The Association between Body Mass Index and Intra-Cortical Myelin: Findings from the Human Connectome Project
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Scanning Protocol
- (1)
- T1 scans were performed using a 3D T1w magnetization-prepared rapid gradient echo (MPRAGE) sequence with the following scan parameters: TR = 2400 ms; TE = 2.14 ms; TI = 1000 ms; 8° flip angle; FOV = 224 × 224 mm; bandwidth = 210 Hz/pixel; echo spacing = 7.6 ms; voxel size = 0.7 mm × 0.7 mm × 0.7 mm; 256 slices; acquisition time = 7:40 min.
- (2)
- T2 scans were performed using a 3D T2w sampling perfection with the application of an optimized contrast featuring a different angle evolution (SPACE) sequence with the following scan parameters: TR = 3200 ms; TE = 565 ms; variable flip angle; FOV = 224 × 224 mm; bandwidth = 744 Hz/pixel; echo spacing = 3.53 ms; voxel size = 0.7 mm × 0.7 mm × 0.7 mm; 256 slices; acquisition time = 8:24 min.
2.3. Image Processing
2.4. Regional and Network Intra-Cortical Myelin Extraction
2.5. Statistical Analysis
3. Results
3.1. Participants’ Demographics
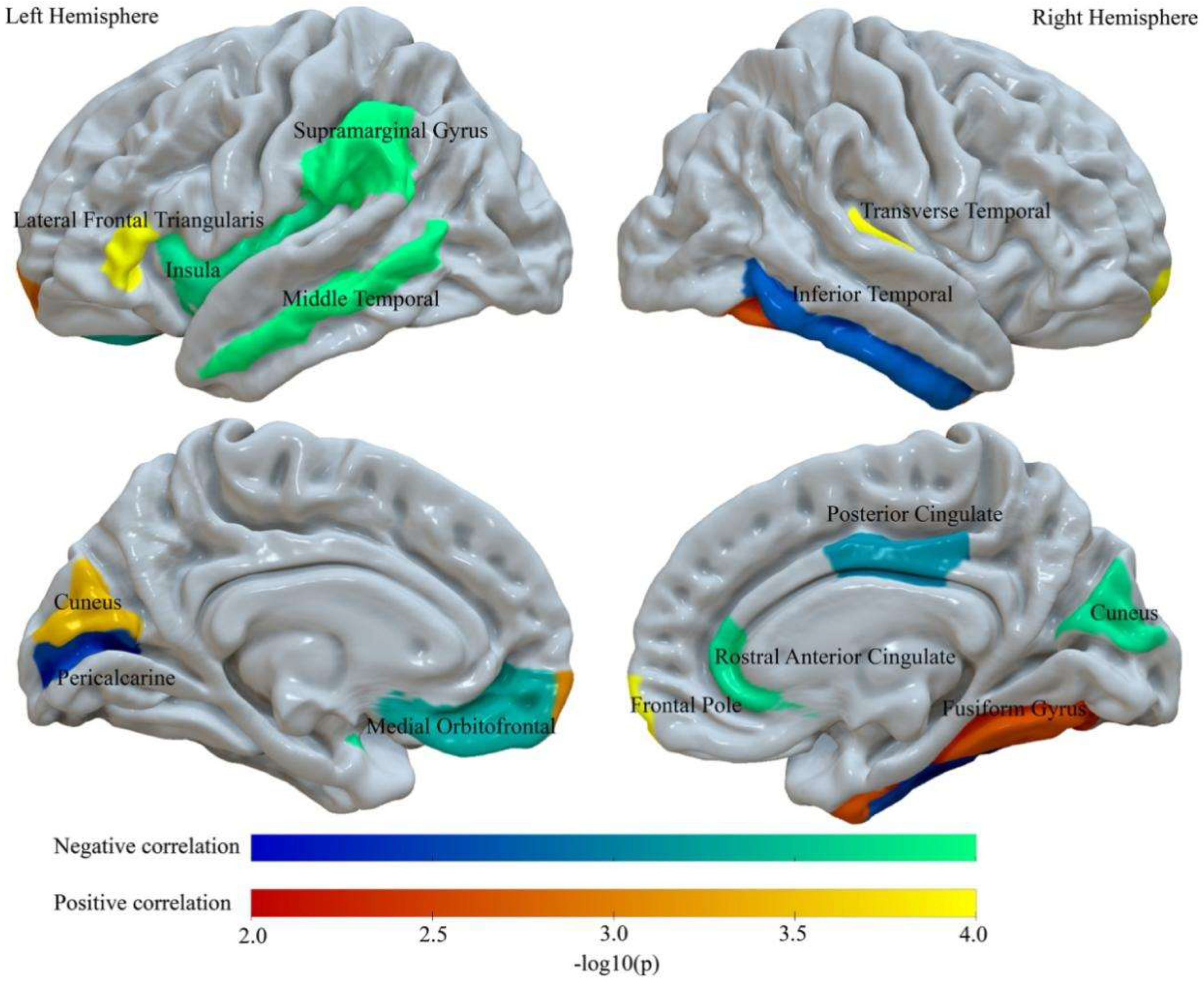
3.2. BMI Relates to Abnormalities of Regional Intra-Cortical Myelin
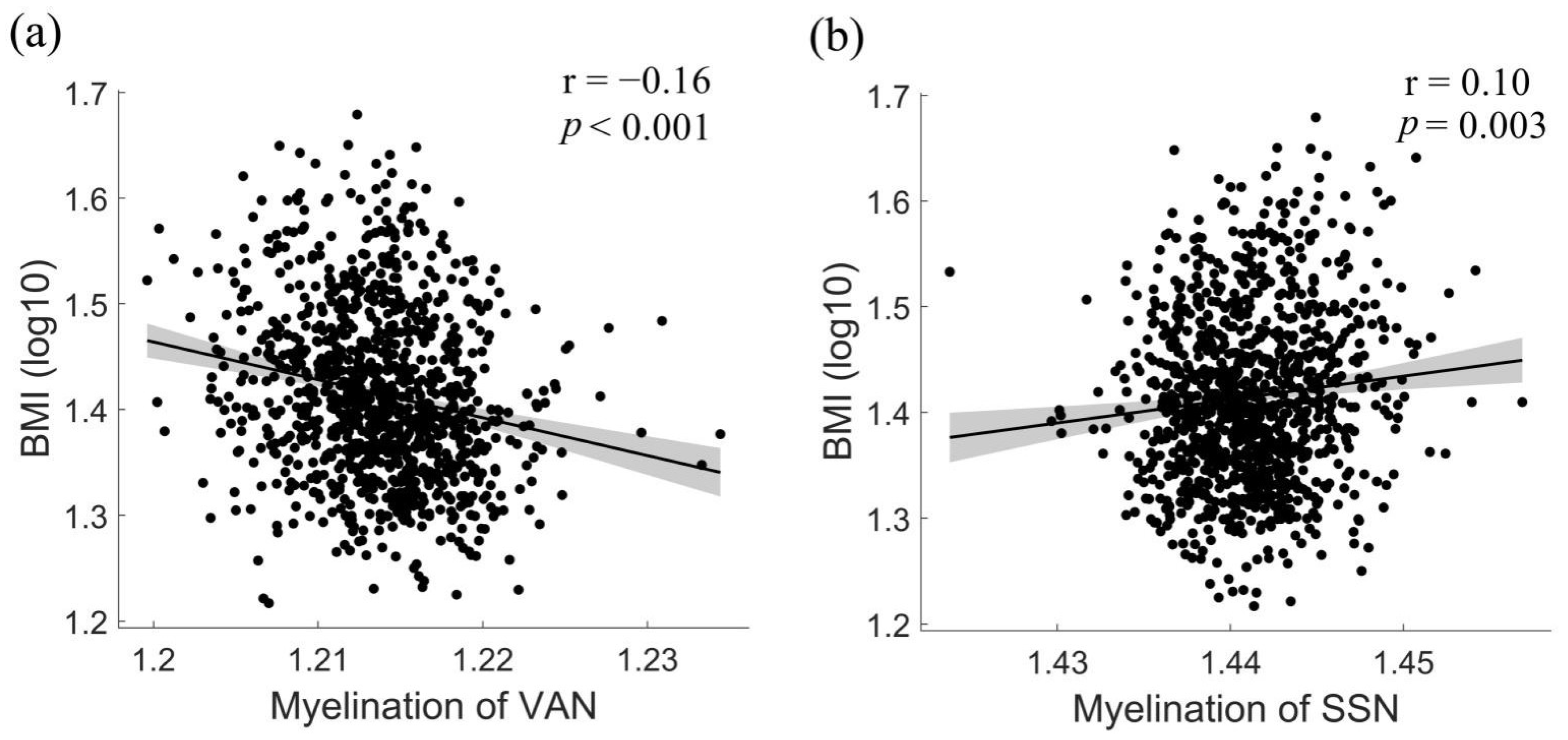
3.3. Associations between BMI and Network-Level Intra-Cortical Myelin Abnormalities
4. Discussion
4.1. Lower Intra-Cortical Myelin in Regions Involved in Reward Processing, Attention, and Salience Detection
4.2. Enhanced Intra-Cortical Myelin in Regions Involved in Somatosensory Processing and Inhibitory Control
4.3. Implications
4.4. Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris, M.J.; Beilharz, J.E.; Maniam, J.; Reichelt, A.C.; Westbrook, R.F. Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Neurosci. Biobehav. Rev. 2015, 58, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Stoeckel, L.E.; Weller, R.E.; Cook, E.W., III; Twieg, D.B.; Knowlton, R.C.; Cox, J.E. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008, 41, 636–647. [Google Scholar] [CrossRef]
- Stice, E.; Yokum, S.; Burger, K.S.; Epstein, L.H.; Small, D.M. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J. Neurosci. 2011, 31, 4360–4366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitropoulos, A.; Tkach, J.; Ho, A.; Kennedy, J. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite 2012, 58, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Rapuano, K.M.; Huckins, J.F.; Sargent, J.D.; Heatherton, T.F.; Kelley, W.M. Individual differences in reward and somatosensory-motor brain regions correlate with adiposity in adolescents. Cereb. Cortex 2016, 26, 2602–2611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devoto, F.; Zapparoli, L.; Bonandrini, R.; Berlingeri, M.; Ferrulli, A.; Luzi, L.; Banfi, G.; Paulesu, E. Hungry brains: A meta-analytical review of brain activation imaging studies on food perception and appetite in obese individuals. Neurosci. Biobehav. Rev. 2018, 94, 271–285. [Google Scholar] [CrossRef]
- McCaffery, J.M.; Haley, A.P.; Sweet, L.H.; Phelan, S.; Raynor, H.A.; Del Parigi, A.; Cohen, R.; Wing, R.R. Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. Am. J. Clin. Nutr. 2009, 90, 928–934. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Yokum, S.; Stice, E.; Harris, J.L.; Brownell, K.D. Relation of obesity to neural activation in response to food commercials. Soc. Cogn. Affect. Neurosci. 2014, 9, 932–938. [Google Scholar] [CrossRef]
- Martin, L.E.; Holsen, L.M.; Chambers, R.J.; Bruce, A.S.; Brooks, W.M.; Zarcone, J.R.; Butler, M.G.; Savage, C.R. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity 2010, 18, 254–260. [Google Scholar] [CrossRef]
- Nave, K.-A.; Werner, H.B. Myelination of the nervous system: Mechanisms and functions. Annu. Rev. cell Dev. Biol. 2014, 30, 503–533. [Google Scholar] [CrossRef]
- Hunt, B.A.; Tewarie, P.K.; Mougin, O.E.; Geades, N.; Jones, D.K.; Singh, K.D.; Morris, P.G.; Gowland, P.A.; Brookes, M.J. Relationships between cortical myeloarchitecture and electrophysiological networks. Proc. Natl. Acad. Sci. USA 2016, 113, 13510–13515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fields, R.D. A new mechanism of nervous system plasticity: Activity-dependent myelination. Nat. Rev. Neurosci. 2015, 16, 756–767. [Google Scholar] [CrossRef]
- Vandewouw, M.M.; Hunt, B.A.; Ziolkowski, J.; Taylor, M.J. The developing relations between networks of cortical myelin and neurophysiological connectivity. NeuroImage 2021, 237, 118142. [Google Scholar] [CrossRef]
- Yang, S.M.; Michel, K.; Jokhi, V.; Nedivi, E.; Arlotta, P. Neuron class–specific responses govern adaptive myelin remodeling in the neocortex. Science 2020, 370, 6523. [Google Scholar] [CrossRef]
- Grydeland, H.; Westlye, L.T.; Walhovd, K.B.; Fjell, A.M. Intracortical Posterior Cingulate Myelin Content Relates to Error Processing: Results from T 1-and T 2-Weighted MRI Myelin Mapping and Electrophysiology in Healthy Adults. Cereb. Cortex 2016, 26, 2402–2410. [Google Scholar] [CrossRef] [Green Version]
- Poggi, G.; Boretius, S.; Möbius, W.; Moschny, N.; Baudewig, J.; Ruhwedel, T.; Hassouna, I.; Wieser, G.L.; Werner, H.B.; Goebbels, S. Cortical network dysfunction caused by a subtle defect of myelination. Glia 2016, 64, 2025–2040. [Google Scholar] [CrossRef] [Green Version]
- Grydeland, H.; Vértes, P.E.; Váša, F.; Romero-Garcia, R.; Whitaker, K.; Alexander-Bloch, A.F.; Bjørnerud, A.; Patel, A.X.; Sederevičius, D.; Tamnes, C.K. Waves of maturation and senescence in micro-structural MRI markers of human cortical myelination over the lifespan. Cereb. Cortex 2019, 29, 1369–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, L.C.; Grabowska, W.A.; Chun, Y.; Risacher, S.L.; Philip, V.M.; Saykin, A.J.; Rizzo, S.J.S.; Howell, G.R.; Initiative, A.s.D.N. Exercise prevents obesity-induced cognitive decline and white matter damage in mice. Neurobiol. Aging 2019, 80, 154–172. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-T.; Tsai, S.-F.; Wu, H.-T.; Huang, H.-Y.; Hsieh, H.-H.; Kuo, Y.-M.; Chen, P.-S.; Yang, C.-S.; Tzeng, S.-F. Chronic exposure to high fat diet triggers myelin disruption and interleukin-33 upregulation in hypothalamus. BMC Neurosci. 2019, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Burguet, J.; Kawaguchi, R.; Havton, L.A.; Hinman, J.D. Obesity restricts oligodendrocyte maturation and impedes repair after white matter stroke. bioRxiv 2018, 283184. [Google Scholar]
- Langley, M.R.; Yoon, H.; Kim, H.N.; Choi, C.-I.; Simon, W.; Kleppe, L.; Lanza, I.R.; LeBrasseur, N.K.; Matveyenko, A.; Scarisbrick, I.A. High fat diet consumption results in mitochondrial dysfunction, oxidative stress, and oligodendrocyte loss in the central nervous system. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165630. [Google Scholar] [CrossRef] [PubMed]
- Bouhrara, M.; Khattar, N.; Elango, P.; Resnick, S.M.; Ferrucci, L.; Spencer, R.G. Evidence of association between obesity and lower cerebral myelin content in cognitively unimpaired adults. Int. J. Obes. 2021, 45, 850–859. [Google Scholar] [CrossRef]
- Nakamura, K.; Chen, J.T.; Ontaneda, D.; Fox, R.J.; Trapp, B.D. T1-/T2-weighted ratio differs in demyelinated cortex in multiple sclerosis. Ann. Neurol. 2017, 82, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Stanley, J.A.; Raz, N. Test–retest reliability and concurrent validity of in vivo myelin content indices: Myelin water fraction and calibrated T1w/T2w image ratio. Hum. Brain Mapp. 2017, 38, 1780–1790. [Google Scholar] [CrossRef] [Green Version]
- Nieuwenhuys, R.; Broere, C.A. A map of the human neocortex showing the estimated overall myelin content of the individual architectonic areas based on the studies of Adolf Hopf. Brain Struct. Funct. 2017, 222, 465–480. [Google Scholar] [CrossRef] [Green Version]
- Rowley, C.D.; Sehmbi, M.; Bazin, P.L.; Tardif, C.L.; Minuzzi, L.; Frey, B.N.; Bock, N.A. Age-related mapping of intracortical myelin from late adolescence to middle adulthood using T1-weighted MRI. Hum. Brain Mapp. 2017, 38, 3691–3703. [Google Scholar] [CrossRef] [Green Version]
- Grydeland, H.; Walhovd, K.B.; Tamnes, C.K.; Westlye, L.T.; Fjell, A.M. Intracortical myelin links with performance variability across the human lifespan: Results from T1-and T2-weighted MRI myelin mapping and diffusion tensor imaging. J. Neurosci. 2013, 33, 18618–18630. [Google Scholar] [CrossRef] [Green Version]
- Sehmbi, M.; Rowley, C.; Minuzzi, L.; Kapczinski, F.; Steiner, M.; Sassi, R.; Bock, N.; Frey, B. Association of intracortical myelin and cognitive function in bipolar I disorder. Acta Psychiatr. Scand. 2018, 138, 62–72. [Google Scholar] [CrossRef]
- Toschi, N.; Passamonti, L.; Bellesi, M. Sleep quality relates to emotional reactivity via intracortical myelination. Sleep 2021, 44, zsaa146. [Google Scholar] [CrossRef]
- Toschi, N.; Passamonti, L. Intra-cortical myelin mediates personality differences. J. Personal. 2019, 87, 889–902. [Google Scholar] [CrossRef] [Green Version]
- Van Essen, D.C.; Smith, S.M.; Barch, D.M.; Behrens, T.E.; Yacoub, E.; Ugurbil, K.; Consortium, W.-M.H. The WU-Minn human connectome project: An overview. Neuroimage 2013, 80, 62–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glasser, M.F.; Van Essen, D.C. Mapping human cortical areas in vivo based on myelin content as revealed by T1-and T2-weighted MRI. J. Neurosci. 2011, 31, 11597–11616. [Google Scholar] [CrossRef] [Green Version]
- Glasser, M.F.; Sotiropoulos, S.N.; Wilson, J.A.; Coalson, T.S.; Fischl, B.; Andersson, J.L.; Xu, J.; Jbabdi, S.; Webster, M.; Polimeni, J.R. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 2013, 80, 105–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [PubMed]
- Fjell, A.M.; Walhovd, K.B. Structural brain changes in aging: Courses, causes and cognitive consequences. Rev. Neurosci. 2010, 21, 187–222. [Google Scholar] [CrossRef]
- Brennan, D.; Wu, T.; Fan, J. Morphometrical brain markers of sex difference. Cereb. Cortex 2021, 31, 3641–3649. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.; Sainsbury, A.; Group, S.C.W. Sex differences in obesity and the regulation of energy homeostasis. Obes. Rev. 2009, 10, 154–167. [Google Scholar] [CrossRef]
- Brito, N.H.; Noble, K.G. Socioeconomic status and structural brain development. Front. Neurosci. 2014, 8, 276. [Google Scholar] [CrossRef] [Green Version]
- Ocklenburg, S.; Beste, C.; Güntürkün, O. Handedness: A neurogenetic shift of perspective. Neurosci. Biobehav. Rev. 2013, 37, 2788–2793. [Google Scholar] [CrossRef] [PubMed]
- Vainik, U.; Baker, T.E.; Dadar, M.; Zeighami, Y.; Michaud, A.; Zhang, Y.; Alanis, J.C.G.; Misic, B.; Collins, D.L.; Dagher, A. Neurobehavioral correlates of obesity are largely heritable. Proc. Natl. Acad. Sci. USA 2018, 115, 9312–9317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nader, D.A.; Sanchez, Z.M. Effects of regular cannabis use on neurocognition, brain structure, and function: A systematic review of findings in adults. Am. J. Drug Alcohol Abus. 2018, 44, 4–18. [Google Scholar] [CrossRef]
- Barry, D.; Clarke, M.; Petry, N.M. Obesity and its relationship to addictions: Is overeating a form of addictive behavior? Am. J. Addict. 2009, 18, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.M.; Webster, M.A.; Vidaurre, D.; Nichols, T.E.; Smith, S.M. Multi-level block permutation. Neuroimage 2015, 123, 253–268. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.; Rolls, E.T.; Ruan, H.; Feng, J. Functional connectivities in the brain that mediate the association between depressive problems and sleep quality. JAMA Psychiatry 2018, 75, 1052–1061. [Google Scholar] [CrossRef]
- M Wheeler, B. Permutation tests for linear models in R. Available online: https://cran.r-project.org/web/packages/lmPerm/vignettes/lmPerm.pdf (accessed on 26 August 2021).
- Winkler, A.M.; Ridgway, G.R.; Webster, M.A.; Smith, S.M.; Nichols, T.E. Permutation inference for the general linear model. Neuroimage 2014, 92, 381–397. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Stice, E.; Spoor, S.; Bohon, C.; Veldhuizen, M.G.; Small, D.M. Relation of reward from food intake and anticipated food intake to obesity: A functional magnetic resonance imaging study. J. Abnorm. Psychol. 2008, 117, 924. [Google Scholar] [CrossRef] [Green Version]
- Berridge, K.C.; Ho, C.-Y.; Richard, J.M.; DiFeliceantonio, A.G. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010, 1350, 43–64. [Google Scholar] [CrossRef] [Green Version]
- Stice, E.; Yokum, S. Neural vulnerability factors that increase risk for future weight gain. Psychol. Bull. 2016, 142, 447. [Google Scholar] [CrossRef] [PubMed]
- Bruce, A.; Holsen, L.; Chambers, R.; Martin, L.; Brooks, W.; Zarcone, J.; Butler, M.; Savage, C.R. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int. J. Obes. 2010, 34, 1494–1500. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Roitzsch, C.; Horstmann, A.; Pössel, M.; Hummel, T. Increased Brain Reward Responsivity to Food-Related Odors in Obesity. Obesity 2021, 29, 1138–1145. [Google Scholar] [CrossRef]
- Carnell, S.; Benson, L.; Pantazatos, S.P.; Hirsch, J.; Geliebter, A. Amodal brain activation and functional connectivity in response to high-energy-density food cues in obesity. Obesity 2014, 22, 2370–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pajevic, S.; Basser, P.J.; Fields, R.D. Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 2014, 276, 135–147. [Google Scholar] [CrossRef] [Green Version]
- Uddin, L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015, 16, 55–61. [Google Scholar] [CrossRef]
- Seeley, W.W. The salience network: A neural system for perceiving and responding to homeostatic demands. J. Neurosci. 2019, 39, 9878–9882. [Google Scholar] [CrossRef] [PubMed]
- Kenny, P.J. Reward mechanisms in obesity: New insights and future directions. Neuron 2011, 69, 664–679. [Google Scholar] [CrossRef] [Green Version]
- Volkow, N.D.; Wang, G.-J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-García, I.; Jurado, M.Á.; Garolera, M.; Segura, B.; Sala-Llonch, R.; Marqués-Iturria, I.; Pueyo, R.; Sender-Palacios, M.J.; Vernet-Vernet, M.; Narberhaus, A. Alterations of the salience network in obesity: A resting-state fMRI study. Hum. Brain Mapp. 2013, 34, 2786–2797. [Google Scholar] [CrossRef]
- Ding, Y.; Ji, G.; Li, G.; Zhang, W.; Hu, Y.; Liu, L.; Wang, Y.; Hu, C.; von Deneen, K.M.; Han, Y. Altered interactions among resting-state networks in individuals with obesity. Obesity 2020, 28, 601–608. [Google Scholar] [CrossRef]
- Sadler, J.R.; Shearrer, G.E.; Burger, K.S. Body mass variability is represented by distinct functional connectivity patterns. NeuroImage 2018, 181, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Huntenburg, J.M.; Bazin, P.-L.; Goulas, A.; Tardif, C.L.; Villringer, A.; Margulies, D.S. A systematic relationship between functional connectivity and intracortical myelin in the human cerebral cortex. Cereb. Cortex 2017, 27, 981–997. [Google Scholar] [CrossRef] [PubMed]
- Doucet, G.E.; Rasgon, N.; McEwen, B.S.; Micali, N.; Frangou, S. Elevated body mass index is associated with increased integration and reduced cohesion of sensory-driven and internally guided resting-state functional brain networks. Cereb. Cortex 2018, 28, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.E.; Airey, D.C.; Young, N.A.; Leitch, D.B.; Kaas, J.H. Neuron densities vary across and within cortical areas in primates. Proc. Natl. Acad. Sci. USA 2010, 107, 15927–15932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fjell, A.M.; Westlye, L.T.; Amlien, I.; Tamnes, C.K.; Grydeland, H.; Engvig, A.; Espeseth, T.; Reinvang, I.; Lundervold, A.J.; Lundervold, A. High-expanding cortical regions in human development and evolution are related to higher intellectual abilities. Cereb. Cortex 2015, 25, 26–34. [Google Scholar] [CrossRef]
- Hill, J.; Inder, T.; Neil, J.; Dierker, D.; Harwell, J.; Van Essen, D. Similar patterns of cortical expansion during human development and evolution. Proc. Natl. Acad. Sci. USA 2010, 107, 13135–13140. [Google Scholar] [CrossRef] [Green Version]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appelhans, B.M.; Woolf, K.; Pagoto, S.L.; Schneider, K.L.; Whited, M.C.; Liebman, R. Inhibiting food reward: Delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity 2011, 19, 2175–2182. [Google Scholar] [CrossRef] [PubMed]

| Variable | |
|---|---|
| Body Mass Index (BMI), mean (SD), Kg/M2 | 26.44 (5.13) |
| Age, mean (SD), years | 28.79 (3.71) |
| Male (Sex), N (%) | 498 (46.72) |
| Handedness, mean (SD) b | 66.03 (43.82) |
| Education, mean (SD), years | 14.92 (1.80) |
| Race N (%) | |
| White | 796 (74.67) |
| Other | 270 (25.33) |
| Evidence of drug consumption on test day, N (%) | 140 (13.13) |
| Fluid Intelligence, mean (SD) c | 16.88 (4.80) |
| Total brain volume, mean (SD), mm3 | 1582972.87 |
| Smoking history, N (%) | |
| Never | 565 (53.00) |
| Smoked 1–19 cigarettes over lifetime | 92 (8.63) |
| Smoked ≥20 cigarettes over lifetime | 126 (11.82) |
| Regular smoker | 283 (26.55) |
| Substance use | |
| Frequency of alcohol use in the last 12 months, mean (SD) d | 4.27 (1.56) |
| Marijuana use, mean (SD), incidents | 1.41 (1.69) |
| Hemoglobin A1C (HbA1c), mean (SD) e | 5.23% (0.30) |
| Region | F | Adj. R2 | Beta | B | CI of B(95%) | DW | Partial r Value | Permutation p Value(−log10) |
|---|---|---|---|---|---|---|---|---|
| Negative Correlations | ||||||||
| LMOFG | 11.08 | 0.062 | −0.10 | −0.24 | (−0.39,−0.10) | 1.97 | −0.097 | 0.0005 (3.30) |
| L MTG | 12.33 | 0.069 | −0.13 | −1.59 | (−2.33,−0.87) | 1.95 | −0.129 | 0.0001 (4) |
| L Perical | 10.64 | 0.060 | −0.08 | −0.39 | (−0.68,−0.11) | 1.95 | −0.082 | 0.0028 (2.55) |
| L SMG | 13.87 | 0.078 | −0.16 | −2.26 | (−3.09,−1.43) | 1.93 | −0.159 | 0.0001 (4) |
| L Insula | 11.87 | 0.073 | −0.12 | −0.68 | (−1.02,−0.34) | 1.95 | −0.113 | 0.0002 (3.70) |
| R Cuneus | 12.41 | 0.07 | −0.13 | −0.73 | (−1.06,−0.40) | 1.96 | −0.132 | 0.0002 (3.70) |
| R ITG | 10.99 | 0.062 | −0.09 | −1.11 | (−1.81,−0.41) | 1.95 | −0.095 | 0.0021 (2.68) |
| R PCG | 11.31 | 0.064 | −0.11 | −0.74 | (−1.15,−0.37) | 1.94 | −0.106 | 0.0008 (3.10) |
| R rACG | 11.77 | 0.066 | −0.12 | −0.76 | (−1.15,−0.37) | 1.94 | −0.115 | 0.0002 (3.70) |
| Positive correlations | ||||||||
| L Cuneus | 11.64 | 0.065 | 0.11 | 0.79 | (0.38,1.21) | 1.94 | 0.113 | 0.0004 (3.40) |
| L lFT | 12.31 | 0.069 | 0.13 | 1.02 | (0.55,1.49) | 1.95 | 0.129 | 0.0001 (4) |
| L FP | 11.24 | 0.063 | 0.10 | 0.29 | (0.12,0.46) | 1.94 | 0.102 | 0.0010 (3) |
| R FG | 10.73 | 0.06 | 0.09 | 1.11 | (0.34,1.88) | 1.95 | 0.085 | 0.0026 (2.59) |
| R FP | 13.80 | 0.078 | 0.16 | 0.47 | (0.30,0.65) | 1.93 | 0.156 | 0.0001 (4) |
| R TTG | 13.05 | 0.073 | 0.15 | 0.35 | (0.21,0.49) | 1.94 | 0.143 | 0.0001 (4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, D.; Wang, Y.; Long, Z.; Jackson, T.; Chang, X.; Zhou, F.; Chen, H. The Association between Body Mass Index and Intra-Cortical Myelin: Findings from the Human Connectome Project. Nutrients 2021, 13, 3221. https://doi.org/10.3390/nu13093221
Dong D, Wang Y, Long Z, Jackson T, Chang X, Zhou F, Chen H. The Association between Body Mass Index and Intra-Cortical Myelin: Findings from the Human Connectome Project. Nutrients. 2021; 13(9):3221. https://doi.org/10.3390/nu13093221
Chicago/Turabian StyleDong, Debo, Yulin Wang, Zhiliang Long, Todd Jackson, Xuebin Chang, Feng Zhou, and Hong Chen. 2021. "The Association between Body Mass Index and Intra-Cortical Myelin: Findings from the Human Connectome Project" Nutrients 13, no. 9: 3221. https://doi.org/10.3390/nu13093221
APA StyleDong, D., Wang, Y., Long, Z., Jackson, T., Chang, X., Zhou, F., & Chen, H. (2021). The Association between Body Mass Index and Intra-Cortical Myelin: Findings from the Human Connectome Project. Nutrients, 13(9), 3221. https://doi.org/10.3390/nu13093221







