Maternal Fructose Intake Exacerbates Cardiac Remodeling in Offspring with Ventricular Pressure Overload
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Transverse Aortic Constriction Operation
2.2. Cardiac Functional Assessment by Echocardiography
2.3. Quantitative Reverse Transcription-Polymerase Chain Reaction
2.4. Western Blot
2.5. Histopathological Staining
2.6. Assessment of Oxiditive Stress in the Myocardium
2.7. Culturing of Cardiac Cells and Immunofluorescent Staining
2.8. Statistical Analysis
3. Results
3.1. Maternal Fructose Exposure Exacerbates Ventricular Pressure Overload-Induced Cardiac Hypertrophy in Adult Offspring
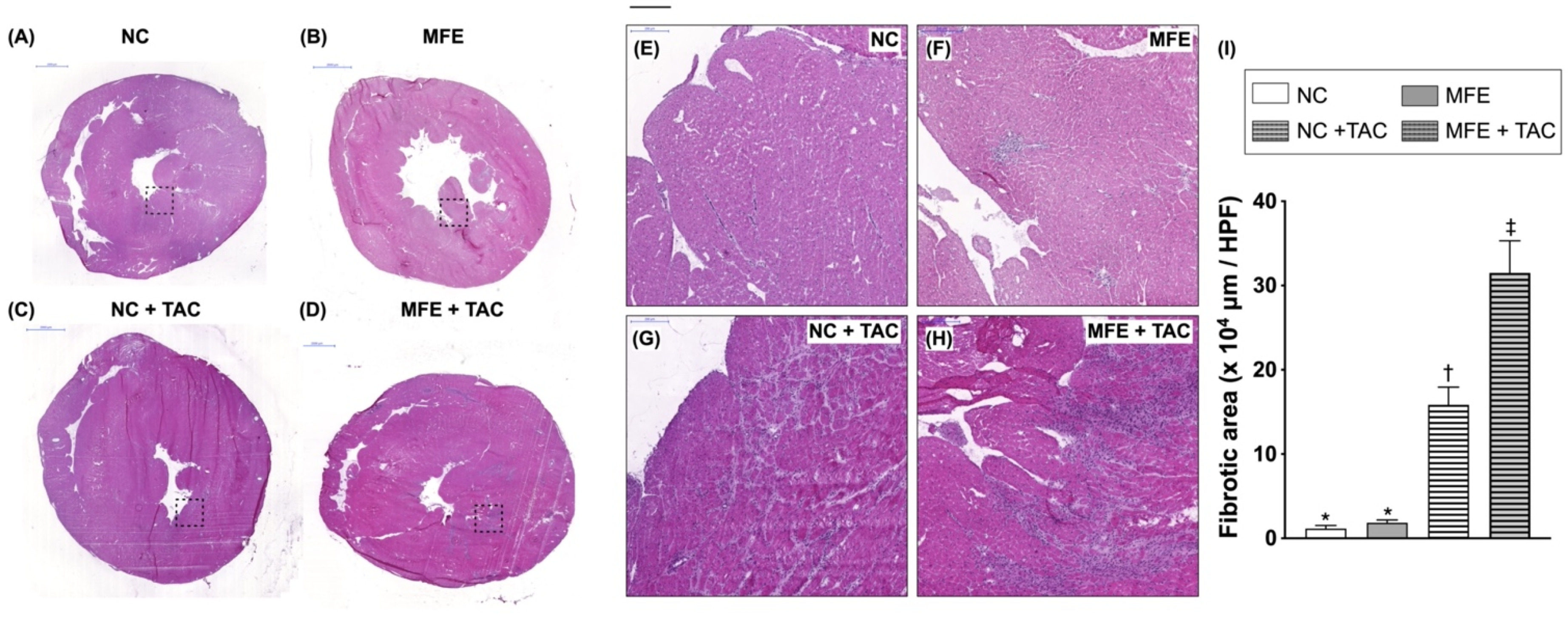
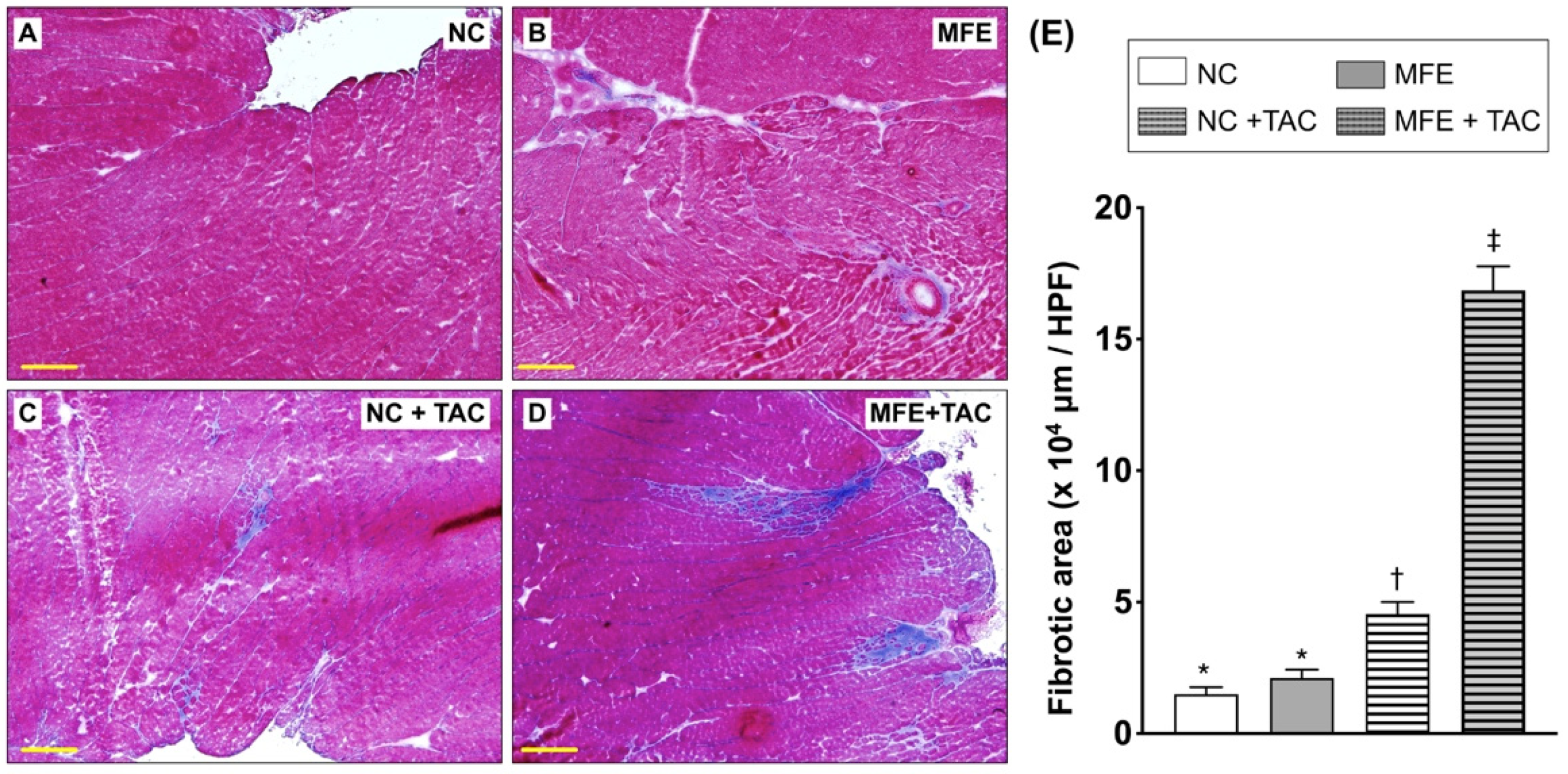
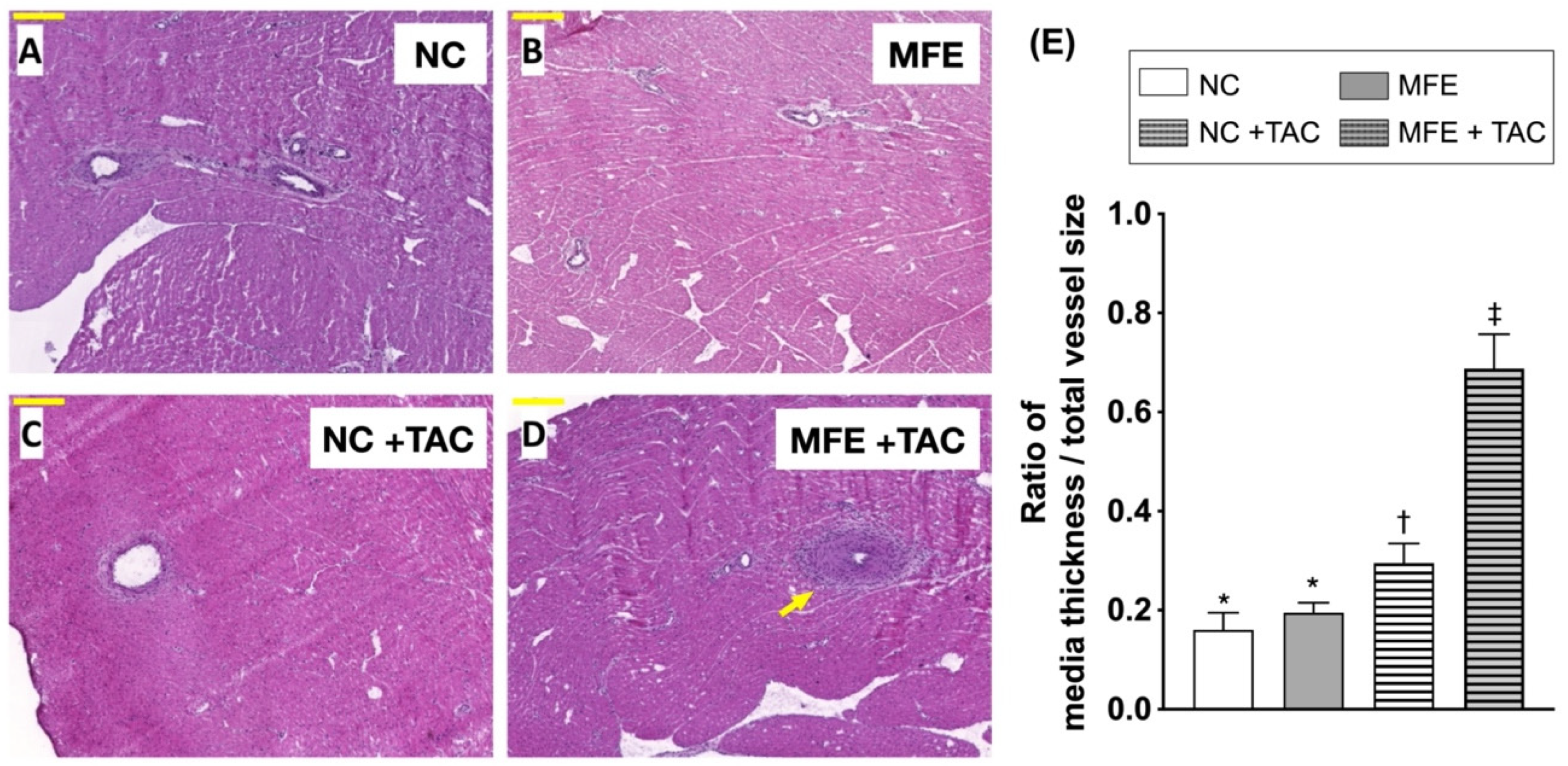
3.2. Maternal Fructose Exposure Exacerbates Ventricular Pressure Overload-Induced Cardiac Remodeling in Adult Offspring
3.3. Maternal Fructose Exposure Increases Oxidative Stress in the Myocardium Subjected to Ventricular Pressure Overload
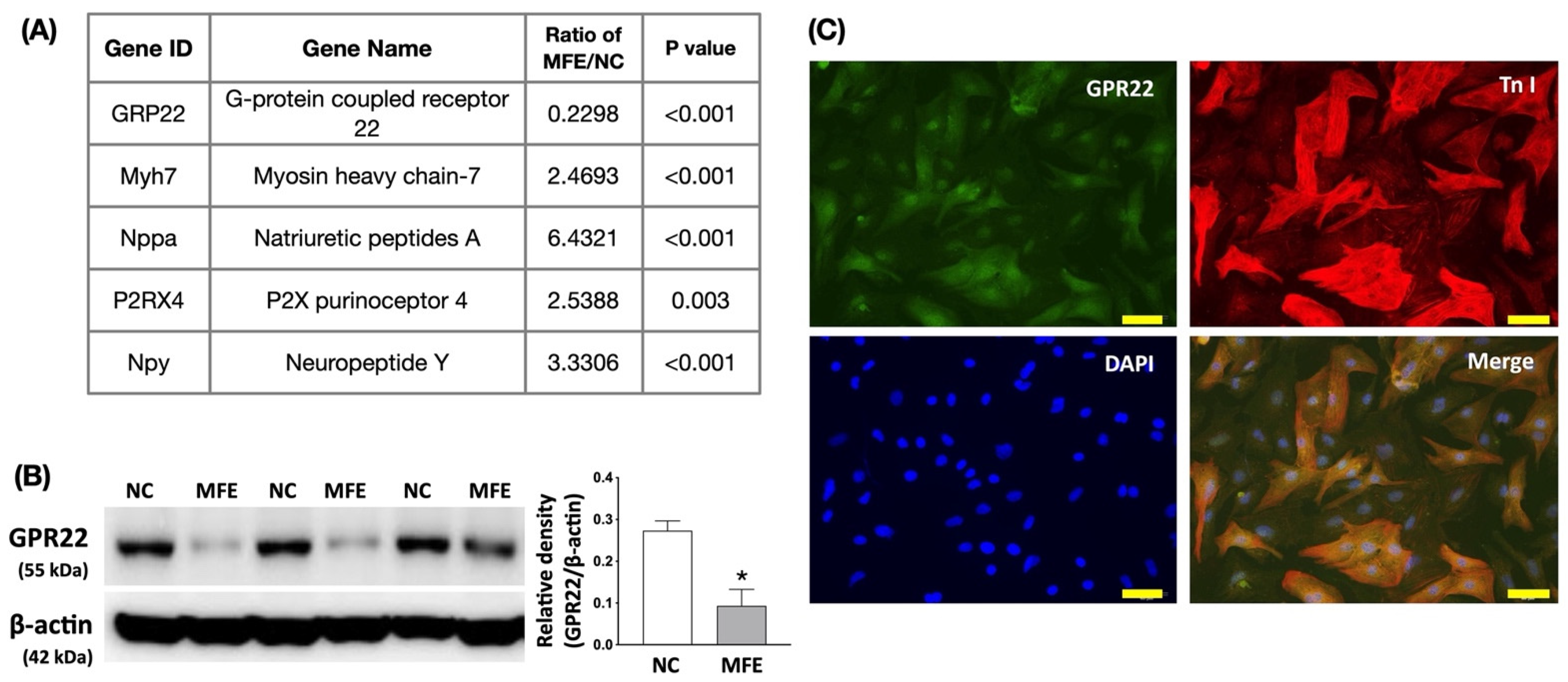
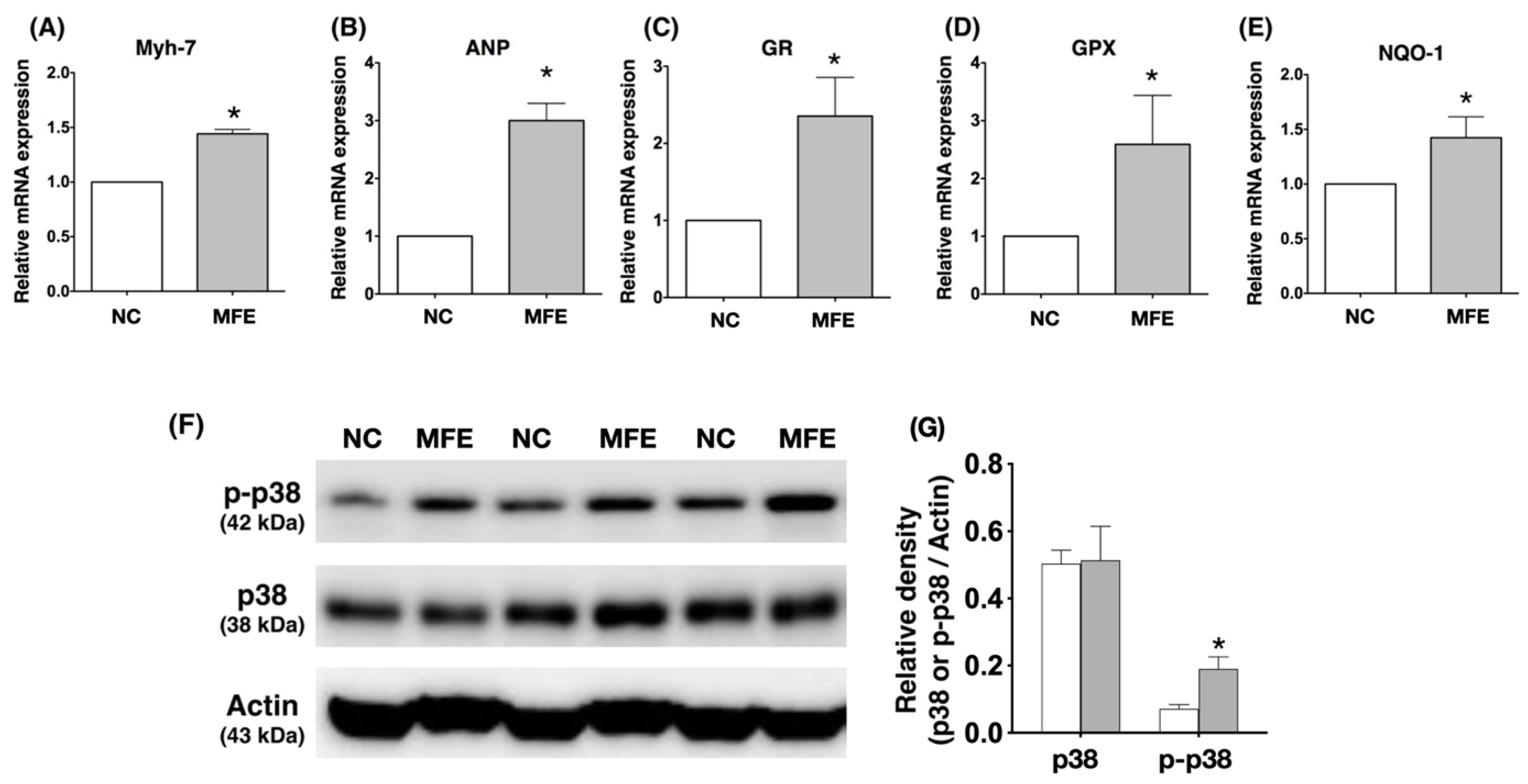
3.4. Maternal Fructose Exposure Regulates the Gene Expression Profiles and Stress Response Signaling in the Myocardium of Adult Offspring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004, 79, 537–543. [Google Scholar] [CrossRef]
- Bantle, J.P. Dietary fructose and metabolic syndrome and diabetes. J. Nutr. 2009, 139, 1263S–1268S. [Google Scholar] [CrossRef]
- Tappy, L.; Le, K.A.; Tran, C.; Paquot, N. Fructose and metabolic diseases: New findings, new questions. Nutrition 2010, 26, 1044–1049. [Google Scholar] [CrossRef]
- Hwang, I.S.; Ho, H.; Hoffman, B.B.; Reaven, G.M. Fructose-induced insulin resistance and hypertension in rats. Hypertension 1987, 10, 512–516. [Google Scholar] [CrossRef]
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 2002, 76, 911–922. [Google Scholar] [CrossRef]
- Deng, J.Y.; Huang, J.P.; Lu, L.S.; Hung, L.M. Impairment of cardiac insulin signaling and myocardial contractile performance in high-cholesterol/fructose-fed rats. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H978–H987. [Google Scholar] [CrossRef]
- Sanchez-Lozada, L.G.; Tapia, E.; Jimenez, A.; Bautista, P.; Cristobal, M.; Nepomuceno, T.; Soto, V.; Avila-Casado, C.; Nakagawa, T.; Johnson, R.J.; et al. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am. J. Physiol. Renal. Physiol. 2007, 292, F423–F429. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Adeli, K. Dietary fructose and the metabolic syndrome. Curr. Opin. Gastroenterol. 2008, 24, 204–209. [Google Scholar] [CrossRef]
- Lucas, A. Programming by early nutrition in man. Ciba Found. Symp. 1991, 156, 38–50. [Google Scholar] [PubMed]
- Williams, S.J.; Campbell, M.E.; McMillen, I.C.; Davidge, S.T. Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R360–R367. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xiao, Y.; Estrella, J.L.; Ducsay, C.A.; Gilbert, R.D.; Zhang, L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J. Soc. Gynecol. Investig. 2003, 10, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Xiao, Y.; Li, G.; Casiano, C.A.; Zhang, L. Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H983–H990. [Google Scholar] [CrossRef] [PubMed]
- Battista, M.C.; Calvo, E.; Chorvatova, A.; Comte, B.; Corbeil, J.; Brochu, M. Intra-uterine growth restriction and the programming of left ventricular remodelling in female rats. J. Physiol. 2005, 565 Pt 1, 197–205. [Google Scholar] [CrossRef]
- Han, H.C.; Austin, K.J.; Nathanielsz, P.W.; Ford, S.P.; Nijland, M.J.; Hansen, T.R. Maternal nutrient restriction alters gene expression in the ovine fetal heart. J. Physiol. 2004, 558 Pt 1, 111–121. [Google Scholar] [CrossRef]
- Wang, J.; Ma, H.; Tong, C.; Zhang, H.; Lawlis, G.B.; Li, Y.; Zang, M.; Ren, J.; Nijland, M.J.; Ford, S.P.; et al. Overnutrition and maternal obesity in sheep pregnancy alter the JNK-IRS-1 signaling cascades and cardiac function in the fetal heart. FASEB J. 2010, 24, 2066–2076. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yan, X.; Zhao, J.X.; Zhu, M.J.; McCormick, R.J.; Ford, S.P.; Nathanielsz, P.W.; Ren, J.; Du, M. Maternal obesity induces fibrosis in fetal myocardium of sheep. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E968–E975. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zheng, Q.; Ford, S.P.; Nathanielsz, P.W.; Ren, J. Maternal obesity, lipotoxicity and cardiovascular diseases in offspring. J. Mol. Cell Cardiol. 2012, 55, 111–116. [Google Scholar] [CrossRef]
- Tain, Y.L.; Leu, S.; Wu, K.L.; Lee, W.C.; Chan, J.Y. Melatonin prevents maternal fructose intake-induced programmed hypertension in the offspring: Roles of nitric oxide and arachidonic acid metabolites. J. Pineal. Res. 2014, 57, 80–89. [Google Scholar] [CrossRef]
- Tain, Y.L.; Wu, K.L.; Lee, W.C.; Leu, S.; Chan, J.Y. Maternal fructose-intake-induced renal programming in adult male offspring. J. Nutr. Biochem. 2015, 26, 642–650. [Google Scholar] [CrossRef]
- Leu, S.; Wu, K.L.H.; Lee, W.C.; Tain, Y.L.; Chan, J.Y.H. The Impact of Maternal Fructose Exposure on Angiogenic Activity of Endothelial Progenitor Cells and Blood Flow Recovery after Critical Limb Ischemia in Rat Offspring. Int. J. Mol. Sci. 2019, 20, 2429. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.W.; Wang, J.; Davis, J.R.; Liaw, C.; Gaidarov, I.; Gatlin, J.; Dalton, N.D.; Gu, Y.; Ross, J., Jr.; Behan, D.; et al. Myocardial expression, signaling, and function of GPR22: A protective role for an orphan G protein-coupled receptor. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H509–H521. [Google Scholar] [CrossRef]
- Zhang, S.; Weinheimer, C.; Courtois, M.; Kovacs, A.; Zhang, C.E.; Cheng, A.M.; Wang, Y.; Muslin, A.J. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J. Clin. Investig. 2003, 111, 833–841. [Google Scholar] [CrossRef]
- Braz, J.C.; Bueno, O.F.; Liang, Q.; Wilkins, B.J.; Dai, Y.S.; Parsons, S.; Braunwart, J.; Glascock, B.J.; Klevitsky, R.; Kimball, T.F.; et al. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J. Clin. Investig. 2003, 111, 1475–1486. [Google Scholar]
- Liang, Q.; Molkentin, J.D. Redefining the roles of p38 and JNK signaling in cardiac hypertrophy: Dichotomy between cultured myocytes and animal models. J. Mol. Cell Cardiol. 2003, 35, 1385–1394. [Google Scholar] [CrossRef]
- Elshenawy, S.; Simmons, R. Maternal obesity and prenatal programming. Mol. Cell Endocrinol. 2016, 435, 2–6. [Google Scholar] [CrossRef]
- Yildiz, M.; Oktay, A.A.; Stewart, M.H.; Milani, R.V.; Ventura, H.O.; Lavie, C.J. Left ventricular hypertrophy and hypertension. Prog. Cardiovasc. Dis. 2020, 63, 10–21. [Google Scholar] [CrossRef]
- Iliev, A.; Kotov, G.; Dimitrova, I.N.; Landzhov, B. Hypertension-induced changes in the rat myocardium during the development of cardiac hypertrophy—A comparison between the left and the right ventricle. Acta Histochem. 2019, 121, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, C.M. Secondary endothelial dysfunction: Hypertension and heart failure. J. Mol. Cell Cardiol. 1999, 31, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Dong, A.; Mueller, P.; Caicedo, J.; Sutton, A.M.; Odetunde, J.; Barrick, C.J.; Klyachkin, Y.M.; Abdel-Latif, A.; Smyth, S.S. Coronary artery remodeling in a model of left ventricular pressure overload is influenced by platelets and inflammatory cells. PLoS ONE 2012, 7, e40196. [Google Scholar]
- Satoh, K.; Nigro, P.; Berk, B.C. Oxidative stress and vascular smooth muscle cell growth: A mechanistic linkage by cyclophilin A. Antioxid. Redox Signal. 2010, 12, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Rababa’h, A.M.; Guillory, A.N.; Mustafa, R.; Hijjawi, T. Oxidative Stress and Cardiac Remodeling: An Updated Edge. Curr. Cardiol. Rev. 2018, 14, 53–59. [Google Scholar] [CrossRef]
- Park, J.H.; Ku, H.J.; Kim, J.K.; Park, J.W.; Lee, J.H. Amelioration of High Fructose-Induced Cardiac Hypertrophy by Naringin. Sci. Rep. 2018, 8, 9464. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Meng, Y.H.; Chang, S.; Zhang, R.Y.; Shi, C. High fructose causes cardiac hypertrophy via mitochondrial signaling pathway. Am. J. Transl. Res. 2016, 8, 4869–4880. [Google Scholar] [PubMed]
- Xie, Y.; Hu, J.; Zhang, X.; Li, C.; Zuo, Y.; Xie, S.; Zhang, Z.; Zhu, S. Neuropeptide Y Induces Cardiomyocyte Hypertrophy via Attenuating miR-29a-3p in Neonatal Rat Cardiomyocytes. Protein Pept. Lett. 2020, 27, 878–887. [Google Scholar] [CrossRef]
- McDermott, B.J.; Bell, D. NPY and cardiac diseases. Curr. Top. Med. Chem. 2007, 7, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Sonin, D.; Jones, L.; Barry, W.H.; Liang, B.T. A beneficial role of cardiac P2X4 receptors in heart failure: Rescue of the calsequestrin overexpression model of cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1096–H1103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cox, E.J.; Marsh, S.A. A systematic review of fetal genes as biomarkers of cardiac hypertrophy in rodent models of diabetes. PLoS ONE 2014, 9, e92903. [Google Scholar]
- Wang, J.; Hao, D.; Zeng, L.; Zhang, Q.; Huang, W. Neuropeptide Y mediates cardiac hypertrophy through microRNA-216b/FoxO4 signaling pathway. Int. J. Med. Sci. 2021, 18, 18–28. [Google Scholar] [CrossRef]
- Ruiz-Hernandez, A.; Sanchez-Munoz, F.; Rodriguez, J.; Calderon-Zamora, L.; Romero-Nava, R.; Huang, F.; Hong, E.; Villafana, S. Expression of orphan receptors GPR22 and GPR162 in streptozotocin-induced diabetic rats. J. Recept. Signal Transduct. Res. 2015, 35, 46–53. [Google Scholar] [CrossRef]

| NC | MFE | NC + TAC a | MFE + TAC | p Value | |
|---|---|---|---|---|---|
| Body weight b (g) | 628.88 ± 59.84 | 625.88 ± 25.98 | 637.88 ± 43.48 | 650.25 ± 49.42 | 0.7391 |
| Heart weight b (g) | 1.449 ± 0.196 | 1.604 ± 0.099 | 1.751 ± 0.118 * | 1.818 ± 0.186 *,† | <0.005 |
| Echocardiography b | |||||
| IVS; d (mm) | 1.492 ± 0.232 | 1.513 ± 0.123 | 1.552 ± 0.225 | 1.646 ± 0.149 | 0.4051 |
| IVS; s (mm) | 2.163 ± 0.271 | 2.257 ± 0.256 | 2.316 ± 0.132 | 2.539 ± 0.333 * | 0.0439 |
| LVPW; d (mm) | 1.858 ± 0.172 | 1.849 ± 0.142 | 2.057 ± 0.786 | 2.175 ± 1.117 | 0.9890 |
| LVPW; s (mm) | 2.422 ± 0.255 | 2.61 ± 0.297 | 2.645 ± 0.368 * | 2.963 ± 0.224 *,† | 0.0058 |
| LVID; d (mm) | 8.899 ± 0.628 | 8.935 ± 0.302 | 9.111 ± 0.57 | 9.346 ± 0.443 | 0.1219 |
| LVID; s (mm) | 5.89 ± 0.684 | 5.749 ± 0.686 | 5.796 ± 0.482 | 5.707 ± 0.692 | 0.8478 |
| LVEF (%) | 60.37 ± 6.57 | 62.53 ± 8.79 | 62.40 ± 5.52 | 63.96 ± 7.84 | 0.8847 |
| SV (μL) | 301.3 ± 36.24 | 310.5 ± 55.14 | 311.8 ± 52.25 | 321.0 ± 52.95 | 0.8634 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leu, S.; Wu, K.L.H.; Lee, W.-C.; Tain, Y.-L.; Chan, J.Y.H. Maternal Fructose Intake Exacerbates Cardiac Remodeling in Offspring with Ventricular Pressure Overload. Nutrients 2021, 13, 3267. https://doi.org/10.3390/nu13093267
Leu S, Wu KLH, Lee W-C, Tain Y-L, Chan JYH. Maternal Fructose Intake Exacerbates Cardiac Remodeling in Offspring with Ventricular Pressure Overload. Nutrients. 2021; 13(9):3267. https://doi.org/10.3390/nu13093267
Chicago/Turabian StyleLeu, Steve, Kay L. H. Wu, Wei-Chia Lee, You-Lin Tain, and Julie Y. H. Chan. 2021. "Maternal Fructose Intake Exacerbates Cardiac Remodeling in Offspring with Ventricular Pressure Overload" Nutrients 13, no. 9: 3267. https://doi.org/10.3390/nu13093267
APA StyleLeu, S., Wu, K. L. H., Lee, W.-C., Tain, Y.-L., & Chan, J. Y. H. (2021). Maternal Fructose Intake Exacerbates Cardiac Remodeling in Offspring with Ventricular Pressure Overload. Nutrients, 13(9), 3267. https://doi.org/10.3390/nu13093267







