Abstract
Magnesium lithospermate B (MLB) is a primary hydrophilic component of Danshen, the dried root of Salvia miltiorrhiza used in traditional medicine, and its beneficial effects on obesity-associated metabolic abnormalities were reported in our previous study. The present study investigated the anti-muscle atrophy potential of MLB in mice with high-fat diet (HFD)-induced obesity. In addition to metabolic abnormalities, the HFD mice had a net loss of skeletal muscle weight and muscle fibers and high levels of muscle-specific ubiquitin E3 ligases, namely the muscle atrophy F-box (MAFbx) and muscle RING finger protein 1 (MuRF-1). MLB supplementation alleviated those health concerns. Parallel changes were revealed in high circulating tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), skeletal TNF receptor I (TNFRI), nuclear factor-kappa light chain enhancer of activated B cells (NF-κB), p65 phosphorylation, and Forkhead box protein O1 (FoxO1) as well as low skeletal phosphoinositide 3-kinase (PI3K) and protein kinase B (Akt) phosphorylation. The study revealed that MLB prevented obesity-associated skeletal muscle atrophy, likely through the inhibition of MAFbx/MuRF-1-mediated muscular degradation. The activation of the PI3K-Akt-FoxO1 pathway and inhibition of the TNF-α/TNFRI/NF-κB pathway were assumed to be beneficial effects of MLB.
1. Introduction
Muscle atrophy occurs as a result of a net loss of muscle mass. In addition to its devastating effects on human health, muscle atrophy is a highly recognized risk factor for physical disabilities and a poor quality of life [1,2]. The majority of muscle mass depends on the counterbalance between protein anabolism and catabolism. Insufficient protein synthesis and overwhelmed protein degradation predispose people to muscle atrophy [3,4]. The prevalence of muscle atrophy is continually increasing because it is a complication of many acute and chronic diseases [5]. Therefore, a more comprehensive understanding of its pathogenic mechanism is necessary for the development of strategies to combat it and its associated sequelae.
Autophagy, caspases, and particularly the ubiquitin-proteasome system are three families of proteolytic enzymes common to protein degradation [6]. The core of the ubiquitin-proteasome system consists of three groups of enzymes, namely the ubiquitin E1 activating enzyme, ubiquitin E2 conjugating enzyme, and ubiquitin E3 ligase. Functional diversity and targeted specificity of the ubiquitin-proteasome system rely on the introduction of ubiquitin E3 ligase [7]. Muscle atrophy F-box (MAFbx)/atrogin-1 and muscle RING finger protein 1 (MuRF-1) are two muscle-specific ubiquitin E3 ligases that are highly expressed in the skeletal muscles under atrophy-prone conditions. Their transcriptional activation is closely linked with muscle atrophy independent of etiologies, and the inhibition of their expression reduces muscle mass loss [8]. Evidence has revealed the crucial roles of nuclear factor kappa light chain enhancer of activated B cells (NF-κB) and the Forkhead box protein O1 (FoxO) family transcription factor in the transcriptional activation of MAFbx and MuRF-1 and the consequences of muscle atrophy [5,8,9]. The phenomena underscore the roles of NF-κB and FoxO intervention as preventive or therapeutic options for patients with muscle atrophy.
Herbal plants are vital sources of biologically active compounds and are beneficial to patients with metabolic disorders [10]. Danshen, the root of the medicinal plant Salvia miltiorrhiza, is traditionally used to improve daily functioning and treat a wide variety of diseases, including coronary heart disease, hepatitis, menstrual disorders, blood circulation diseases, and cardiovascular diseases [11]. Magnesium lithospermate B (MLB), a derivative of a caffeic acid tetramer metabolized in the liver and excreted through the bile, is the primary hydrophilic component of Danshen [12]. It exerts pharmacological effects, such as anti-ischemia reperfusion, and contains antioxidant, anti-inflammatory, and antitumor properties [13,14,15]. Although the components of Danshen have indicated the promotion of muscle anabolism and prevention of muscle wasting [16,17], a study focused on MLB has not yet been conducted. MLB’s anti-inflammatory properties result from the inhibition of NF-κB [18], and MLB promotes Akt activity, a critical negative regulator of the FoxO family of transcription factors [19]. Its effects on NF-κB and Akt suggest that MLB may benefit the treatment of muscle atrophy.
Skeletal muscle atrophy is a common complication of obesity, a metabolic disease characterized by insulin resistance and inflammation, with poor outcomes [20,21,22]. Protein degradation appears to have a dominant role in obesity-associated skeletal muscle atrophy [23]. Previous studies revealed that MLB diminishes high-fat diet (HFD)-induced metabolic abnormalities through reducing obesity, fatty liver, glucose intolerance, and insulin resistance [24], and aging and obesity-induced ER stress, insulin resistance, and inflammasome formation in the liver [25]. To expand the study of MLB’s nutraceutical potential, we hypothesized that MLB may weaken skeletal muscle atrophy through acting on MAFbx and MuRF-1. To complete our working hypothesis, obesity-associated skeletal muscle atrophy was modeled in mice through an HFD and daily administrations of MLB.
2. Materials and Methods
2.1. Animals
Seven-week-old male C57BL/6J mice were purchased from BioLasco, Taiwan Co., Ltd. (Taipei, Taiwan). The mice were maintained on a 12:12 light–dark cycle with unrestricted access to regular food (5008 Rodent LabDiet, PMI Nutrition International Inc., St. Louis, MO, USA) and water. After a 1-week acclimation period, the mice were randomly divided into three groups. The control group (n = 8) consisted of mice that continued to eat regular food and the high-fat (HF) group (n = 8) comprised mice that ate high-fat food (high-fat Rodent TestDiet, PMI Nutrition International Inc., St. Louis, MO, USA); 67% of calories were obtained from fat. The HF + MLB group (n = 8) consisted of mice that consumed HF food and daily MLB supplements (100 mg/kg body weight/day) that were dissolved in water and administrated through oral gavage. MLB, purified from S. miltiorrhiza, was purchased from KO DA Pharmaceutical Co., Ltd. (Taoyuan, Taiwan) with a purity of approximately 85%.
2.2. Fasting Blood Glucose Measurement
After 17 weeks of supplementation, the mice fasted overnight. A blood droplet from a tail clipping and a glucometer with test strips (Roche Ltd., Basel, Switzerland) were used to measure each mouse’s fasting blood glucose level.
2.3. Serum Analyses
Blood samples were collected through cardiac puncture and were centrifuged at 3000 rpm for 10 min to separate the serum. The serum levels of aspartate transaminase (AST), alanine transaminase (ALT), total cholesterol, and triacylglycerol were measured using a clinical chemistry analyzer (Hitachi Autoanalyzer 7070, Hitachi Ltd., Tokyo, Japan). The levels of insulin (Crystal Chem Inc., Elk Grove Village, Illinois, USA), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6; Quantikine R&D Systems, Minneapolis, MN, USA) were measured using enzyme-linked immunosorbent assay (ELISA) kits, following the procedures provided by the respective manufacturers.
2.4. HOMA-IR
HOMA-IR, a method developed by Matthews et al. (1985) [26], is an index used to quantify insulin resistance. The index is calculated as follows: HOMA-IR = fasting glucose (mg/dL) × serum insulin (mU/L)/405.
2.5. Histological Examination
At the end of the 17-week experimental period, the mice were euthanized under anesthesia with Zoletil 50 (40 mg/kg, IP; Virbac Laboratories, Carros, France), and the epididymal fat and gastrocnemius (GC), extensor digitorum longus (EDL), and soleus (SOL) muscles were quickly removed and weighed. The resected tissues, namely the epididymal fat and GC muscles, were fixed in 10% formalin and embedded with paraffin. Hematoxylin and eosin (H&E) staining was performed according to standard procedures. Histological images were captured with a light microscope (Olympus, BX43, Tokyo, Japan) equipped with a digital camera (Canon EOS 600 D, Tokyo, Japan). The H&E-stained sections were used for cross-sectional area (CSA) analyses. The numbers of adipocytes in the epididymal adipose tissues and the numbers of fibers in the GC muscles were counted in a total number of 100 per animal. Semiautomatic quantification of the minimal Feret’s diameter of fibers was performed as described previously [27]. The variance coefficient of minimal Feret’s diameter from each section was defined to evaluate the muscle fiber size variability among the groups and then calculated as the ratio between the standard deviation (×1000) and the mean of the diameter and was expressed as arbitrary units.
2.6. Tissue Preparation and Western Blot Analysis
The GC muscle tissues were homogenized with a lysis buffer (1% Triton X-100; 50 mM Tris-HCl, pH 7.6; 150 mM NaCl) and 1% protease inhibitor cocktail for protein extraction. The obtained proteins were separated through sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred to polyvinylidene difluoride membranes. The blots were then incubated with antibodies, namely phosphatidylinositol 3-kinase (PI3K) p85g (Cell Signaling Technology, Inc., Danvers, MA, USA), protein kinase B (Akt; Thermo Fisher Scientific, Rockford, IL, USA), phospho-Akt (ser473; Cell Signaling Technology, Inc., Danvers, MA, USA), MAFbx/Atrogin-1 (Santa Cruz Biotechnology, Dallas, TX, USA), MURF-1 (Santa Cruz Biotechnology, Dallas, USA), FOXO1 (Proteintech, Rosemont, USA), phospho-FOXO1 (Cell Signaling Technology, Inc., Danvers, MA, USA), TNF-α (PeproTech, East Windsor, Mercer County, NJ, USA), TNFRI (Santa Cruz Biotechnology, Dallas, TX, USA), NF-κB p65 (Santa Cruz Biotechnology, Dallas, TX, USA), phospho-NF-κB p65 (Santa Cruz Biotechnology, Dallas, TX, USA), IL-6 (Santa Cruz Biotechnology, Dallas, TX, USA), and GAPDH (Santa Cruz Biotechnology, Dallas, TX, USA). After incubation with horseradish peroxidase-labeled IgG, the blots were developed using ECL Western blotting reagents and quantified with optical densitometry (Image Master ID) of the developed autoradiographs.
2.7. RNA Isolation and Quantitative Real-Time Reverse Transcriptase Polymerase Chain Reaction (RT-qPCR)
Total RNAs were isolated from excised epididymal adipose tissues using a TriZol RNA isolation reagent (Invitrogen, Carlsbad, CA, USA) and subjected to conventional cDNA synthesis and real-time PCR. The Ct values obtained were used to calculate relative levels of gene expression based on the ΔΔCT method. Oligonucleotides for PCR were: TNF-α, 5′-TCCCAACAAGGAGGAGAAGT and 5′-TGGTATGAAGTGGCAAATCG; IL-6, 5′-AGGTTCCATGTGCAAGTGTCT and 5′-GACAGCCCTGGTCAAAGGTT, and β-actin, 5′-AGAGGGAAATCGTGCGTGAC, and 5′-CAATAGTGATGACCTGGCCGT.
2.8. Statistical Analyses
One-way analysis of variance (ANOVA) was used for statistical analysis, and the differences between the three groups were determined using Bonferroni’s post hoc test (SPSS Statistics version 22). The results were deemed statistically significant at p < 0.05. All data are presented as the mean ± SD.
3. Results
3.1. MLB Supplementation Attenuated HFD-Induced Adiposity
The results revealed that an HFD increased the mice’s weight (Figure 1A), but had no clear effect on their average food intake (Figure 1B), compared with the mice that were fed a regular diet. Daily MLB supplementation limited the mice’s weight gain (Figure 1A). The percentage of the epididymal fat weight of the total weight of the HFD mice was significantly higher than that of the control group (Figure 1C). MLB supplementation reduced the HFD-induced increase in epididymal fat weight (Figure 1C). H&E staining revealed larger adipocytes (Figure 1D), macrophage infiltration, and increased CSAs (Figure 1D,E) in the epididymal fat pads of the HFD mice compared with those in the control group, and MLB supplementation decreased the size of the adipocytes in the HFD mice. These results suggested that MLB supplementation alleviated HFD-induced adiposity.
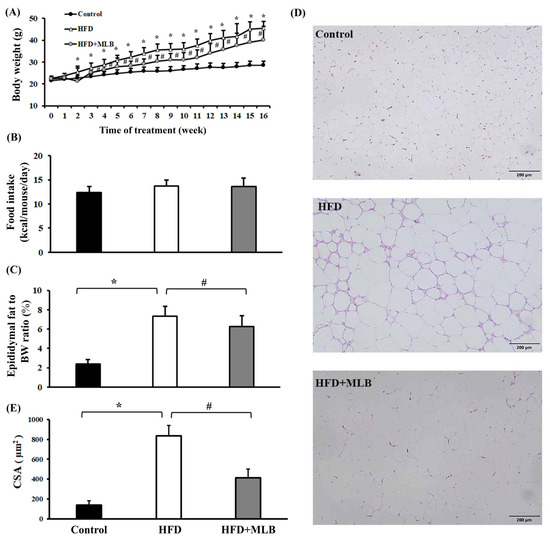
Figure 1.
MLB supplementation attenuated HFD-induced adiposity. Over the course of 16 weeks, the mice’s body weight and food intake were recorded. Weekly changes in body weight (A) and food intake (B) are depicted and summarized. At the end of the experiment, the excised epididymal adipose tissues were weighed (C) and subjected to histological examination. Representative photomicrographs of H&E stain are displayed (D) and the relative epididymal adipose cross-sectional area (CSA) data are illustrated (E). * p < 0.05 vs. control group. # p < 0.05 vs. HFD group. n = 8. Scale bar: 200 μm.
3.2. MLB Supplementation Reduced HFD-Induced Insulin Resistance, Dyslipidemia, and Liver Injury
Several parameters reflecting the insulin action, lipid profile, and liver function were assessed to examine the effects of MLB on insulin resistance, lipid metabolism, and liver injury. At the end of the 17-week experiment, the HFD mice exhibited increased levels of fasting blood glucose, serum insulin, serum cholesterol, serum triglycerides, serum AST and serum ALT, all of which were alleviated by MLB supplementation (Figure 2A,B,D–G), indicating that MLB can induce hypoglycemia, hypoinsulinemia, and hypolipidemia, and improve liver function. A parallel HOMA-IR assessment indicated that the HFD mice exhibited impaired insulin sensitivity, and MLB supplementation reduced their HFD-induced insulin resistance (Figure 2C). These results indicated that MLB supplementation may have reduced hyperglycemia, hyperinsulinemia, insulin resistance, dyslipidemia, and liver injury in HFD mice.
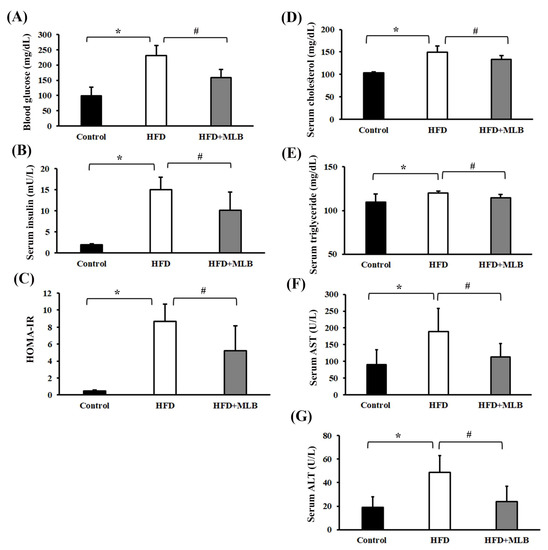
Figure 2.
MLB supplementation improved HFD-induced insulin resistance, dyslipidemia, and liver injury. At the end of the experiment, serum samples were subjected to analyses to measure fasting glucose (A), insulin (B), cholesterol (D), triglycerides (E), AST (F), and ALT (G). The value of HOMA-IR is depicted in (C). * p < 0.05 vs. control group. # p < 0.05 vs. HFD group. n = 8.
3.3. MLB Supplementation Reduced HFD-Increased Proinflammatory Cytokines
Inflammation is a result of muscle wasting, and an elevated expression of proinflammatory cytokines correlates with several clinical disorders, including obesity and insulin resistance [28,29]. In our study, the ELISA data illustrated the effects of MLB supplementation on IL-6 and TNF-α levels in the serum of mice with HFD-induced obesity. A significant increase was noted in the IL-6 and TNF-α levels in the serum of the HFD mice compared with the control group (Figure 3A,B). MLB supplementation significantly decreased the production of HFD-induced IL-6 and TNF-α (Figure 3A,B). These findings revealed that MLB supplementation could attenuate an inflammatory response in mice with HFD-induced obesity by lowering the levels of circulating proinflammatory cytokines.
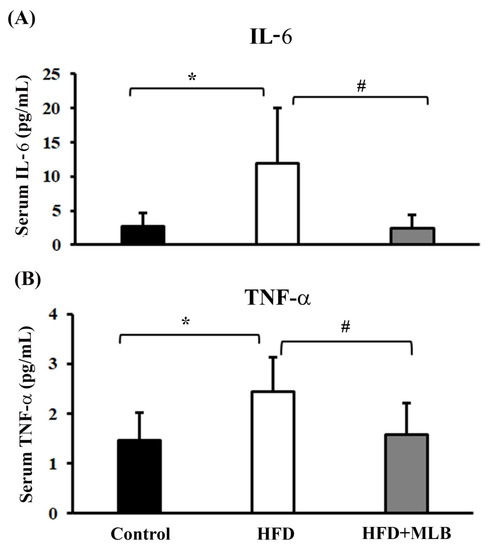
Figure 3.
MLB supplementation reduced HFD-induced proinflammatory cytokines. At the end of the experiment, serum samples were subjected to measurements of IL-6 (A) and TNF-α (B) with commercially available enzyme immunosorbent assay kits. * p < 0.05 vs. control group. # p < 0.05 vs. HFD group. n = 8.
3.4. MLB Supplementation Attenuated HFD-Induced Muscle Atrophy
Skeletal muscle atrophy is a common complication of obesity [30,31]. The potential effects of MLB on skeletal muscle atrophy were investigated in mice that had HFD-induced obesity. The percentage of the weights of the GC (Figure 4A), SOL (Figure 4B), and EDL muscles (Figure 4C) of total body weight decreased in the HFD mice. Histological examination revealed a reduced CSA and an increased variance coefficient of the GC muscle fibers (Figure 4D–F) in the HFD mice compared with the other groups of mice. Those muscular changes were alleviated by MLB supplementation (Figure 4), which, according to the results, could attenuate HFD-induced muscle atrophy.
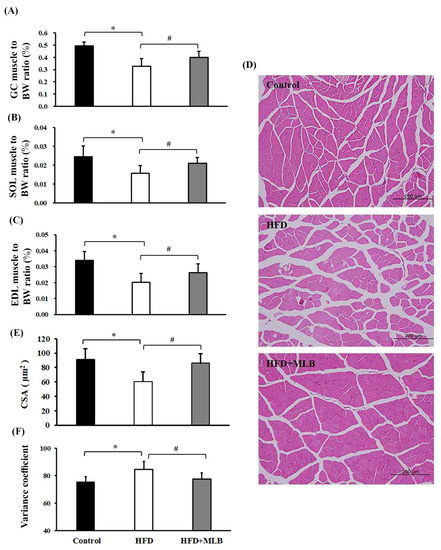
Figure 4.
MLB supplementation attenuated HFD-induced muscle atrophy. At the end of the experiment, the skeletal muscles, namely the gastrocnemius (GC), extensor digitorum longus (EDL), and soleus (SOL) muscles, were removed and weighed. The ratios of the weight of the GC, SOL, and EDL muscles to the body weight are depicted (A–C). The representative hematoxylin and eosin (H&E) staining of GC muscles from each group are presented (D), and the relative muscle cross-sectional area (CSA) data are illustrated (E). Measurement of variance coefficients of the fiber size in the cross-sectional samples of three groups using Feret’s diameter as the geometrical parameter (F). Values are expressed as the mean ± SD. * p < 0.05 vs. control group. # p < 0.05 vs. HFD group. n = 8.
3.5. MLB Supplementation Attenuated HFD-Increased E3 Ligases
To further examine the effects of MLB on HFD-induced muscle atrophy, we investigated the changes in the muscle-specific ubiquitin E3 ligases in the mice’s skeletal muscles. The representative blots of the Western blot analysis are presented in Figure 5A. Quantitative results revealed that an HFD significantly increased the expression of MAFbx (Figure 5B) and MuRF-1 (Figure 5C), and the changes were attenuated by MLB supplementation. The results indicated that MLB attenuated muscle atrophy in HFD mice, likely through the inhibition of the ubiquitin E3 ligase expression.
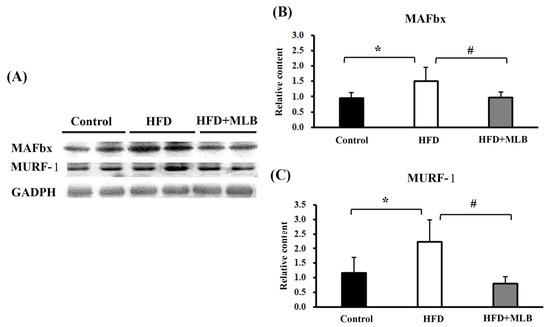
Figure 5.
MLB supplementation attenuated HFD-induced muscle atrophy through inhibiting protein degradation in skeletal muscles. Proteins were isolated from skeletal muscles and subjected to a Western blot with antibodies against MAFbx, MURF-1, and GAPDH. Two representative samples from each group are presented (A), and the quantitative data are depicted (B,C). Values are expressed as the mean ± SD. * p < 0.05 vs. control group. # p < 0.05 vs. HFD group. n = 8.
3.6. MLB Supplementation Attenuated HFD-Activated FoxO1 Signaling
The PI3K/Akt/FoxO axis signaling played a substantial role in the regulation of the MAFbx and MuRF-1 expression [3]. The HFD mice exhibited decreased PI3K protein content (Figure 6A,B), Akt phosphorylation (Figure 6A,C), and FoxO1 phosphorylation (Figure 6A,D), and increased FoxO1 protein content (Figure 6A,E) in their skeletal muscles. The changes in the PI3K/Akt/FoxO signaling molecules were attenuated through MLB supplementation (Figure 6). The results indicated that MLB attenuated HFD-induced FoxO1 activation through the promotion of Akt signaling.
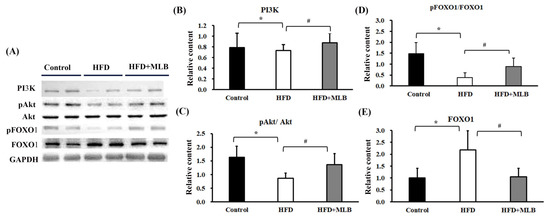
Figure 6.
MLB supplementation attenuated HFD-induced muscle atrophy through the upregulation of PI3K/Akt/FOXO1 signaling. Proteins were isolated from skeletal muscles and subjected to a Western blot with antibodies against PI3K, pAkt, Akt, pFOXO1, FOXO1, and GAPDH. Two representative samples from each group are presented (A), and the quantitative data are depicted (B–E). Values are expressed as the mean ± SD. * p < 0.05 vs. control group. # p < 0.05 vs. HFD group. n = 8.
3.7. MLB Supplementation Attenuated HDF-Activated TNF-α/NF-κB Signaling
TNF-α is a cachectic factor that induces MAFbx and MuRF-1 expressions, which leads to the induction of muscle atrophy in an NF-κB-dependent mechanism [32,33]. Although the TNF-α levels were not significantly altered (Figure 7A,B) in the skeletal muscles of the HFD mice, an elevated TNF-RI protein content (Figure 7A,C) and p65 phosphorylation (Figure 7A,D) were noted. An elevated expression of IL-6 protein was also revealed (Figure 7A,E). Intriguingly, HFD mice elevated TNF-α mRNA expression (Figure 7F) in the epididymal adipose tissues, while having little effect on IL-6 mRNA (Figure 7G). Those changes were attenuated by MLB supplementation (Figure 7). The results indicated that MLB attenuated HFD-activated TNF-α/NF-κB and IL-6 signaling through different modes of action.
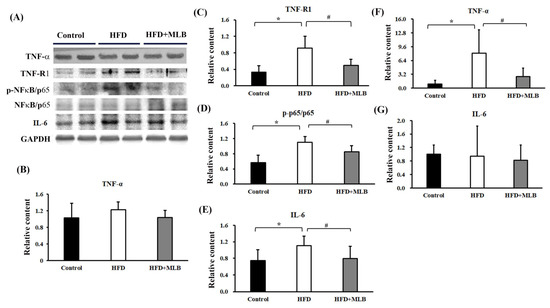
Figure 7.
MLB supplementation attenuated HFD-induced muscle atrophy through the downregulation of TNF-α/NF-κB signaling. Proteins were isolated from the skeletal muscles and subjected to a Western blot with antibodies against TNF-α, TNFRI, NF-κB/p65, p-NF-κB/p65, IL-6, and GAPDH. Two representative samples from each group are presented (A), and the quantitative data are depicted (B–E). Total RNAs were extracted from epididymal adipose tissues and subjected to RT-qPCR for the measurement of TNF-α (F) and IL-6 (G) mRNA levels. Values are expressed as mean ± SD. * p < 0.05 vs. control group. # p < 0.05 vs. HFD group. n = 8.
4. Discussion
MLB reduced obesity, aging, and diabetes by reducing hepatic inflammation and insulin resistance [24,25]. This study demonstrated that MLB supplementation attenuated obesity-associated skeletal muscle atrophy in HFD mice through the regulation of the PI3K/Akt/FoxO1 and TNF-α/NF-κB signaling pathways, leading to the inhibition of muscle-specific ubiquitin E3 ligase expression.
Muscle atrophy is a serious health concern, and the majority of the skeletal muscular protein structure is governed by protein synthesis and degradation. Under insufficient nutrition or malnutrition, the impaired IGF-1-mediated macromolecular synthesis creates an obstacle to muscle mass maintenance. However, abnormal protein degradation has caused most cases of muscle atrophy [34]. Consistent with relevant studies [35,36], the HFD mice exhibited signs of skeletal muscle atrophy with concurrent activation of MAFbx and MuRF-1. This study presented the first evidence that daily MLB supplementation diminished skeletal muscle atrophy, and the improvement correlated with the reduction in skeletal MAFbx and MuRF-1 expression. The results revealed that the ubiquitin-proteasome system played a substantial role in obesity-associated skeletal muscle atrophy, and MLB had an anti-atrophy effect through negatively affecting skeletal MAFbx and MuRF-1 expression.
The etiologies of muscle atrophy are multifactorial. Obese people often develop muscle atrophy [22,23], and those with excessive caloric intakes and deposition of fat are prone to develop insulin resistance and chronic inflammation [20,21]. At the molecular level, obesity is associated with impaired Akt activity, enhanced NF-κB and FoxO1, and increased TNF-α and IL-6 levels [37], all of which are related to muscle atrophy [37]. We reported that MLB reduced obesity, fatty liver, glucose intolerance, and insulin resistance in HFD mice [24]. In this study, the HFD mice that received MLB supplements exhibited activated skeletal Akt, inhibited skeletal NF-κB and FoxO1, and reduced circulating TNF-α and IL-6. Thus, MLB contains diverse pharmacological properties that are attributed to diminished obesity-associated biochemical and molecular changes.
The FoxO family transcription factors have integrative roles in glucose and lipid metabolism. FoxO1, a typical FoxO family member, regulates hepatic gluconeogenesis, glycogenolysis, and lipogenesis in response to the insulin signal. The insulin-PI3K-Akt pathway negatively regulates FoxO1 transcription factors. Conversely, decreased PI3K-Akt activity prevents the degradation of FoxO1 and enforces FoxO1 transcriptional activity [3]. Furthermore, FoxO1 actively participates in the transcriptional activation of MAFbx and MuRF-1 expression [3]. An inhibited PI3K-Akt pathway and FoxO1 phosphorylation and increased FoxO1 protein content were observed in the skeletal muscles of the HFD mice, and MLB reversed the inactivation of the PI3K-Akt pathway. MLB’s improvement of the HFD mice’s insulin resistance strongly suggested that the restoration of the PI3K-Akt pathway not only played a role in glucose metabolism but also had an inhibitory effect on the FoxO1/MAFbx and MuRF-1 pathways and reduced obesity-associated muscle atrophy.
Skeletal muscle atrophy is highly associated with inflammatory responses and cytokines [38,39]; particularly, TNF-α is a recognized cachetic factor [40,41]. Evidence indicated that the tissue expressions of MAFbx and MuRF-1 messenger ribonucleic acid (mRNA) correlated with elevated TNF-α levels in an inflammatory catabolic state [42,43]. The presence of TNF-α promoted MAFbx/MuRF-1 mRNA expression, in vivo and in vitro [44,45]. TNF-α increased the effectiveness of the ubiquitin-proteasome pathway and caused muscular degradation in the C2C12 myotubes and skeletal muscles through the activation of NF-κB signaling [32,33]. By contrast, anti-TNF treatments reduced rat skeletal muscle wasting [40]. Upon activation, TNF-α transduced intracellular signals through engagement with TNF Receptor I (TNFRI) and converged onto NF-κB [40,41]. In this study, the HFD mice exhibited an increased circulating TNF-α level, adipose TNF-α mRNA level, skeletal TNFRI expression, and skeletal NF-κB p65 phosphorylation. Parallel elevation was demonstrated in the skeletal MAFbx and MuRF-1 expressions and muscle atrophy, and MLB supplementation alleviated those TNF-α-related inflammatory changes. Therefore, MLB’s inhibition of the TNF-α/TNFRI/NF-κB pathway represented an alternative means of decreasing the MAFbx and MuRF-1 expression and muscle atrophy. However, the levels of skeletal TNF-α remained consistent with no remarkable change among groups. Moreover, MLB alleviated metabolic abnormality and hepatic inflammation in obese mice [24,25]. All aforementioned data indicate that the anti-atrophy effect of MLB is probably secondary to the reduction in tissue inflammation, such as hepatic and adipose tissues.
5. Conclusions
This study revealed that daily MLB supplements reduced obesity-associated skeletal muscle atrophy, likely through the inhibition of MuRF-1- and MAFbx-mediated muscular degradation. The activation of the PI3K-Akt-FoxO1 pathway and inhibition of the TNF-α/TNFRI/NF-κB pathway were assumed to be attributed to MLB. In addition to improving obesity-associated metabolic abnormalities, as reported in our previous study [24], the data presented in this study further highlights MLB’s potential to combat obesity-associated muscle atrophy.
Author Contributions
C.-J.C. and W.-Y.C. conceived and designed the experiments; T.-L.C., Z.-Y.L., K.-Y.L., W.-C.H., C.-F.J., P.-H.P. and Y.-H.K. performed the experiments; C.-J.C. and W.-Y.C. wrote the paper; W.-Y.C. edited the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from Taichung Veterans General Hospital and National Chung Hsing University (TCVGH-NCHU1107607), and was partly supported by the Higher Education Sprout Project, Ministry of Education (MOE), Taiwan.
Institutional Review Board Statement
The animal experiments were conducted in accordance with the Guideline for the Care and Use of Laboratory Animals published by the Council of Agriculture Executive Yuan of Taiwan on 22 June 2018 and were reviewed and approved by the Institutional Animal Care and Use Committee of National Chung Hsing University (IACUC Approval No: 109-033).
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- McGregor, R.A.; Cameron-Smith, D.; Poppitt, S.D. It is not just muscle mass: A review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev. Healthspan 2014, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Keller, K.; Engelhardt, M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J. 2013, 3, 346–350. [Google Scholar] [CrossRef]
- McKinnell, I.W.; Rudnicki, M.A. Molecular mechanisms of muscle atrophy. Cell 2004, 119, 907–910. [Google Scholar] [CrossRef] [Green Version]
- Egerman, M.A.; Glass, D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Enber, N.; Sliziuk, V.; Scherbakov, N.; Sandek, A. Muscle wasting in ageing and chronic illness. ESC Heart Fail. 2015, 2, 58–68. [Google Scholar]
- Kocaturk, N.M.; Gozuacik, D. Crosstalk between mammalian autophagy and the ubiquitin-proteasome system. Front. Cell Dev. Biol. 2018, 6, 128. [Google Scholar] [CrossRef]
- Callis, J. The ubiquitination machinery of the ubiquitin system. Arab. Book 2014, 12, e0174. [Google Scholar] [CrossRef] [Green Version]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [Green Version]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Rommel, C.; Bodine, S.C.; Clarke, B.A.; Rossman, R.; Nunez, L.; Stitt, T.N.; Yancopoulos, G.D.; Glass, D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 2001, 3, 1009–1013. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Li, L.N. Biologically active components from traditional Chinese medicines. Pure Appl. Chem. 1998, 70, 547–554. [Google Scholar] [CrossRef]
- Wu, W.Y.; Wang, Y.P. Pharmacological actions and therapeutic applications of Salvia miltiorrhiza depside salt and its active components. Acta Pharmacol. Sin. 2012, 33, 1119–1130. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Cheung, C.M.; Yang, J.M.; Or, P.M.; Lee, W.Y.; Yeung, J.H. Danshen (Salvia miltiorrhiza) water extract inhibits paracetamol-induced toxicity in primary rat hepatocytes via reducing CYP2E1 activity and oxidative stress. J. Pharm. Pharmacol. 2015, 67, 980–989. [Google Scholar] [CrossRef]
- Han, J.Y.; Fan, J.Y.; Horie, Y.; Miura, S.; Cui, D.H.; Ishii, H.; Hibi, T.; Tsuneki, H.; Kimura, I. Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol. Ther. 2008, 117, 280–295. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Q.; Zhang, H.; Wan, L.; Xin, B.; Cao, Y.; Zhang, J.; Guo, C. Cryptotanshinone prevents muscle wasting in CT26-induced cancer cachexia through inhibiting STAT3 signaling pathway. J. Ethnopharmacol. 2020, 260, 113066. [Google Scholar] [CrossRef]
- Zhao, P.; Soukup, S.T.; Hegevoss, J.; Ngueu, S.; Kulling, S.E.; Diel, P. Anabolic effect of the traditional Chinese medicine compound tanshinone IIA on myotube hypertrophy is mediated by estrogen receptor. Planta Med. 2015, 81, 578–585. [Google Scholar] [CrossRef]
- Cheng, C.C.; Yang, S.P.; Lin, W.S.; Ho, L.J.; Lai, J.H.; Cheng, S.M.; Lin, W.Y. Magnesium lithospermate B mediates anti-inflammation targeting activator protein-1 and nuclear factor-kappa B signaling pathways in human peripheral T lymphocytes. Int. Immunopharmacol. 2012, 13, 354–361. [Google Scholar] [CrossRef]
- Quan, W.; Wu, B.; Bai, Y.; Zhang, X.; Yin, J.; Xi, M.; Guan, Y.; Shao, Q.; Chen, Y.; Wu, Q.; et al. Magnesium lithospermate B improves myocardial function and prevents simulated ischemia/reperfusion injury-induced H9c2 cardiomyocytes apoptosis through Akt-dependent pathway. J. Ethnopharmacol. 2014, 151, 714–721. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha-and obesity-induced insulin resistance. Science 1996, 271, 665–668. [Google Scholar] [CrossRef]
- Baracos, V.E.; Arribas, L. Sarcopenic obesity: Hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann. Oncol. 2018, 29, ii1–ii9. [Google Scholar] [CrossRef]
- Bollinger, L.M.; Powell, J.J.; Houmard, J.A.; Witczak, C.A.; Brault, J.J. Skeletal muscle myotubes in severe obesity exhibit altered ubiquitin-proteasome and autophagic/lysosomal proteolytic flux. Obesity (Silver Spring) 2015, 23, 1185–1193. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.J.; Lo, Y.H.; Chen, Y.T.; Lai, N.W.; Lin, N.H.; Chung, T.Y.; Chen, W.Y.; Tzen, T.C. Magnesium lithospermate B improves metabolic changes in high-fat diet-fed rats with metabolic syndrome. J. Funct. Foods 2015, 14, 163–173. [Google Scholar] [CrossRef]
- Jeong, J.W.; Lee, B.; Dae Hyun Kim, D.H.; Jeong, H.O.; Moon, K.M.; Kim, M.J.; Yokozawa, T.; Chung, H.Y. Mechanism of action of Magnesium Lithospermate B against aging and obesity-induced ER stress, insulin resistance, and inflammsome formation in the liver. Molecules 2018, 23, 2098. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Briguet, A.; Courdier-Fruh, I.; Foster, M.; Meier, T.; Magyar, J.P. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul. Disord. 2004, 14, 675–682. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Johns, N.; Stephens, N.A.; Fearon, K.C. Muscle wasting in cancer. Int. J. Biochem. Cell Biol. 2013, 45, 2215–2229. [Google Scholar] [CrossRef]
- Onishi, S.; Ishino, M.; Kitazawa, H.; Yoto, A.; Shimba, Y.; Mochizuki, Y.; Unno, K.; Meguro, S.; Tokimitsu, I.; Miura, S. Green tea extracts ameliorate high-fat diet-induced muscle atrophy in senescence-accelerated mouse prone-8 mice. PLoS ONE 2018, 13, e0195753. [Google Scholar] [CrossRef]
- Pijet, B.; Pijet, M.; Litwiniuk, A.; Gajewska, M.; Pajak, B.; Orzechowski, A. TNF-α and IFN-s-dependent muscle decay is linked to NF-κB- and STAT-1α-stimulated Atrogin1 and MuRF1 genes in C2C12 myotubes. Mediat. Inflamm. 2013, 2013, 171437. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.Y.; Chen, W.Y.; Lee, F.Y.; Huang, C.J.; Sheu, W.H. Activation of ubiquitin-proteasome pathway is involved in skeletal muscle wasting in a rat model with biliary cirrhosis: Potential role of TNF-α. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E493–E501. [Google Scholar] [CrossRef]
- Sandri, M. Protein breakdown in muscle wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int. J. Biochem. Cell Biol. 2013, 45, 2121–2129. [Google Scholar] [CrossRef] [Green Version]
- Nakao, R.; Abe, T.; Yamamoto, S.; Oishi, K. Ketogenic diet induces skeletal muscle atrophy via reducing muscle protein synthesis and possibly activating proteolysis in mice. Sci. Rep. 2019, 9, 19652. [Google Scholar] [CrossRef] [Green Version]
- Roseno, S.L.; Davis, P.R.; Bollinger, L.M.; Powell, J.J.; Witczak, C.A.; Brault, J.J. Short-term, high-fat diet accelerates disuse atrophy and protein degradation in a muscle-specific manner in mice. Nutr. Metab. 2015, 12, 39. [Google Scholar] [CrossRef] [Green Version]
- Roy, B.; Curtis, M.E.; Fears, L.S.; Nahashon, S.N.; Fentress, H.M. Molecular mechanisms of obesity-induced osteoporosis and muscle atrophy. Front. Physiol. 2016, 7, 439. [Google Scholar] [CrossRef] [Green Version]
- Bruunsgaard, H.; Pedersen, M.; Pedersen, B.K. Aging and proinflammatory cytokines. Curr. Opin. Hematol. 2001, 8, 131–136. [Google Scholar] [CrossRef]
- Stentz, F.B.; Umpierrez, G.E.; Cuervo, R.; Kitabchi, A.E. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes 2004, 53, 2079–2086. [Google Scholar] [CrossRef] [Green Version]
- Steffen, B.T.; Lees, S.J.; Booth, F.W. Anti-TNF treatment reduces rat skeletal muscle wasting in monocrotaline-induced cardiac cachexia. J. Appl. Physiol. 2008, 105, 1950–1958. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Al-Lamki, R.S. Tumor necrosis factor receptor 2: Its contribution to acute cellular rejection and clear cell renal carcinoma. BioMed Res. Int. 2013, 2013, 821310. [Google Scholar] [CrossRef] [Green Version]
- Webster, J.M.; Kempen, L.J.; Hardy, R.S.; Langen, R.C. Inflammation and skeletal muscle wasting during cachexia. Front. Physiol. 2020, 11, 597675. [Google Scholar] [CrossRef]
- Anker, S.D.; Rauchhaus, M. Insights into the pathogenesis of chronic heart failure: Immune activation and cachexia. Curr. Opin. Cardiol. 1999, 14, 211–216. [Google Scholar] [CrossRef]
- Wang, D.T.; Yin, Y.; Yang, Y.J.; Lv, P.J.; Shi, Y.; Lu, L.; Wei, L.B. Resveratrol prevents TNF-α-induced muscle atrophy via regulation of Akt/mTOR/FoxO1 signaling in C2C12 myotubes. Int. Immunopharmacol. 2014, 19, 206–213. [Google Scholar] [CrossRef]
- Collins, R.A.; Grounds, M.D. The role of tumor necrosis factor-alpha (TNF-α) in skeletal muscle regeneration: Studies in TNF-α(-/-) and TNF-α(-/-)/LT-α(-/-) mice. J. Histochem. Cytochem. 2001, 49, 989–1001. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
