Role of Whole Grain Consumption in Glycaemic Control of Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction and Study Quality
2.3. Statistical Methods
3. Results
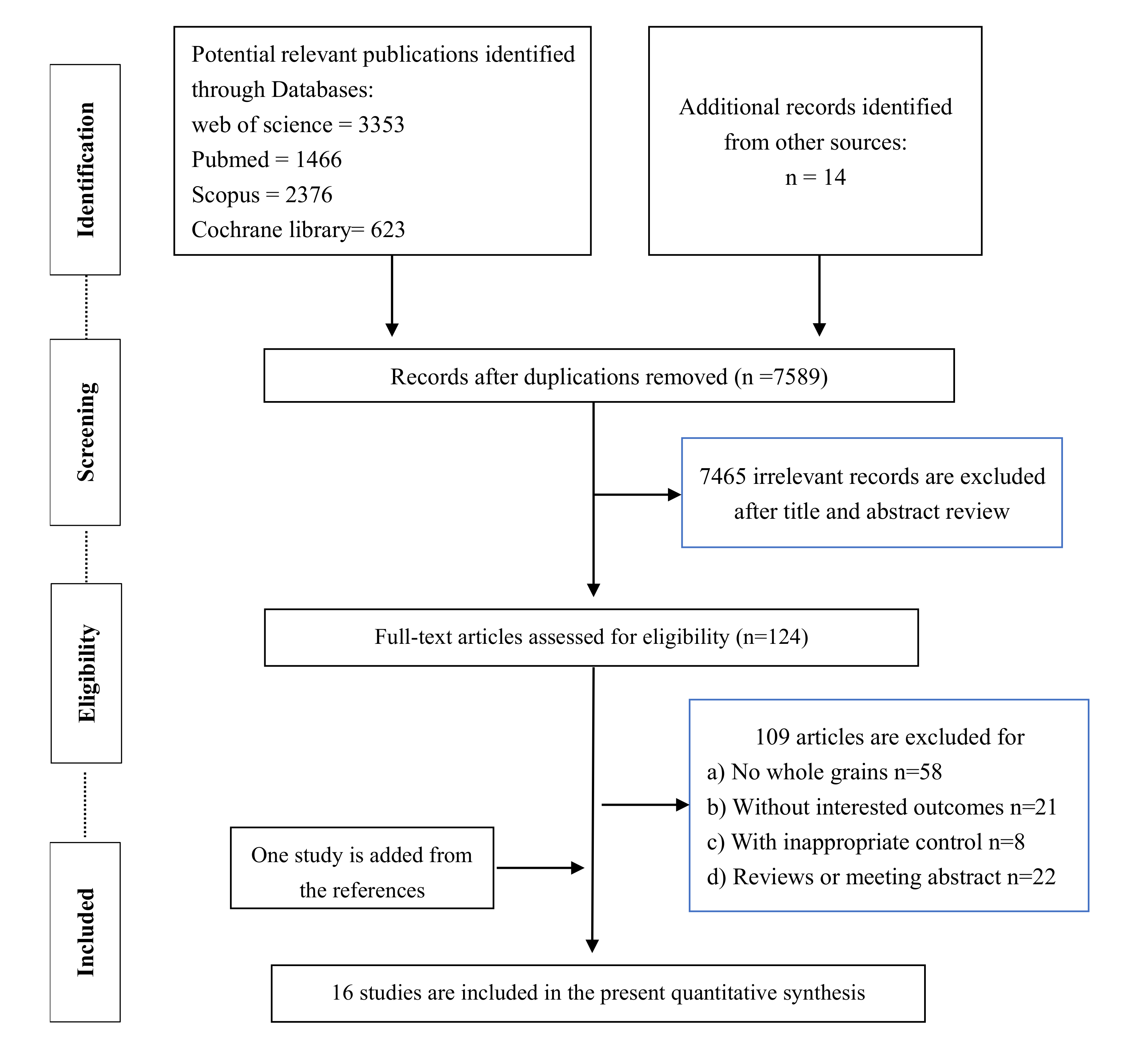
3.1. Literature Selection
3.2. Study Characteristics
3.3. Effects of Whole Grain Consumption on Parameters Involve Glycaemic Control
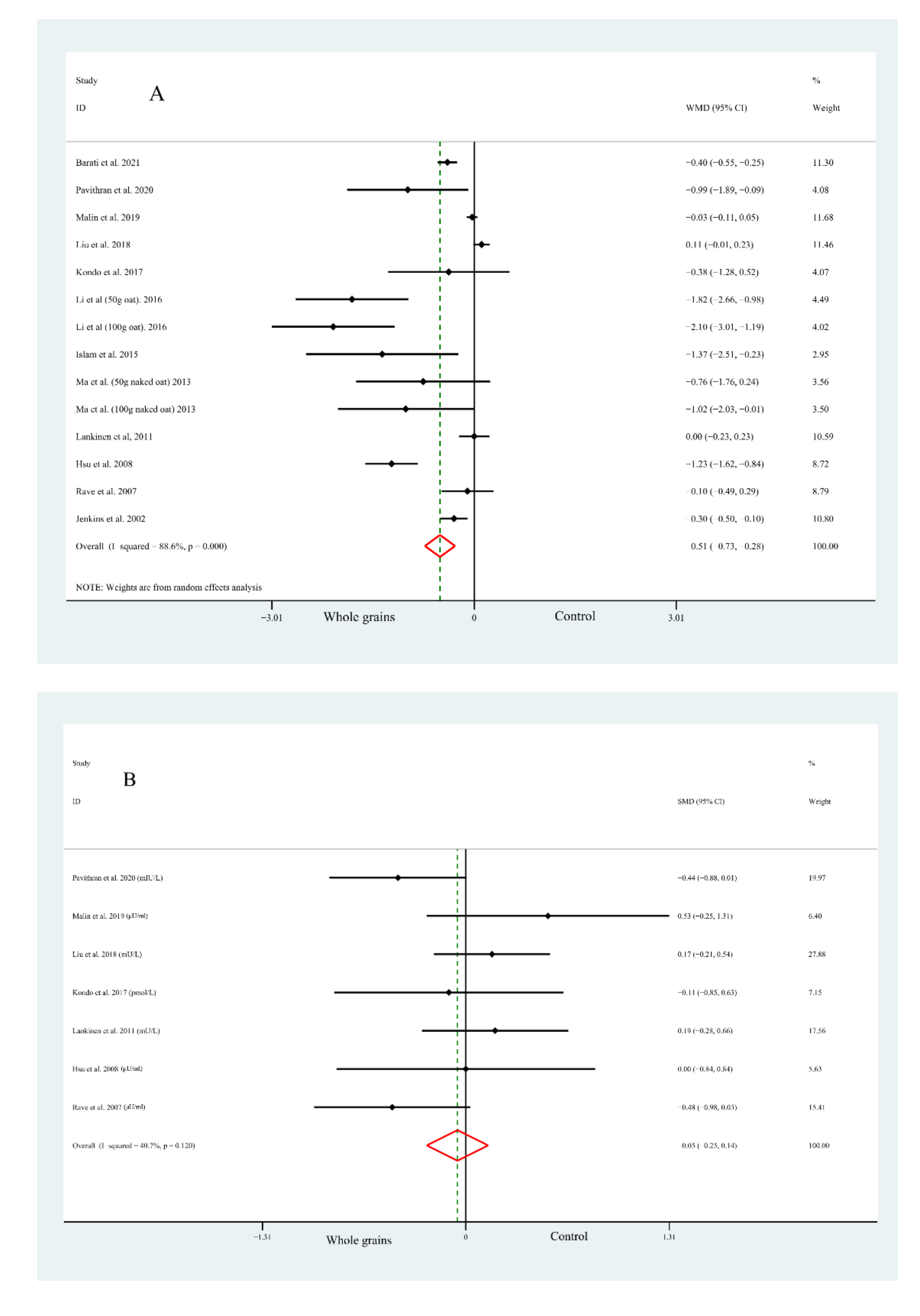
3.3.1. Fast Plasma GLUCOSE Concentrations
3.3.2. Fast Plasma Insulin Concentrations
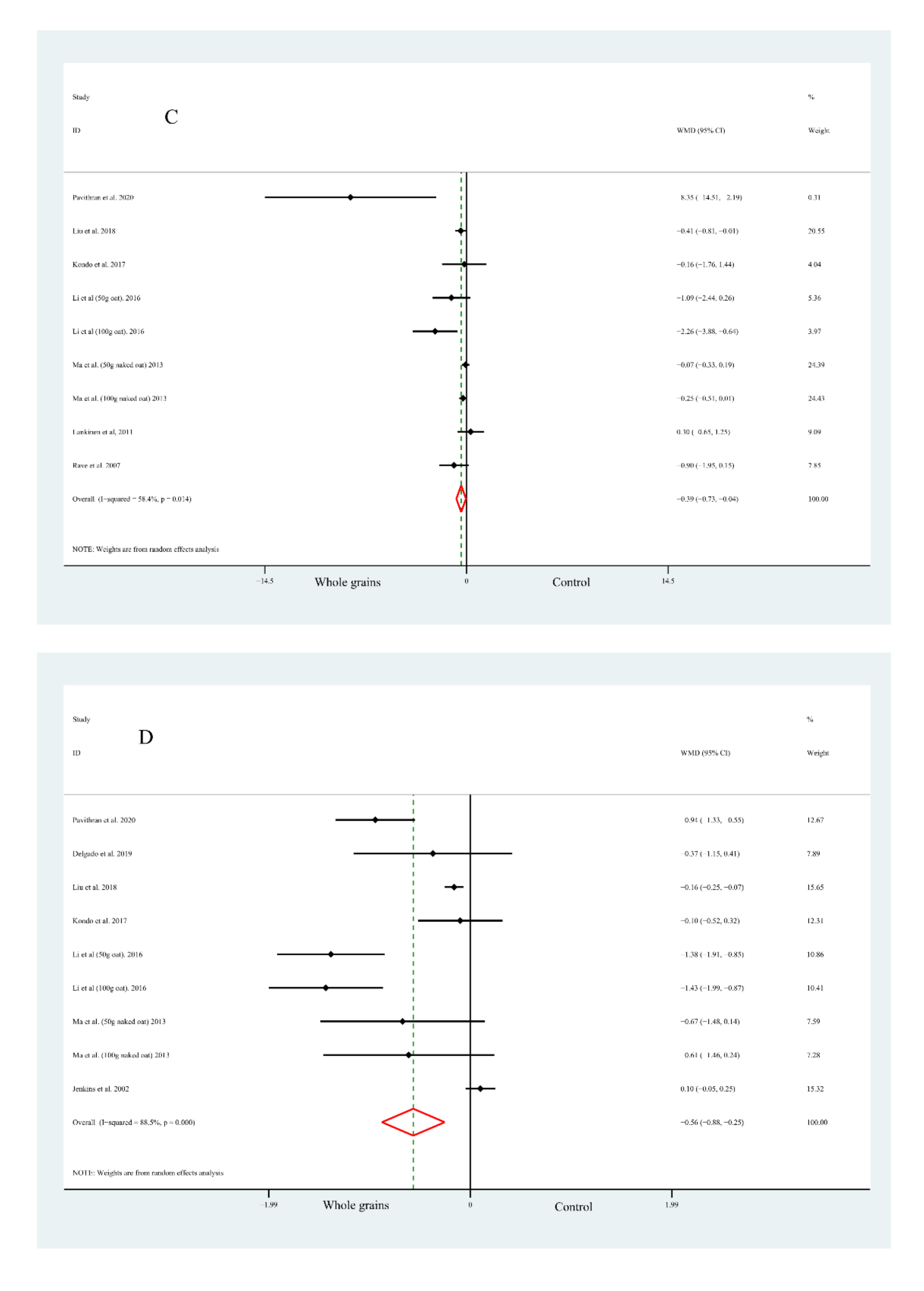
3.3.3. Homeostasis Model Assessment of Insulin Resistance
3.3.4. Glycosylated Haemoglobin
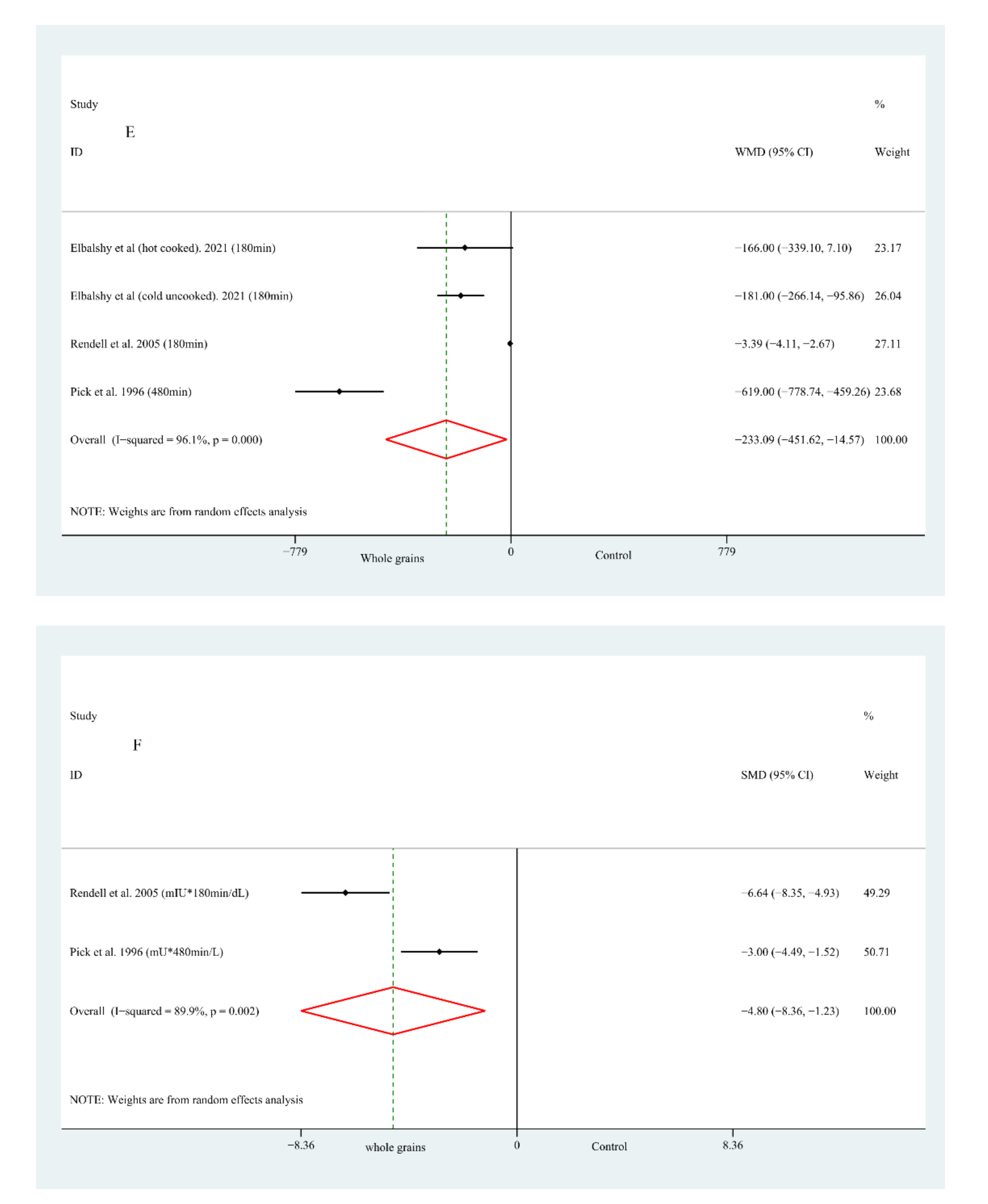
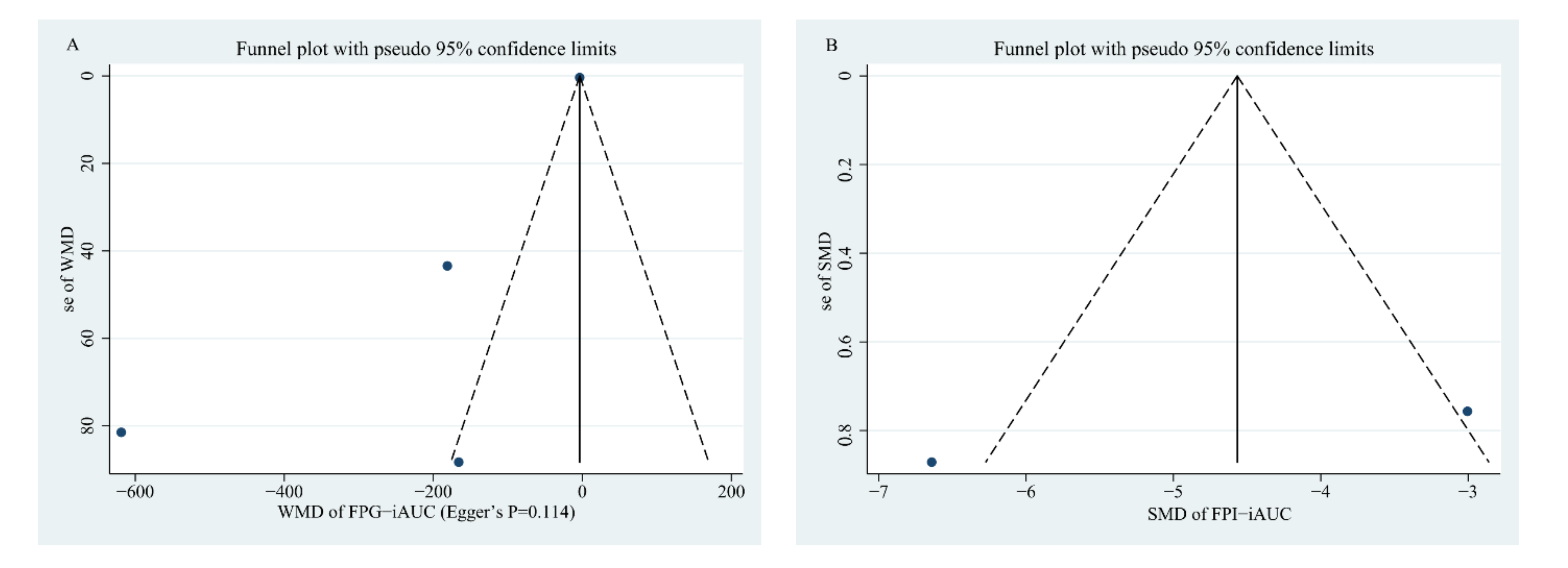
3.3.5. Glucose/Insulin Incremental Area under the Curve
3.4. Sensitivity Analysis
3.5. Subgroup Analysis
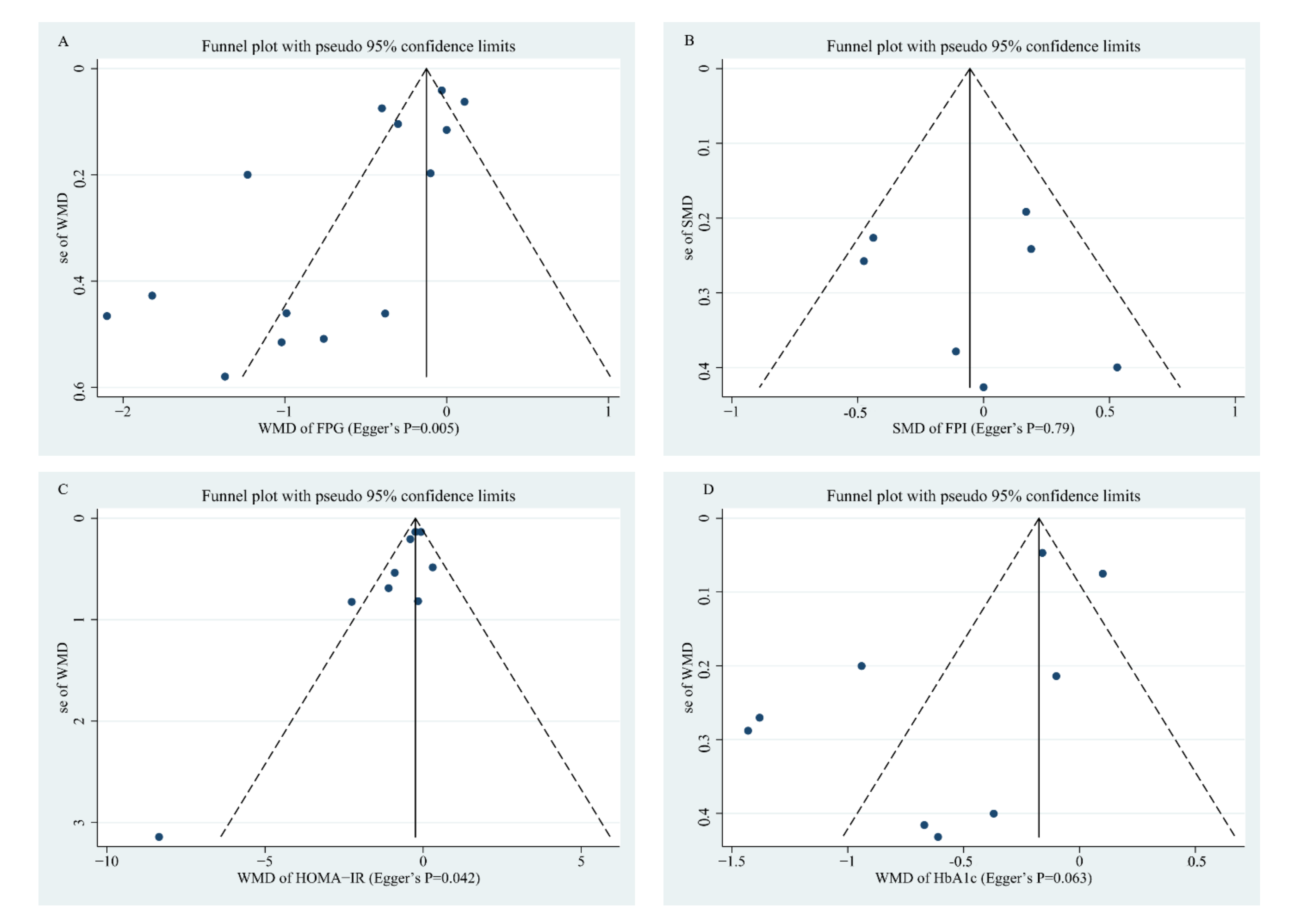
3.6. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- World Health Organization. Global Report on Diabetes; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Li, Y.Z.; Teng, D.; Shi, X.G.; Qin, G.J.; Qin, Y.F.; Quan, H.B.; Shi, B.Y.; Sun, H.; Ba, J.M.; Chen, B.; et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: National cross sectional study. BMJ Br. Med. J. 2020, 369, m997. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Arcidiacono, B.; Chiefari, E.; Brunetti, A.; Indolfi, C.; Foti, D.P. Type 2 Diabetes Mellitus and Cardiovascular Disease: Genetic and epigenetic Links. Front. Endocrinol. 2018, 9, 2. [Google Scholar] [CrossRef]
- Dal Canto, E.; Ceriello, A.; Ryden, L.; Ferrini, M.; Hansen, T.B.; Schnell, O.; Standl, E.; Beulens, J.W.J. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur. J. Prev. Cardiol. 2019, 26, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ Br. Med. J. 2016, 353, i2716. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.D.; Sang, S.M. Phytochemicals in whole grain wheat and their health-promoting effects. Mol. Nutr. Food Res. 2017, 61, 1600852. [Google Scholar] [CrossRef] [PubMed]
- Calinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [Green Version]
- Durazzo, A.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Lisciani, S.; Gambelli, L.; Aguzzi, A.; Novellino, E.; Santini, A.; et al. Dietary Lignans: Definition, Description and Research Trends in Databases Development. Molecules 2018, 23, 3251. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, L.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ Br. Med. J. 2018, 361, k2179. [Google Scholar] [CrossRef] [Green Version]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.P.; Zhang, F.; Ding, X.Y.; Wu, G.J.; Lam, Y.Y.; Wang, X.J.; Fu, H.Q.; Xue, X.H.; Lu, C.H.; Ma, J.L.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanegas, S.M.; Meydani, M.; Barnett, J.B.; Goldin, B.; Kane, A.; Rasmussen, H.; Brown, C.; Vangay, P.; Knights, D.; Jonnalagadda, S.; et al. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am. J. Clin. Nutr. 2017, 105, 635–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.; Hernández, M.A.G.; Goossens, G.H.; Reijnders, D.; Holst, J.J.; Jocken, J.W.E.; van Eijk, H. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci. Rep. 2019, 9, 12515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lappi, J.; Mykkanen, H.; Knudsen, K.E.B.; Kirjavainen, P.; Katina, K.; Pihlajamaki, J.; Poutanen, K.; Kolehmainen, M. Postprandial glucose metabolism and SCFA after consuming wholegrain rye bread and wheat bread enriched with bioprocessed rye bran in individuals with mild gastrointestinal symptoms. Nutr. J. 2014, 13, 104. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, A.C.; Johansson-Boll, E.V.; Borck, I.M.E. Increased gut hormones and insulin sensitivity index following a 3-d intervention with a barley kernel-based product: A randomised cross-over study in healthy middle-aged subjects. Br. J. Nutr. 2015, 114, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef]
- Li, X.; Cai, X.; Ma, X.; Jing, L.; Gu, J.; Bao, L.; Li, J.; Xu, M.; Zhang, Z.; Li, Y. Short- and Long-Term Effects of Wholegrain Oat Intake on Weight Management and Glucolipid Metabolism in Overweight Type-2 Diabetics: A Randomized Control Trial. Nutrients 2016, 8, 549. [Google Scholar] [CrossRef] [Green Version]
- McGeoch, S.C.; Johnstone, A.M.; Lobley, G.E.; Adamson, J.; Hickson, K.; Holtrop, G.; Fyfe, C.; Clark, L.F.; Pearson, D.W.M.; Abraham, P.; et al. A randomized crossover study to assess the effect of an oat-rich diet on glycaemic control, plasma lipids and postprandial glycaemia, inflammation and oxidative stress in Type 2 diabetes. Diabet. Med. 2013, 30, 1314–1323. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ Br. Med. J. 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Elbalshy, M.M.; Reynolds, A.N.; Mete, E.; Robinson, C.; Oey, I.; Silcock, P.; Haszard, J.J.; Perry, T.L.; Mann, J.; Te Morenga, L. Gelatinisation and milling whole-wheat increases postprandial blood glucose: Randomised crossover study of adults with type 2 diabetes. Diabetologia 2021, 64, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Barati, Z.; Iravani, M.; Karandish, M.; Haghighizadeh, M.H.; Masihi, S. The effect of oat bran consumption on gestational diabetes: A randomized controlled clinical trial. BMC Endocr. Disord. 2021, 21, 67. [Google Scholar] [CrossRef]
- Pavithran, N.; Kumar, H.; Menon, A.S.; Pillai, G.K.; Sundaram, K.R.; Ojo, O. South Indian Cuisine with Low Glycemic Index Ingredients Reduces Cardiovascular Risk Factors in Subjects with Type 2 Diabetes. Int. J. Environ. Res. Public Health 2020, 17, 6232. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Kullman, E.L.; Scelsi, A.R.; Godin, J.P.; Ross, A.B.; Kirwan, J.P. A Whole-Grain Diet Increases Glucose-Stimulated Insulin Secretion Independent of Gut Hormones in Adults at Risk for Type 2 Diabetes. Mol. Nutr. Food Res. 2019, 63, 1800967. [Google Scholar] [CrossRef]
- Delgado, G.; Kleber, M.E.; Krämer, B.K.; Morcos, M.; Humpert, P.M.; Wiegand, K.; Mauldin, A.; Kusterer, K.; Enghöfer, M.; März, W.; et al. Dietary Intervention with Oatmeal in Patients with uncontrolled Type 2 Diabetes Mellitus—A Crossover Study. Exp. Clin. Endocrinol. Diabetes 2019, 127, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiu, J.; Yue, Y.; Li, K.; Ren, G. Dietary black-grained wheat intake improves glycemic control and inflammatory profile in patients with type 2 diabetes: A randomized controlled trial. Ther. Clin. Risk Manag. 2018, 14, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Kondo, K.; Morino, K.; Nishio, Y.; Ishikado, A.; Arima, H.; Nakao, K.; Nakagawa, F.; Nikami, F.; Sekine, O.; Nemoto, K.I.; et al. Fiber-rich diet with brown rice improves endothelial function in type 2 diabetes mellitus: A randomized controlled trial. PLoS ONE 2017, 12, e0179869. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Kamruzzaman, M.; Islam, M.S.; Elahi, M.T.; Rahman, S.S.; Paul, D.K.; Chaudhury, M.A.Z.; Rouf, S.A.; Samad, M.A. Impact of Bread Made from Mix Cereals and Pulses on the Glycemic Profile in Type 2 Diabetic Patients—A Randomized Controlled Trial. Curr. Nutr. Food Sci. 2015, 11, 136–144. [Google Scholar] [CrossRef]
- Ma, X.; Gu, J.; Zhang, Z.; Jing, L.; Xu, M.; Dai, X.; Jiang, Y.; Li, Y.; Bao, L.; Cai, X.; et al. Effects of Avena nuda L. on metabolic control and cardiovascular disease risk among Chinese patients with diabetes and meeting metabolic syndrome criteria: Secondary analysis of a randomized clinical trial. Eur. J. Clin. Nutr. 2013, 67, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Lankinen, M.; Schwab, U.; Kolehmainen, M.; Paananen, J.; Poutanen, K.; Mykkänen, H.; Seppänen-Laakso, T.; Gylling, H.; Uusitupa, M.; Orešič, M. Whole grain products, fish and bilberries alter glucose and lipid metabolism in a randomized, controlled trial: The Sysdimet study. PLoS ONE 2011, 6, e22646. [Google Scholar] [CrossRef] [Green Version]
- Hsu, T.F.; Kise, M.; Wang, M.F.; Ito, Y.; Yang, M.D.; Aoto, H.; Yoshihara, R.; Yokoyama, J.; Kunii, D.; Yamamoto, S. Effects of pre-germinated brown rice on blood glucose and lipid levels in free-living patients with impaired fasting glucose or type 2 diabetes. J. Nutr. Sci. Vitaminol. 2008, 54, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Rave, K.; Roggen, K.; Dellweg, S.; Heise, T.; tom Dieck, H. Improvement of insulin resistance after diet with a whole-grain based dietary product: Results of a randomized, controlled cross-over study in obese subjects with elevated fasting blood glucose. Br. J. Nutr. 2007, 98, 929–936. [Google Scholar] [CrossRef]
- Rendell, M.; Vanderhoof, J.; Venn, M.; Shehan, M.A.; Arndt, E.; Rao, C.S.; Gill, G.; Newman, R.K.; Newman, C.W. Effect of a barley breakfast cereal on blood glucose and insulin response in normal and diabetic patients. Plant Foods Hum. Nutr. 2005, 60, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Augustin, L.S.A.; Martini, M.C.; Axelsen, M.; Faulkner, D.; Vidgen, E.; Parker, T.; Lau, H.; Connelly, P.W.; et al. Effect of wheat bran on glycemic control and risk factors for cardiovascular disease in type 2 diabetes. Diabetes Care 2002, 25, 1522–1528. [Google Scholar] [CrossRef] [Green Version]
- Pick, M.E.; Hawrysh, Z.J.; Gee, M.I.; Toth, E.; Garg, M.L.; Hardin, R.T. Oat bran concentrate bread products improve long-term control of diabetes: A pilot study. J. Am. Diet. Assoc. 1996, 96, 1254–1261. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef]
- Sa’ad-Aldin, K.; Altamimi, M. Effect of whole-grain plant-based diet on the diabetes mellitus type 2 features in newly diagnosed patients: A pilot study. Int. J. Diabetes Dev. Ctries. 2019, 39, 535–546. [Google Scholar] [CrossRef]
- Assoc, A.D. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 36, S67–S74. [Google Scholar] [CrossRef] [Green Version]
- Muller, M.; Canfora, E.E.; Blaak, E.E. Gastrointestinal Transit Time, Glucose Homeostasis and Metabolic Health: Modulation by Dietary Fibers. Nutrients 2018, 10, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, N.N.; Purslow, P.P.; Tosh, S.M.; Bakovic, M. Oat beta-glucan depresses SGLT1-and GLUT2-mediated glucose transport in intestinal epithelial cells (IEC-6). Nutr. Res. 2016, 36, 541–552. [Google Scholar] [CrossRef]
- Qin, J.J.; Li, Y.R.; Cai, Z.M.; Li, S.H.; Zhu, J.F.; Zhang, F.; Liang, S.S.; Zhang, W.W.; Guan, Y.L.; Shen, D.Q.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, F.; Bloks, V.W.; Groen, A.K. Beyond intestinal soap-bile acids in metabolic control. Nat. Rev. Endocrinol. 2014, 10, 488–498. [Google Scholar] [CrossRef]
- Kumar, D.P.; Asgharpour, A.; Mirshahi, F.; Park, S.H.; Liu, S.C.; Imai, Y.M.; Nadler, J.L.; Grider, J.R.; Murthy, K.S.; Sanyal, A.J. Activation of Transmembrane Bile Acid Receptor TGR5 Modulates Pancreatic Islet Cells to Promote Glucose Homeostasis. J. Biol. Chem. 2016, 291, 6626–6640. [Google Scholar] [CrossRef] [Green Version]
- Caron, S.; Samanez, C.H.; Dehondt, H.; Ploton, M.; Briand, O.; Lien, F.; Dorchies, E.; Dumont, J.; Postic, C.; Cariou, B.; et al. Farnesoid X Receptor Inhibits the Transcriptional Activity of Carbohydrate Response Element Binding Protein in Human Hepatocytes. Mol. Cell. Biol. 2013, 33, 2202–2211. [Google Scholar] [CrossRef] [Green Version]
- Marshall, S.; Petocz, P.; Duve, E.; Abbott, K.; Cassettari, T.; Blumfield, M.; Fayet-Moore, F. The Effect of Replacing Refined Grains with Whole Grains on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with GRADE Clinical Recommendation. J. Acad. Nutr. Diet. 2020, 120, 1859. [Google Scholar] [CrossRef]
- Janus, A.; Szahidewicz-Krupska, E.; Mazur, G.; Doroszko, A. Insulin Resistance and Endothelial Dysfunction Constitute a Common Therapeutic Target in Cardiometabolic Disorders. Mediat. Inflamm. 2016, 2016, 3634948. [Google Scholar] [CrossRef] [Green Version]
- Wirstrom, T.; Hilding, A.; Gu, H.F.; Ostenson, C.G.; Bjorklund, A. Consumption of whole grain reduces risk of deteriorating glucose tolerance, including progression to prediabetes. Am. J. Clin. Nutr. 2013, 97, 179–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.S.; Kim, H.; Jung, M.H.; Hong, S.; Song, J. Consumption of barley beta-glucan ameliorates fatty liver and insulin resistance in mice fed a high-fat diet. Mol. Nutr. Food Res. 2010, 54, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Saad, M.J.A.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef]
- Faits, T.; Walker, M.E.; Rodriguez-Morato, J.; Meng, H.C.; Gervis, J.E.; Galluccio, J.M.; Lichtenstein, A.H.; Johnson, W.E.; Matthan, N.R. Exploring changes in the human gut microbiota and microbial-derived metabolites in response to diets enriched in simple, refined, or unrefined carbohydrate-containing foods: A post hoc analysis of a randomized clinical trial. Am. J. Clin. Nutr. 2020, 112, 1631–1641. [Google Scholar] [CrossRef]
- Ginos, B.N.R.; Navarro, S.L.; Schwarz, Y.; Gu, H.W.; Wang, D.F.; Randolph, T.W.; Shojaie, A.; Hullar, M.A.J.; Lampe, P.D.; Kratz, M.; et al. Circulating bile acids in healthy adults respond differently to a dietary pattern characterized by whole grains, legumes and fruits and vegetables compared to a diet high in refined grains and added sugars: A randomized, controlled, crossover feeding study. Metab. Clin. Exp. 2018, 83, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Vetrani, C.; Vitale, M.; Godos, J.; Riccardi, G.; Grosso, G. Whole Grain Intake and Glycaemic Control in Healthy Subjects: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2017, 9, 769. [Google Scholar] [CrossRef]
- Bao, L.; Cai, X.X.; Xu, M.H.; Li, Y. Effect of oat intake on glycaemic control and insulin sensitivity: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2014, 112, 457–466. [Google Scholar] [CrossRef]
- Shen, X.L.; Zhao, T.; Zhou, Y.Z.; Shi, X.Q.; Zou, Y.; Zhao, G.H. Effect of Oat beta-Glucan Intake on Glycaemic Control and Insulin Sensitivity of Diabetic Patients: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, A.J.; Vandermey, J.S.; Robinson, L.E.; Graham, T.E.; Bakovic, M.; Duncan, A.M. Effects of breads of varying carbohydrate quality on postprandial glycaemic, incretin and lipidaemic response after first and second meals in adults with diet-controlled type 2 diabetes. J. Funct. Foods 2014, 6, 116–125. [Google Scholar] [CrossRef]
| First Author, Year (Country) | Sample Size (Male/ Female) | Age (Year) | Intervention Meals | Amounts of Whole Grains | Matching Foods | Type of Diabetes Mellitus | Outcomes Evaluated | Duration (Week) | Study Design |
|---|---|---|---|---|---|---|---|---|---|
| Elbalshy et al., 2021[26] (New Zealand) | 18 (11/7) | 63.10 ± 9.80 | I1: Hot cooked Coarse kibbled whole grain I2: Cold uncooked Coarse kibbled whole grain | 50 g | Finely milled whole grain | T2DM | G-iAUC | None | Randomized crossover trial |
| Barati et al., 2021[27] (Iran) | 104 (0/104) | C = 28.72 ± 4.13; I = 29.23 ± 3.80 | Standard diet with oat bran | 30 g | Standard diet without oat bran | Gestational diabetes | FPG | 4 weeks | Randomized controlled trial |
| Pavithran et al., 2020 [28] (India) | 80 (52/28) | C = 51.93 ± 7.43; I = 54.43 ± 7.57 | Local low GI whole grain | None | Usual diet | T2DM | FPG; FPI; HOMA-IR; HbA1c | 24 weeks | Randomized controlled trial |
| Malin et al., 2019 [29] (USA) | 13 (10/3) | 37.20 ± 1.80 | Whole grain | 100 | Refined-grain diet | Prediabetic adults | FPG; FPI; | 8 weeks | Randomized controlled crossover trial |
| Delgado et al., 2019 [30] (Germany) | 15 (8/7) | 58.6 (10.1) | Oatmeal | 100 | Usual diet | T2DM | HbA1c | 12 weeks | Randomized controlled crossover trial |
| Liu et al., 2018 [31] (China) | 110 (50/60) | C = 57.40 ± 8.80; I = 58.50 ± 10.20 | Black-grained wheat | 50 | Rice | T2DM | FPG; HbA1c; FPI; HOMA-IR | 5-weeks | Randomized controlled trial |
| Kondo et al., 2017 [32] (Japan) | 28 (18/10) | C = 68.10 ± 6.80; I = 65.20 ± 8.70 | Brown rice | 28–30 kcal/kg | White rice | T2DM | FPG; FPI; HbA1c; HOMA-IR | 8 weeks | Randomized controlled trial |
| Li et al., 2016 [21] (China) | 219 (113/106) | C = 59.00 ± 3.94; I1 = 59.72 ± 6.10; I2 = 59.44 ± 6.78 | Whole grain oat | 50;100 | Usual care | T2DM | FPG; HbA1c; HOMA-IR | 1 years; 30 days | Randomized controlled trial |
| Islam et al., 2015 [33] (Bangladesh) | 24 (16/8) | 52.83 ± 5.88 | Composite flour bread | None | Normal wheat flour | T2DM | FPG; | 4 weeks | Randomized controlled trial |
| Ma et al., 2013 [34] (China) | 260 (112/148) | C = 59.30 ± 6.60; I1 = 59.40 ± 6.10; I2 = 60.30 ± 6.00 | Organic naked oat with whole germ | 50;100 | Systematic diet plans | T2DM | FPG; HbA1c; HOMA-IR | 30 days | Randomized controlled trial |
| Lankinen et al., 2011 [35] (Finland) | 106 (52/54) | C = 59.00 ± 7.00; I = 58.00 ± 8.00; | Whole grain enriched diet | None | Refined wheat breads | Patients with impaired glucose metabolism | FPG; FPI; HOMA-IR | 12 weeks | Randomized controlled trial |
| Hsu et al., 2008 [36] (Taiwan, China) | 11 (6/5) | 51.50 ± 16.20 | Pre-germinated brown rice | 540 | White rice | Patients with impaired fasting glucose and type 2 diabetes | FPG; FPI; | 12 weeks | Randomized controlled crossover trial |
| Rave et al., 2007 [37] (Germany) | 31 (13/18) | 51.00 ± 13.00 | Whole grain-based diet- ary product with reduced starch content derived from double-fermented wheat | 200 | nutrient-dense meal replacement product | Obese subjects with elevated fasting blood glucose | FPG; FPI; HOMA-IR | 4 weeks | Randomized controlled crossover trial |
| Rendell et al., 2005 [38] (USA) | 18 (12/6) | 62.00 ± 3.00 | Prowash barley flakes; | None | A liquid meal replacer | T2DM | G-iAUC; I-iAUC | None | Randomized controlled crossover trial |
| Jenkins et al., 2002 [39] (Canada) | 23 (16/7) | 63.00 ± 1.00 | Wheat bran | None | Controlled diet with low fiber | T2DM | FPG; HbA1c | 12 weeks | Randomized crossover study |
| Pick et al., 1996 [40] (Canada) | 8 (8/0) | 46.00 ± 1.00 | Oat bran concentrate | None | White bread | Subjects with non-insulin-dependent diabetes | G-iAUC; I-iAUC | 24 weeks | Randomized crossover study |
| Fast Plasma Glucose | Fast Plasma Insulin 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of Studies | WMD (95% CI) | Heterogeneity | No. of Studies | WMD (95% CI) | Heterogeneity | |||
| p | p | |||||||
| Study design | ||||||||
| Parallel | 10 | −0.68 (−1.03, −0.33) | 88.0 | <0.001 | 4 | −0.02 (−0.25, 0.21) | 42.3 | 0.158 |
| Crossover | 4 | −0.39 (−0.79, 0.01) | 92.2 | <0.001 | 3 | −0.14 (−0.52, 0.24) | 56.8 | 0.099 |
| Type | ||||||||
| T2DM | 9 | −0.84 (−1.29, −0.40) | 87.4 | <0.001 | 3 | −0.09 (−0.36, 0.18) | 52.4 | 0.122 |
| Others | 5 | −0.32 (−0.62, −0.02) | 92.1 | <0.001 | 4 | −0.01S (−0.31, 0.28) | 48.1 | 0.123 |
| Matching foods | ||||||||
| Standard diet 2 | 5 | −0.22 (−0.44, −0.00) | 85.5 | <0.001 | 2 | 0.28 (−0.12, 0.69) | 0 | 0.464 |
| Others | 9 | −0.87 (−1.43, −0.31) | 90.8 | <0.001 | 5 | −0.16 (−0.39, 0.07) | 34.7 | 0.190 |
| Duration | ||||||||
| <8 weeks | 6 | −0.36 (−0.72, 0.00) | 86.4 | <0.001 | 2 | −0.06 (−0.36, 0.24) | 75.2 | 0.045 |
| ≥8 weeks | 8 | −0.70 (−1.07, −0.33) | 91.0 | <0.001 | 5 | −0.05 (−0.31, 0.21) | 34.2 | 0.193 |
| National economic levels | ||||||||
| Developing | 10 | −0.84 (−1.20, −0.47) | 91 | <0.001 | 3 | −0.08 (−0.35, 0.20) | 52.7 | 0.121 |
| Developed | 4 | −0.03 (−0.11, 0.04) | 0 | 0.857 | 4 | −0.03 (−0.32, 0.26) | 48.6 | 0.120 |
| HOMA-IR | HbA1c | |||||||
| Study design | ||||||||
| Parallel | 8 | −0.34 (−0.70. 0.02) | 60.5 | 0.013 | 7 | −0.74 (−1.19, −0.28) | 88.4 | <0.001 |
| Crossover | 1 | −0.90 (−1.95, 0.15) | ~ | ~ | 2 | 0.03 (−0.30, 0.36) | 24.9 | 0.249 |
| Type | ||||||||
| T2DM | 7 | −0.42 (−0.80, −0.03) | 63.6 | 0.011 | 9 | −0.58 (−0.88, −0.25) | 88.5 | <0.001 |
| Others | 2 | −0.28 (−1.45, 0.90) | 63.6 | 0.097 | 0 | ~ | ~ | ~ |
| Matching foods | ||||||||
| Standard diet 2 | 1 | 0.30 (−0.65, 1.25) | ~ | ~ | 1 | 0.10 (−0.05, 0.25) | ~ | ~ |
| Others | 8 | −0.46 (−0.83, −0.09) | 61.0 | 0.012 | 8 | −0.70 (−1.11, −0.28) | 86.5 | <0.001 |
| Duration | ||||||||
| <8 weeks | 4 | −0.24 (−0.43, −0.04) | 19.9 | 0.290 | 3 | −0.26 (−0.54, 0.02) | 20.9 | 0.283 |
| ≥8 weeks | 5 | −1.08 (−2.46, 0.31) | 72.2 | 0.006 | 6 | −0.67 (−1.27, −0.08) | 92.6 | <0.001 |
| National economic levels | ||||||||
| Developing | 6 | −0.45 (−0.86, −0.03) | 69.9 | 0.006 | 7 | −0.66 (−1.03, −0.30) | 91.3 | <0.001 |
| Developed | 3 | −0.24 (−1.02, 0.54) | 27.5 | 0.252 | 2 | −0.16 (−0.53, 0.21) | 0 | 0.552 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Fu, L.; Pan, D.; Lu, Y.; Yang, C.; Wang, Y.; Wang, S.; Sun, G. Role of Whole Grain Consumption in Glycaemic Control of Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 109. https://doi.org/10.3390/nu14010109
Xu D, Fu L, Pan D, Lu Y, Yang C, Wang Y, Wang S, Sun G. Role of Whole Grain Consumption in Glycaemic Control of Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2022; 14(1):109. https://doi.org/10.3390/nu14010109
Chicago/Turabian StyleXu, Dengfeng, Lingmeng Fu, Da Pan, Yifei Lu, Chao Yang, Yuanyuan Wang, Shaokang Wang, and Guiju Sun. 2022. "Role of Whole Grain Consumption in Glycaemic Control of Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 14, no. 1: 109. https://doi.org/10.3390/nu14010109
APA StyleXu, D., Fu, L., Pan, D., Lu, Y., Yang, C., Wang, Y., Wang, S., & Sun, G. (2022). Role of Whole Grain Consumption in Glycaemic Control of Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 14(1), 109. https://doi.org/10.3390/nu14010109








