.; Cotan, D.; Jurado, I.; Oropesa-Avila, M.; Sanchez-Martin, P.; Savaris, R.F.; Tan, J.; Sanchez-Alcazar, J.A.; Tan, S.L.; Horcajadas, J.A. The Effect of Copper on Endometrial Receptivity and Induction of Apoptosis on Decidualized Human Endometrial Stromal Cells. Reprod. Sci. 2018, 25, 985–999. [Google Scholar] [CrossRef]
- Arancibia, V.; Pena, C.; Allen, H.E.; Lagos, G. Characterization of copper in uterine fluids of patients who use the copper T-380A intrauterine device. Clin. Chim. Acta 2003, 332, 69–78. [Google Scholar] [CrossRef]
- Hubacher, D. Copper intrauterine device use by nulliparous women: Review of side effects. Contraception 2007, 75, S8–S11. [Google Scholar] [CrossRef]
- Jimenez, M.F.; Passos, E.P.; Fagundes, P.A.; de Freitas, F.M.; Arbo, E.; Cunha-Filho, J.S. Effect of the copper-intrauterine device (TCu 380A) on subendometrial microvascularization and uterine artery blood flow. Fertil. Steril. 2006, 86, 1780–1782. [Google Scholar] [CrossRef]
- Noda, Y.; Ota, K.; Shirasawa, T.; Shimizu, T. Copper/zinc superoxide dismutase insufficiency impairs progesterone secretion and fertility in female mice. Biol. Reprod. 2012, 86, 1–8. [Google Scholar] [CrossRef]
- Aoun, A.; Darwiche, F.; Al Hayek, S.; Doumit, J. The Fluoride Debate: The Pros and Cons of Fluoridation. Prev. Nutr. Food Sci. 2018, 23, 171–180. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Duan, Q.; Jiao, J.; Chen, X.; Wang, X. Association between water fluoride and the level of children’s intelligence: A dose-response meta-analysis. Public Health 2018, 154, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, T.; Rawls, H.R. Fluoride exposure in early life as the possible root cause of disease in later life. J. Clin. Pediatr. Dent. 2018, 42, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Barbier, O.; Arreola-Mendoza, L.; Del Razo, L.M. Molecular mechanisms of fluoride toxicity. Chem. Biol. Interact. 2010, 188, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Flora, S.J. Effects of fluoride on the tissue oxidative stress and apoptosis in rats: Biochemical assays supported by IR spectroscopy data. Toxicology 2008, 254, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Peckham, S.; Awofeso, N. Water fluoridation: A critical review of the physiological effects of ingested fluoride as a public health intervention. Sci. World J. 2014, 2014, 293019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadbent, J.M.; Thomson, W.M.; Ramrakha, S.; Moffitt, T.E.; Zeng, J.; Foster Page, L.A.; Poulton, R. Community Water Fluoridation and Intelligence: Prospective Study in New Zealand. Am. J. Public Health 2015, 105, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Solanki, Y.S.; Agarwal, M.; Gupta, A.B.; Gupta, S.; Shukla, P. Fluoride occurrences, health problems, detection, and remediation methods for drinking water: A comprehensive review. Sci. Total Environ. 2021, 807, 150601. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Lanphear, B.; Hornung, R.; Flora, D.; Martinez-Mier, E.A.; Neufeld, R.; Ayotte, P.; Muckle, G.; Till, C. Association Between Maternal Fluoride Exposure During Pregnancy and IQ Scores in Offspring in Canada. JAMA Pediatr. 2019, 173, 940–948. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, Y.; He, J.; Chen, X.; Ding, Y.; Wang, Y.; Liu, X. The toxicity mechanism of sodium fluoride on fertility in female rats. Food Chem. Toxicol. 2013, 62, 566–572. [Google Scholar] [CrossRef]
- Dhar, V.; Bhatnagar, M. Physiology and toxicity of fluoride. Indian J. Dent. Res. 2009, 20, 350–355. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Sao Paulo Med. J. 2015, 133, 164–165. [Google Scholar] [CrossRef] [Green Version]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef] [Green Version]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Newman, R.; Waterland, N.; Moon, Y.; Tou, J.C. Selenium Biofortification of Agricultural Crops and Effects on Plant Nutrients and Bioactive Compounds Important for Human Health and Disease Prevention—A Review. Plant Foods Hum. Nutr. 2019, 74, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef]
- Wu, S.; Wang, M.; Deng, Y.; Qiu, J.; Zhang, X.; Tan, J. Associations of toxic and essential trace elements in serum, follicular fluid, and seminal plasma with In vitro fertilization outcomes. Ecotoxicol. Environ. Saf. 2020, 204, 110965. [Google Scholar] [CrossRef]
- Maeda, E.; Murata, K.; Kumazawa, Y.; Sato, W.; Shirasawa, H.; Iwasawa, T.; Izumo, K.; Tatsuta, N.; Sakamoto, M.; Terada, Y. Associations of environmental exposures to methylmercury and selenium with female infertility: A case-control study. Environ. Res. 2019, 168, 357–363. [Google Scholar] [CrossRef]
- Showell, M.G.; Mackenzie-Proctor, R.; Jordan, V.; Hart, R.J. Antioxidants for female subfertility. Cochrane Database Syst. Rev. 2020, 8, CD007807. [Google Scholar] [CrossRef]
- Mantovani, G.; Isidori, A.M.; Moretti, C.; Di Dato, C.; Greco, E.; Ciolli, P.; Bonomi, M.; Petrone, L.; Fumarola, A.; Campagna, G.; et al. Selenium supplementation in the management of thyroid autoimmunity during pregnancy: Results of the “SERENA study”, a randomized, double-blind, placebo-controlled trial. Endocrine 2019, 66, 542–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qazi, I.H.; Angel, C.; Yang, H.; Pan, B.; Zoidis, E.; Zeng, C.-J.; Han, H.; Zhou, G.-B. Selenium, Selenoproteins, and Female Reproduction: A Review. Molecules 2018, 23, 3053. [Google Scholar] [CrossRef] [Green Version]
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef] [Green Version]
- Edassery, S.L.; Shatavi, S.V.; Kunkel, J.P.; Hauer, C.; Brucker, C.; Penumatsa, K.; Yu, Y.; Dias, J.A.; Luborsky, J.L. Autoantigens in ovarian autoimmunity associated with unexplained infertility and premature ovarian failure. Fertil. Steril. 2010, 94, 2636–2641. [Google Scholar] [CrossRef] [Green Version]
- Harrison, J.H.; Conrad, H.R. Effect of dietary calcium on selenium absorption by the nonlactating dairy cow. J. Dairy Sci. 1984, 67, 1860–1864. [Google Scholar] [CrossRef]
- Khera, A.; Dong, L.F.; Holland, O.; Vanderlelie, J.; Pasdar, E.A.; Neuzil, J.; Perkins, A.V. Selenium supplementation induces mitochondrial biogenesis in trophoblasts. Placenta 2015, 36, 863–869. [Google Scholar] [CrossRef]
- Khera, A.; Vanderlelie, J.J.; Perkins, A.V. Selenium supplementation protects trophoblast cells from mitochondrial oxidative stress. Placenta 2013, 34, 594–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, M.; van Leer, L.; Vanderlelie, J.J.; Perkins, A.V. Selenium supplementation protects trophoblast cells from oxidative stress. Placenta 2012, 33, 1012–1019. [Google Scholar] [CrossRef] [Green Version]
- Khera, A.; Vanderlelie, J.J.; Holland, O.; Perkins, A.V. Overexpression of Endogenous Anti-Oxidants with Selenium Supplementation Protects Trophoblast Cells from Reactive Oxygen Species-Induced Apoptosis in a Bcl-2-Dependent Manner. Biol. Trace Elem. Res. 2017, 177, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Bizerea, T.O.; Dezsi, S.G.; Marginean, O.; Stroescu, R.; Rogobete, A.; Bizerea-Spiridon, O.; Ilie, C. The Link Between Selenium, Oxidative Stress and Pregnancy Induced Hypertensive Disorders. Clin. Lab. 2018, 64, 1593–1610. [Google Scholar] [CrossRef]
- Mertz, W. Chromium in human nutrition: A review. J. Nutr. 1993, 123, 626–633. [Google Scholar] [CrossRef]
- Vincent, J.B. Quest for the molecular mechanism of chromium action and its relationship to diabetes. Nutr. Rev. 2000, 58, 67–72. [Google Scholar] [CrossRef]
- Wallach, S. Clinical and biochemical aspects of chromium deficiency. J. Am. Coll. Nutr. 1985, 4, 107–120. [Google Scholar] [CrossRef]
- Yoshida, M. Is chromium an essential trace element in human nutrition? Nihon Eiseigaku Zasshi 2012, 67, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.B. Recent advances in the nutritional biochemistry of trivalent chromium. Proc. Nutr. Soc. 2004, 63, 41–47. [Google Scholar] [CrossRef]
- Di Bona, K.R.; Love, S.; Rhodes, N.R.; McAdory, D.; Sinha, S.H.; Kern, N.; Kent, J.; Strickland, J.; Wilson, A.; Beaird, J.; et al. Chromium is not an essential trace element for mammals: Effects of a “low-chromium” diet. J. Biol. Inorg. Chem. 2011, 16, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Trace Elements. Scottish Trace Element and Micronutrient Diagnostic and Research Laboratory—Chromium. Available online: https://www.trace-elements.co.uk/chromium.asp (accessed on 13 September 2021).
- Freund, H.; Atamian, S.; Fischer, J.E. Chromium deficiency during total parenteral nutrition. JAMA 1979, 241, 496–498. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Cefalu, W.T. Current concepts about chromium supplementation in type 2 diabetes and insulin resistance. Curr. Diab. Rep. 2010, 10, 145–151. [Google Scholar] [CrossRef]
- Shmitova, L.A. Content of hexavalent chromium in the biological substrates of pregnant women and puerperae engaged in the manufacture of chromium compounds. Gig. Tr. Prof. Zabol. 1980, 2, 33–35. [Google Scholar]
- Remy, L.L.; Byers, V.; Clay, T. Reproductive outcomes after non-occupational exposure to hexavalent chromium, Willits California, 1983-2014. Environ Health 2017, 16, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marat, I.; Arstan, M.; Galymzhan, Y.; Timur, J.; Yerbolat, I.; Almasbek, Y. Impact of chromium and boron compounds on the reproductive function in rats. Toxicol. Ind. Health 2018, 34, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Faghfoori, Z.; Fazelian, S.; Shadnoush, M.; Goodarzi, R. Nutritional management in women with polycystic ovary syndrome: A review study. Diabetes Metab. Syndr. 2017, 11 (Suppl. 1), S429–S432. [Google Scholar] [CrossRef]
- Pourghassem Gargari, B.; Houjeghani, S.; Farzadi, L.; Houjeghani, S.; Safaeiyan, A. Relationship between Serum Leptin, Ghrelin and Dietary Macronutrients in Women with Polycystic Ovary Syndrome. Int. J. Fertil. Steril. 2015, 9, 313–321. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Kumar, C.S.; Rani, M.U.; Kavita, K.; Boobalan, G.; Reddy, A.G. Evaluation of protective action of alpha-tocopherol in chromium-induced oxidative stress in female reproductive system of rats. J. Nat. Sci. Biol. Med. 2013, 4, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, R.; Samuel, J.; Arosh, J.; Lee, J.; Aruldhas, M.; Banu, S. Chromium toxicity induces ovarian follicular developmental arrest, apoptosis, and deregulated steroidogenesis: Vitamin c restores follicular survival and function. Biol. Reprod. 2007, 77, 215. [Google Scholar] [CrossRef]
- Rao, M.V.; Chawla, S.L.; Sharma, S.R. Protective role of vitamin E on nickel and/or chromium induced oxidative stress in the mouse ovary. Food Chem. Toxicol. 2009, 47, 1368–1371. [Google Scholar] [CrossRef]
- Elbetieha, A.; Al-Hamood, M.H. Long-term exposure of male and female mice to trivalent and hexavalent chromium compounds: Effect on fertility. Toxicology 1997, 116, 39–47. [Google Scholar] [CrossRef]
- Bataineh, H.N.; Bataineh, Z.M.; Daradka, H. Short-term exposure of female rats to industrial metal salts: Effect on implantation and pregnancy. Reprod. Med. Biol. 2007, 6, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Bataineh, H.; al-Hamood, M.H.; Elbetieha, A.; Bani Hani, I. Effect of long-term ingestion of chromium compounds on aggression, sex behavior and fertility in adult male rat. Drug Chem. Toxicol. 1997, 20, 133–149. [Google Scholar] [CrossRef]
- National Research Council, Committee to Assess the Health Implications of Perchlorate Ingestion. Health Implications of Perchlorate Ingestion; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Lincoln, S.R.; Ke, R.W.; Kutteh, W.H. Screening for hypothyroidism in infertile women. J. Reprod. Med. 1999, 44, 455–457. [Google Scholar]
- Mills, J.L.; Buck Louis, G.M.; Kannan, K.; Weck, J.; Wan, Y.; Maisog, J.; Giannakou, A.; Wu, Q.; Sundaram, R. Delayed conception in women with low-urinary iodine concentrations: A population-based prospective cohort study. Hum. Reprod. 2018, 33, 426–433. [Google Scholar] [CrossRef]
- Zimmermann, M.B. Iodine deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef] [Green Version]
- Schaffner, M.; Muhlberger, N.; Conrads-Frank, A.; Qerimi Rushaj, V.; Sroczynski, G.; Koukkou, E.; Heinsbaek Thuesen, B.; Volzke, H.; Oberaigner, W.; Siebert, U.; et al. Benefits and Harms of a Prevention Program for Iodine Deficiency Disorders: Predictions of the Decision-Analytic EUthyroid Model. Thyroid 2021, 31, 494–508. [Google Scholar] [CrossRef]
- Threapleton, D.E.; Waiblinger, D.; Snart, C.J.P.; Taylor, E.; Keeble, C.; Ashraf, S.; Bi, S.; Ajjan, R.; Azad, R.; Hancock, N.; et al. Prenatal and Postpartum Maternal Iodide Intake from Diet and Supplements, Urinary Iodine and Thyroid Hormone Concentrations in a Region of the United Kingdom with Mild-to-Moderate Iodine Deficiency. Nutrients 2021, 13, 230. [Google Scholar] [CrossRef]
- Skoracka, K.; Ratajczak, A.E.; Rychter, A.M.; Dobrowolska, A.; Krela-Kazmierczak, I. Female Fertility and the Nutritional Approach: The Most Essential Aspects. Adv. Nutr. 2021, 12, 2372–2386. [Google Scholar] [CrossRef] [PubMed]
- Mumford, S.L.; Chavarro, J.E.; Zhang, C.; Perkins, N.J.; Sjaarda, L.A.; Pollack, A.Z.; Schliep, K.C.; Michels, K.A.; Zarek, S.M.; Plowden, T.C.; et al. Dietary fat intake and reproductive hormone concentrations and ovulation in regularly menstruating women. Am. J. Clin. Nutr. 2016, 103, 868–877. [Google Scholar] [CrossRef] [Green Version]
- Willett, W.C.; Stampfer, M.J.; Manson, J.E.; Colditz, G.A.; Speizer, F.E.; Rosner, B.A.; Sampson, L.A.; Hennekens, C.H. Intake of trans fatty acids and risk of coronary heart disease among women. Lancet 1993, 341, 581–585. [Google Scholar] [CrossRef]
- Wise, L.A.; Wesselink, A.K.; Tucker, K.L.; Saklani, S.; Mikkelsen, E.M.; Cueto, H.; Riis, A.H.; Trolle, E.; McKinnon, C.J.; Hahn, K.A.; et al. Dietary Fat Intake and Fecundability in 2 Preconception Cohort Studies. Am. J. Epidemiol. 2018, 187, 60–74. [Google Scholar] [CrossRef] [Green Version]
- Wathes, D.C.; Abayasekara, D.R.; Aitken, R.J. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 2007, 77, 190–201. [Google Scholar] [CrossRef]
- Hammiche, F.; Vujkovic, M.; Wijburg, W.; de Vries, J.H.; Macklon, N.S.; Laven, J.S.; Steegers-Theunissen, R.P. Increased preconception omega-3 polyunsaturated fatty acid intake improves embryo morphology. Fertil. Steril. 2011, 95, 1820–1823. [Google Scholar] [CrossRef]
- Mumford, S.L.; Browne, R.W.; Kim, K.; Nichols, C.; Wilcox, B.; Silver, R.M.; Connell, M.T.; Holland, T.L.; Kuhr, D.L.; Omosigho, U.R.; et al. Preconception Plasma Phospholipid Fatty Acids and Fecundability. J. Clin. Endocrinol. Metab. 2018, 103, 4501–4510. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.H.; Karmon, A.E.; Gaskins, A.J.; Arvizu, M.; Williams, P.L.; Souter, I.; Rueda, B.R.; Hauser, R.; Chavarro, J.E.; Team, E.S. Serum omega-3 fatty acids and treatment outcomes among women undergoing assisted reproduction. Hum. Reprod. 2018, 33, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Mohan, V.; Modi, D.R.; Sinha, R.A.; Rastogi, L.; Kumar, P.; Godbole, M.M. Iodine plus n-3 fatty acid supplementation augments rescue of postnatal neuronal abnormalities in iodine-deficient rat cerebellum. Br. J. Nutr. 2013, 110, 659–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berbel, P.; Mestre, J.L.; Santamaria, A.; Palazon, I.; Franco, A.; Graells, M.; Gonzalez-Torga, A.; de Escobar, G.M. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: The importance of early iodine supplementation. Thyroid 2009, 19, 511–519. [Google Scholar] [CrossRef]
- Babu, S.; Sinha, R.A.; Mohan, V.; Rao, G.; Pal, A.; Pathak, A.; Singh, M.; Godbole, M.M. Effect of hypothyroxinemia on thyroid hormone responsiveness and action during rat postnatal neocortical development. Exp. Neurol. 2011, 228, 91–98. [Google Scholar] [CrossRef]
- Kobayashi, N.; Orisaka, M.; Cao, M.; Kotsuji, F.; Leader, A.; Sakuragi, N.; Tsang, B.K. Growth differentiation factor-9 mediates follicle-stimulating hormone-thyroid hormone interaction in the regulation of rat preantral follicular development. Endocrinology 2009, 150, 5566–5574. [Google Scholar] [CrossRef]
- Slebodzinski, A.B. Ovarian iodide uptake and triiodothyronine generation in follicular fluid. The enigma of the thyroid ovary interaction. Domest. Anim. Endocrinol. 2005, 29, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Furlanetto, T.W.; Nguyen, L.Q.; Jameson, J.L. Estradiol increases proliferation and down-regulates the sodium/iodide symporter gene in FRTL-5 cells. Endocrinology 1999, 140, 5705–5711. [Google Scholar] [CrossRef]
- Mathews, D.M.; Johnson, N.P.; Sim, R.G.; O’Sullivan, S.; Peart, J.M.; Hofman, P.L. Iodine and fertility: Do we know enough? Hum. Reprod. 2021, 36, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.P.; Bhattu, S.; Wagner, A.; Blake, D.A.; Chamley, L.W. Lipiodol alters murine uterine dendritic cell populations: A potential mechanism for the fertility-enhancing effect of lipiodol. Fertil. Steril. 2005, 83, 1814–1821. [Google Scholar] [CrossRef]
- van Dyk, E.; Lange, A.L. The detrimental effect of iodine as an intra-uterine instillation in mares. J. S. Afr. Vet. Assoc. 1986, 57, 205–210. [Google Scholar]
- Abel, M.H.; Caspersen, I.H.; Sengpiel, V.; Jacobsson, B.; Meltzer, H.M.; Magnus, P.; Alexander, J.; Brantsæter, A.L. Insufficient maternal iodine intake is associated with subfecundity, reduced foetal growth, and adverse pregnancy outcomes in the Norwegian Mother, Father and Child Cohort Study. BMC Med. 2020, 18, 211. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. Manganese, Molybdenum, Boron, Chromium, and Other Trace Elements. In Present Knowledge in Nutrition, 10th ed.; Erdman, J.W., Jr., MacDonald, I.A., Zeisel, S.H., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 586–607. [Google Scholar]
- Secretariat, W.H.O.; Andersson, M.; de Benoist, B.; Delange, F.; Zupan, J. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: Conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007, 10, 1606–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NIH. Office of Dietary Supplements—Manganese. 2017. Available online: https://ods.od.nih.gov/factsheets/Manganese-HealthProfessional/ (accessed on 20 November 2021).
- Buchman, A.R. Manganese. In Modern Nutrition in Health and Disease, 11th ed.; Ross, A.C., Caballero, B., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2014; pp. 238–244. [Google Scholar]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid. Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef] [Green Version]
- Aschner, J.L.; Aschner, M. Nutritional aspects of manganese homeostasis. Mol. Asp. Med. 2005, 26, 353–362. [Google Scholar] [CrossRef]
- McGough, D.; Jardine, L. A two-generation inhalation reproductive toxicity study upon the exposure to manganese chloride. Neurotoxicology 2017, 58, 194–202. [Google Scholar] [CrossRef]
- Xie, J.; Tian, C.; Zhu, Y.; Zhang, L.; Lu, L.; Luo, X. Effects of inorganic and organic manganese supplementation on gonadotropin-releasing hormone-I and follicle-stimulating hormone expression and reproductive performance of broiler breeder hens. Poult. Sci. 2014, 93, 959–969. [Google Scholar] [CrossRef]
- Faure, C.; Leveille, P.; Dupont, C.; Julia, C.; Chavatte-Palmer, P.; Alifert, G.; Sutton, A.; Levy, R. Are superoxide dismutase 2 and nitric oxide synthase polymorphisms associated with idiopathic infertility? Antioxid. Redox Signal. 2014, 21, 565–569. [Google Scholar] [CrossRef]
- Suzuki, T.; Sugino, N.; Fukaya, T.; Sugiyama, S.; Uda, T.; Takaya, R.; Yajima, A.; Sasano, H. Superoxide dismutase in normal cycling human ovaries: Immunohistochemical localization and characterization. Fertil. Steril. 1999, 72, 720–726. [Google Scholar] [CrossRef]
- Krupinska, I. Aluminium Drinking Water Treatment Residuals and Their Toxic Impact on Human Health. Molecules 2020, 25, 641. [Google Scholar] [CrossRef]
- Skogheim, T.S.; Weyde, K.V.F.; Engel, S.M.; Aase, H.; Suren, P.; Oie, M.G.; Biele, G.; Reichborn-Kjennerud, T.; Caspersen, I.H.; Hornig, M.; et al. Metal and essential element concentrations during pregnancy and associations with autism spectrum disorder and attention-deficit/hyperactivity disorder in children. Environ. Int. 2021, 152, 106468. [Google Scholar] [CrossRef]
- Igbokwe, I.O.; Igwenagu, E.; Igbokwe, N.A. Aluminium toxicosis: A review of toxic actions and effects. Interdiscip. Toxicol. 2019, 12, 45–70. [Google Scholar] [CrossRef] [Green Version]
- Cabus, N.; Oguz, E.O.; Tufan, A.C.; Adiguzel, E. A histological study of toxic effects of aluminium sulfate on rat hippocampus. Biotech. Histochem. 2015, 90, 132–139. [Google Scholar] [CrossRef]
- Ljunggren, K.G.; Lidums, V.; Sjögren, B. Blood and urine concentrations of aluminium among workers exposed to aluminium flake powders. Br. J. Ind. Med. 1991, 48, 106–109. [Google Scholar] [CrossRef] [Green Version]
- Hirata-Koizumi, M.; Fujii, S.; Ono, A.; Hirose, A.; Imai, T.; Ogawa, K.; Ema, M.; Nishikawa, A. Evaluation of the reproductive and developmental toxicity of aluminium ammonium sulfate in a two-generation study in rats. Food Chem. Toxicol. 2011, 49, 1948–1959. [Google Scholar] [CrossRef]
- Marwa, M.; Adrian, F.; Nedra, B.; Samira, M.; Horea, M.; Walid-Habib, T.; Baati, R.; Leila, T. The role of lysosomes in the phenomenon of concentration of aluminum and indium in the female reproductive system. An ultrastructural study. J. Trace Elem. Med. Biol. 2017, 44, 59–64. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Harano, T.; Harano, K. The higher structure and physiological significance of hemoglobin. Nihon Rinsho 1996, 54, 2305–2310. [Google Scholar]
- Kasvosve, I.; Delanghe, J. Total iron binding capacity and transferrin concentration in the assessment of iron status. Clin. Chem. Lab. Med. 2002, 40, 1014–1018. [Google Scholar] [CrossRef]
- Knovich, M.A.; Storey, J.A.; Coffman, L.G.; Torti, S.V.; Torti, F.M. Ferritin for the clinician. Blood Rev. 2009, 23, 95–104. [Google Scholar] [CrossRef]
- Wally, J.; Buchanan, S.K. A structural comparison of human serum transferrin and human lactoferrin. Biometals 2007, 20, 249–262. [Google Scholar] [CrossRef] [Green Version]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Stehle, P.; Stoffel-Wagner, B.; Kuhn, K.S. Parenteral trace element provision: Recent clinical research and practical conclusions. Eur. J. Clin. Nutr. 2016, 70, 886–893. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.B. The Biochemistry of Chromium. J. Nutr. 2000, 130, 715–718. [Google Scholar] [CrossRef]
- WHO; UNICEF; ICCIDD. Assessment of the Iodine Deficiency Disorders and Monitoring Their Elimination. 2007 WHO/NHD/01.1. Available online: https://www.who.int/publications-detail-redirect/9789241595827 (accessed on 20 October 2021).
- Leung, A.M.; Braverman, L.E. Consequences of excess iodine. Nat. Rev. Endocrinol. 2014, 10, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Vieja, A.; Santisteban, P. Role of iodide metabolism in physiology and cancer. Endocr.-Relat. Cancer 2018, 25, R225–R245. [Google Scholar] [CrossRef] [PubMed]
- Southern, A.P.; Jwayyed, S. Iodine Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Greger, J.L. Nutrition versus toxicology of manganese in humans: Evaluation of potential biomarkers. Neurotoxicology 1999, 20, 205–212. [Google Scholar] [PubMed]
- Paul, C.; Lagana, A.S.; Maniglio, P.; Triolo, O.; Brady, D.M. Inositol’s and other nutraceuticals’ synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: State-of-the-art and future perspectives. Gynecol. Endocrinol. 2016, 32, 431–438. [Google Scholar] [CrossRef]
- Pizzorno, J. Environmental Toxins and Infertility. Integr. Med. 2018, 17, 8–11. [Google Scholar]
- Sofo, V.; Gotte, M.; Lagana, A.S.; Salmeri, F.M.; Triolo, O.; Sturlese, E.; Retto, G.; Alfa, M.; Granese, R.; Abrao, M.S. Correlation between dioxin and endometriosis: An epigenetic route to unravel the pathogenesis of the disease. Arch. Gynecol. Obs. 2015, 292, 973–986. [Google Scholar] [CrossRef]
- Mallozzi, M.; Leone, C.; Manurita, F.; Bellati, F.; Caserta, D. Endocrine Disrupting Chemicals and Endometrial Cancer: An Overview of Recent Laboratory Evidence and Epidemiological Studies. Int. J. Environ. Res. Public Health 2017, 14, 334. [Google Scholar] [CrossRef] [Green Version]
- Dunnick, J.K.; Sanders, J.M.; Kissling, G.E.; Johnson, C.L.; Boyle, M.H.; Elmore, S.A. Environmental chemical exposure may contribute to uterine cancer development: Studies with tetrabromobisphenol A. Toxicol. Pathol. 2015, 43, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Machalek, D.A.; Wark, J.D.; Tabrizi, S.N.; Hopper, J.L.; Bui, M.; Dite, G.S.; Cornall, A.M.; Pitts, M.; Gertig, D.; Erbas, B.; et al. Genetic and Environmental Factors in Invasive Cervical Cancer: Design and Methods of a Classical Twin Study. Twin Res. Hum. Genet. 2017, 20, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Type of Cancer | Causes | References |
|---|---|---|
| Cervical | Human papillomavirus (HPV) infection | [51] |
| Tobacco smoke | [93] | |
| Oral contraception | [93] | |
| Ovarian | BRCA Gene Mutation | [94] |
| Postmenopausal hormone-replacement therapy | [95] | |
| Tobacco smoke | [95] | |
| Corpus uteri | Obesity | [63] |
| Estrogen hormone replacement therapy | [60,65] | |
| Estrogen-producing tumors | [66] | |
| Tamoxifen | [62,64] | |
| Lynch syndrome | [62,96] | |
| Vaginal | Human papillomavirus type 16 | [77,78] |
| Smoking | [79] | |
| Chronic irritation | [79] | |
| Vaginal and cervical intraepithelial neoplasia (CIN) | [80,81] | |
| Cervical carcinoma | [80] | |
| Vulvar | Human papillomavirus (HPV) infection | [97] |
| Chronic inflammation | [87] | |
| Autoimmune-related processes | [87] |
| Element | Ref. | Physiological Significance | Reference Ranges (SI Units) | Reference Ranges (Pregnancy) | Toxicity | Serum/Plasma/Urine2 | Essential for Female Repr. Sytem | Contraindications | Factor of Influence |
|---|---|---|---|---|---|---|---|---|---|
| Iron [Fe] | [239] | Iron is required for numerous crucial cell processes such as DNA synthesis, energy production, and proper functioning of nuclei. It is a crucial component of hemoglobin and myoglobin, involved in hematopoiesis, significant in the formation and maturation of red blood cells, responsible for oxygen transport | 10.7–26.9 μmol/L | Serum | Y | ++ | |||
| Hemoglobin (Hb) | [240] | Normal physiological function and prevention of anemia. Advanced allosteric protein is the transportation of oxygen and carbon dioxide between the lung and the tissues | 7.45–9.30 mmol/L | Micro-/Macrocytic RBC abnormalities, contributing factors will ascertain relevant pathologies. | Whole Blood | Y | Various types of anaemias and haematological pathologies and metabolism. | ++ | |
| TIBC | [241] | Considered as a measure of transferrin (Tf) concentration in serum or plasma. Diagnosis for IDA. | 44.8–71.6 μmol/L | Serum | Y | ++ | |||
| Ferritin | [242] | Storage protein of iron and acts on the homeostasis and sequesteration | 15–200 μg/L | Plasma | Y | liver disease, rheumatoid arthritis, other inflammatory conditions or hyperthyroidism | +++ | ||
| Transferrin | [243] | Blood-plasma glycoprotein, which plays a central role in iron metabolism and is responsible for ferric-ion delivery | 2.0–3.8 g/L | Serum | Y | ++ | |||
| Zinc | [244] | Biological processes, as a structural, catalytic, and intracellular and intercellular signaling component | 7.7–23.0 μmol/L | ≤23.0 μmol/L *** | ≥23.0 μmol/L *** | Serum | Y | Nutritional deficiency from breast feeding, synergic interuption from iron supplementation, and prolonged pregnacy, subfertility. | +++ |
| Copper | [245,246] | As a catalytic cofactor in the redox chemistry of enzymes, mitochondrial respiration, iron absorption, free radical scavenging, and elastin cross-linking, it plays a critical role in cell physiology | 11.0–22.0 μmol/L | ≤22.0 μmol/L *** | ≥23.0 μmol/L *** | Serum | N/A | Contraception elements to reduce risks of pregnancy, hormonal amplitude, mood swings and induced cyclical bleeding. Anaemia, leukopenia, bone abnormalities, decreased pigmentation of skin and hair, neurological derangement | ++ |
| Fluoride | [157] | Accumulates in the body’s hard tissues and is known to serve a crucial role in the mineralization of bone and teeth | 1.0–100.0 mg/L | N/A | ≥100.0 mg/L *** | Urine | N | Child assessment for neurocognitive dysfunction, potential endometrial and impaired embryo implantation. | ++ |
| Selenium | [161] | Antioxidant and promoter for active thyroid hormone synthesis | 0.74–2.97 μmol/L | ≤2.97 μmol/L *** | ≥300 μmol/L *** | Whole Blood | Y | Pre-eclampsia, intrauterine growth restriction, gestational diabetes, and premature labour stem from deficiency. Hypertensive diseases in pregnancy. | +++ |
| Chromium | [247] | At the molecular level, chromium may have a function in maintaining normal glucose and lipid metabolism. In response to an insulin-mediated chromic ion flow, the oligopeptide chromodulin binds chromic ions, and the metal-saturated oligopeptide can attach to an insulin-stimulated insulin receptor, activating the receptor’s tyrosine kinase activity | 13.4–538.6 nmol/L | N/A | ≥550 nmol/L *** | Whole Blood | N | Toxicosis potential to induce lung and other forms of cancer, can aid in insulin resistance. | + |
| Iodine | [223,248,249,250,251] | Centrally around thyroid metabolism and function, crucial for the synthesis of thyroid hormones | 100–199 μg/L * | 150–249 μg/L ** | >500 μg/L considered excessive in pregnant women | Urine | Y | Hypo-/ Hyperthyroidism. Reduced Iodine levels may induce pre-eclampsia. Miscarriage, stillbirth, preterm delivery and fetal congenital abnormalities and pappilar cancer | ++ |
| Manganese | [226,252] | Involved in the creation and activation of several enzymes, as well as the control of glucose and lipid metabolism | 182–218 nmol/L | >218 nmol/L *** | Mn shortage and intoxication have been linked to negative metabolic and neuropsychiatric consequences. The prevalence of metabolic illnesses, such as type 2 diabetes mellitus (T2DM), obesity, insulin resistance, atherosclerosis, hyperlipidemia, nonalcoholic fatty liver disease (NAFLD), and hepatic steatosis, has risen substantially in recent decades | Whole Blood | N/A, further studies needed. | Mn is a hazardous trace element as well as a vital trace element involved in human health and development. Adverse metabolic and neuropsychiatric effects | ++ |
| Aluminum | [235,236,237,238] | N/A, considered as industrial material and no biological significance | 1–3 µg/L | N/A | >50 µg/L | Serum/Whole Blood | N/A | N/A, further studies required. | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dring, J.C.; Forma, A.; Chilimoniuk, Z.; Dobosz, M.; Teresiński, G.; Buszewicz, G.; Flieger, J.; Cywka, T.; Januszewski, J.; Baj, J. Essentiality of Trace Elements in Pregnancy, Fertility, and Gynecologic Cancers—A State-of-the-Art Review. Nutrients 2022, 14, 185. https://doi.org/10.3390/nu14010185
Dring JC, Forma A, Chilimoniuk Z, Dobosz M, Teresiński G, Buszewicz G, Flieger J, Cywka T, Januszewski J, Baj J. Essentiality of Trace Elements in Pregnancy, Fertility, and Gynecologic Cancers—A State-of-the-Art Review. Nutrients. 2022; 14(1):185. https://doi.org/10.3390/nu14010185
Chicago/Turabian StyleDring, James Curtis, Alicja Forma, Zuzanna Chilimoniuk, Maciej Dobosz, Grzegorz Teresiński, Grzegorz Buszewicz, Jolanta Flieger, Tomasz Cywka, Jacek Januszewski, and Jacek Baj. 2022. "Essentiality of Trace Elements in Pregnancy, Fertility, and Gynecologic Cancers—A State-of-the-Art Review" Nutrients 14, no. 1: 185. https://doi.org/10.3390/nu14010185
APA StyleDring, J. C., Forma, A., Chilimoniuk, Z., Dobosz, M., Teresiński, G., Buszewicz, G., Flieger, J., Cywka, T., Januszewski, J., & Baj, J. (2022). Essentiality of Trace Elements in Pregnancy, Fertility, and Gynecologic Cancers—A State-of-the-Art Review. Nutrients, 14(1), 185. https://doi.org/10.3390/nu14010185







