Protective and Recovery Effects of Resveratrol Supplementation on Exercise Performance and Muscle Damage following Acute Plyometric Exercise
Abstract
1. Introduction
2. Materials and Methods
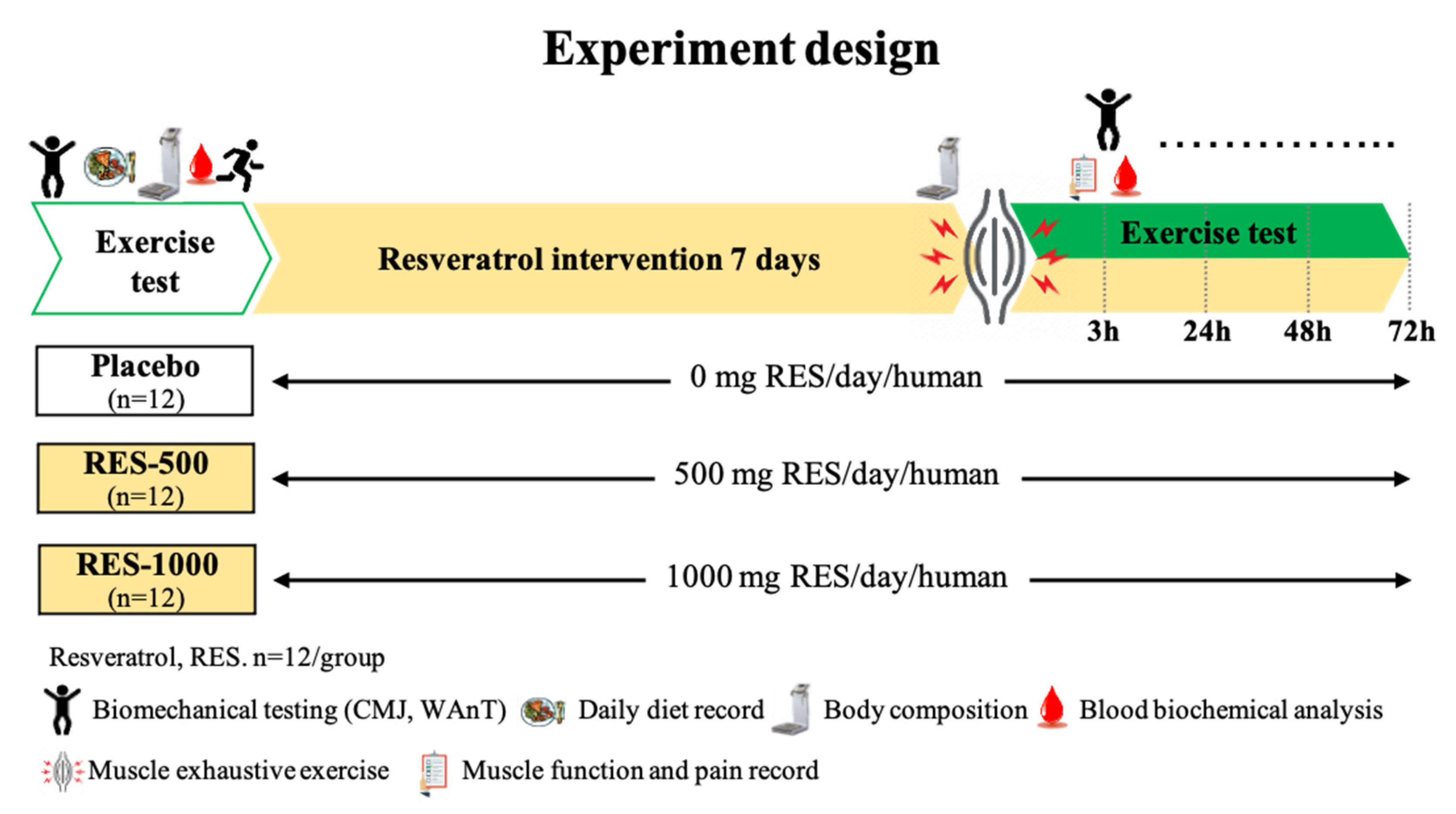
2.1. Experimental Design
2.2. Participants
2.3. Anthropometric Measurements
2.4. Plyometric-Exercise-Induced Muscle Damage (PEIMD)
2.5. The Countermovement Jump (CMJ) Test
2.6. Wingate Anaerobic Test (WAnT)
2.7. Muscle Soreness
2.8. Clinical Biochemistry, Hematology, and Inflammation Cytokines Analysis
2.9. Statistical Analysis
3. Results
3.1. Subject Characteristics
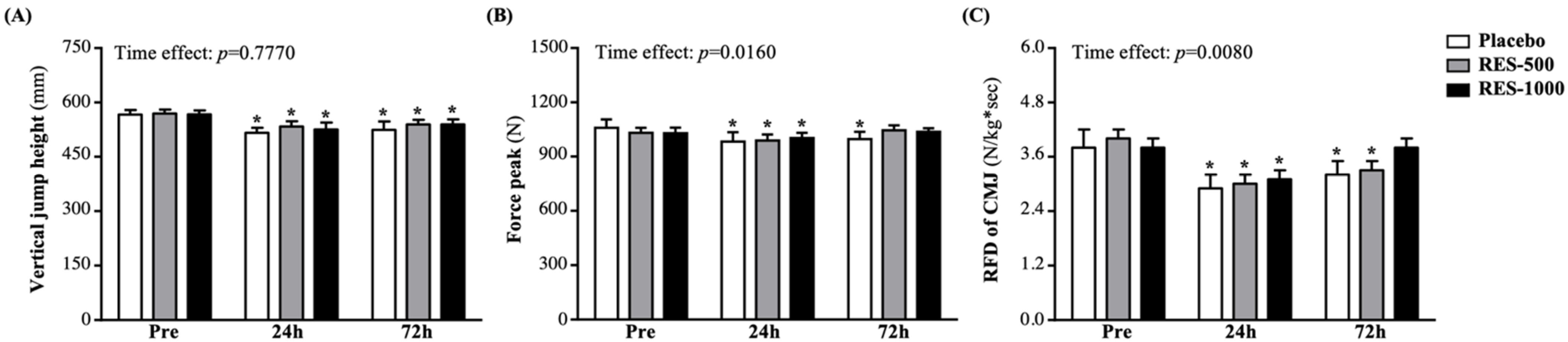
3.2. Effect of RES Supplementation on Vertical Jump Height of Exercise Performance
3.3. Effect of RES Supplementation on Wingate Anaerobic Test (WAnT) of Exercise Performance
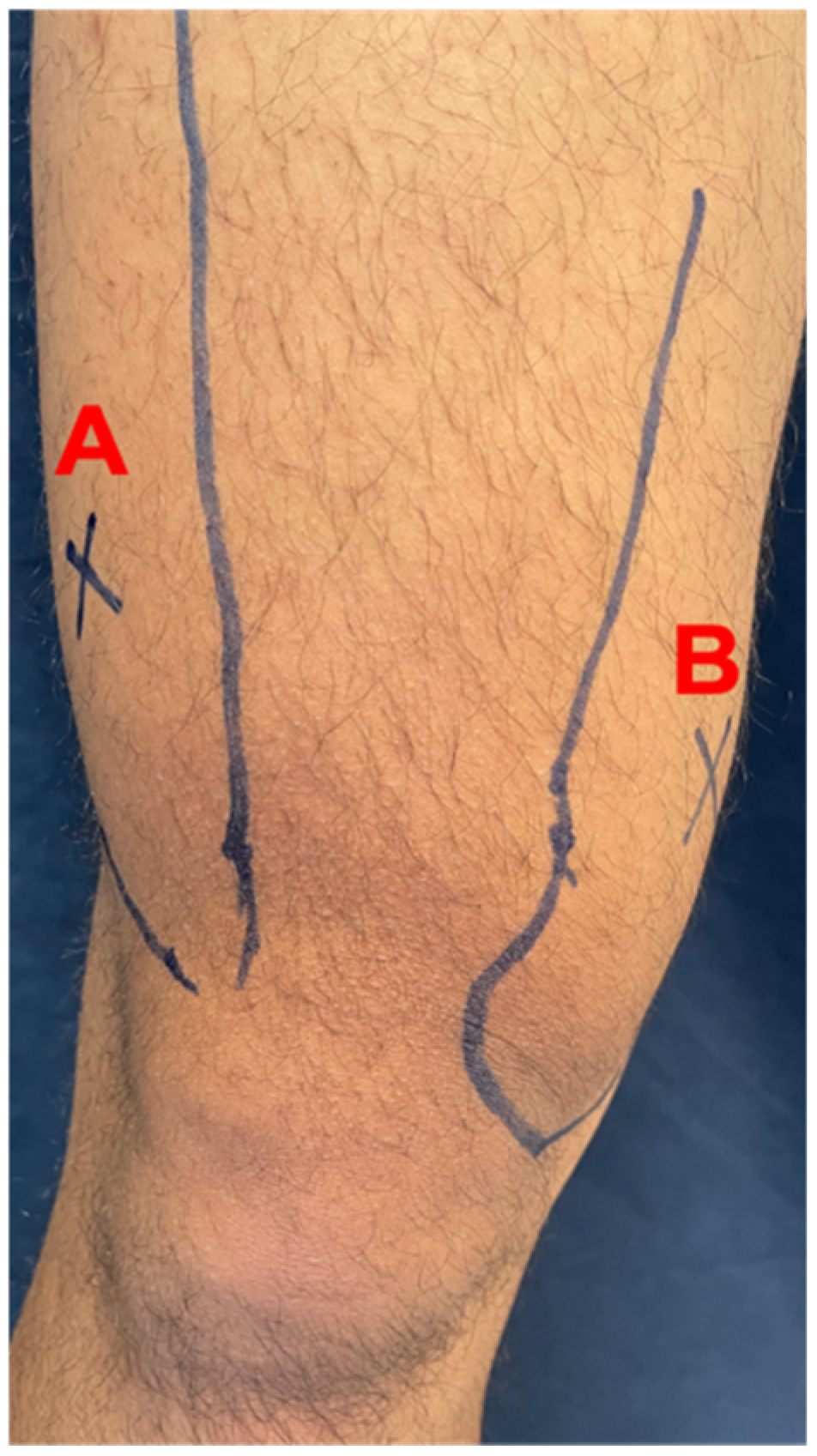
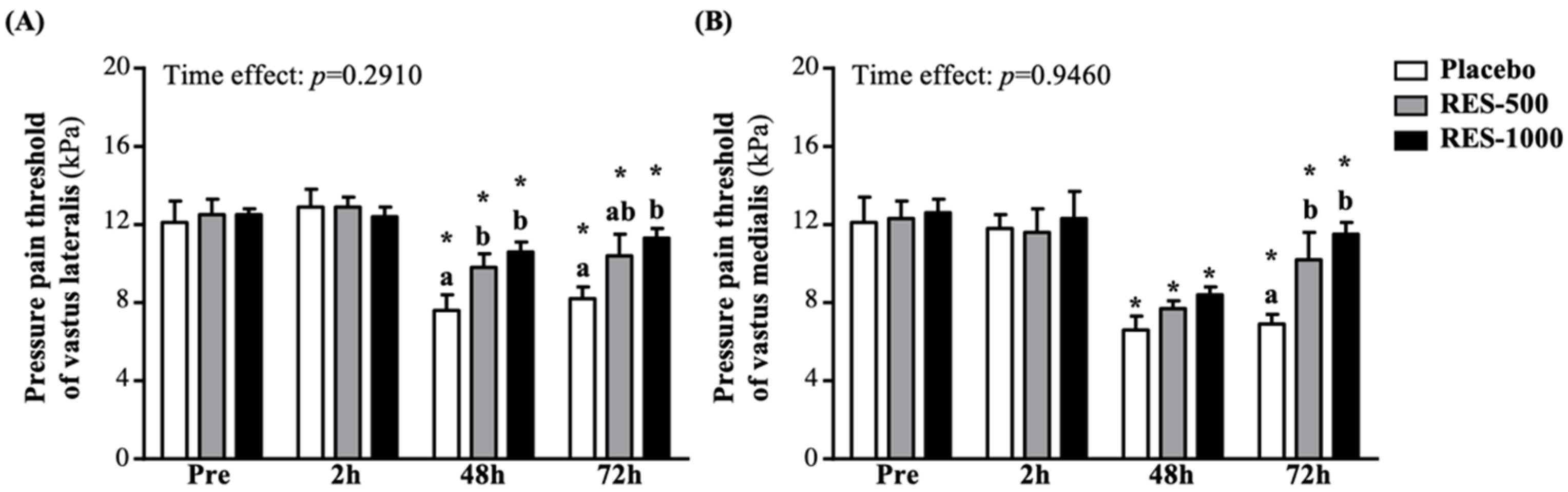
3.4. Effect of RES Supplementation on Pressure Pain Thresholds (PPT)
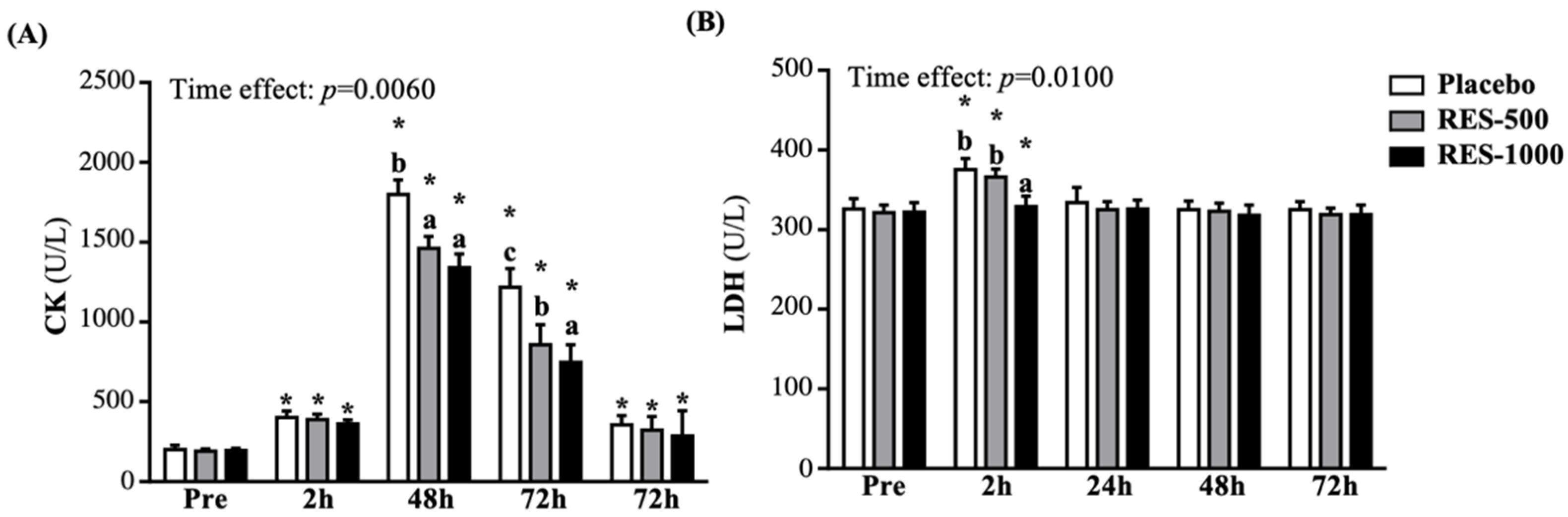
3.5. Effect of RES Supplementation on Muscle Damage Biomarkers
3.6. Effect of RES Supplementation on Blood Biochemistry Markers
3.7. Effect of RES Supplementation on Complete Blood Count (CBC) Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Komi, P.V. Stretch-shortening cycle: A powerful model to study normal and fatigued muscle. J. Biomech. 2000, 33, 1197–1206. [Google Scholar] [CrossRef]
- Silva, A.F.; Clemente, F.M.; Lima, R.; Nikolaidis, P.T.; Rosemann, T.; Knechtle, B. The effect of plyometric training in volleyball players: A systematic review. Int. J. Environ. Res. Public Health. 2019, 16, 2960. [Google Scholar] [CrossRef] [PubMed]
- Hody, S.; Croisier, J.L.; Bury, T.; Rogister, B.; Leprince, P. Eccentric muscle contractions: Risks and benefits. Front. Physiol. 2019, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.W.; Smith, A.; Macnaughton, L.S.; French, D.N. Strength and conditioning and concurrent training practices in elite rugby union. J. Strength Cond Res. 2016, 30, 3354–3366. [Google Scholar] [CrossRef]
- Hortobágyi, T.; Houmard, J.; Fraser, D.; Dudek, R.; Lambert, J.; Tracy, J. Normal forces and myofibrillar disruption after repeated eccentric exercise. J. Appl. Physiol. (1985) 1998, 84, 492–498. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81, S52–S69. [Google Scholar] [CrossRef]
- Dominguez-Balmaseda, D.; Diez-Vega, I.; Larrosa, M.; San Juan, A.F.; Issaly, N.; Moreno-Pérez, D.; Burgos, S.; Sillero-Quintana, M.; Gonzalez, C.; Bas, A.; et al. Effect of a Blend of Zingiber officinale Roscoe and Bixa orellana L. Herbal supplement on the recovery of delayed-onset muscle soreness induced by unaccustomed eccentric resistance training: A randomized, triple-blind, placebo-controlled trial. Front. Physiol. 2020, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, K.; Newton, M. Difference in the magnitude of muscle damage between maximal and submaximal eccentric loading. J. Strength Cond Res. 2002, 16, 202–208. [Google Scholar]
- Lee, J.; Goldfarb, A.H.; Rescino, M.H.; Hegde, S.; Patrick, S.; Apperson, K. Eccentric exercise effect on blood oxidative-stress markers and delayed onset of muscle soreness. Med. Sci. Sports Exerc. 2002, 34, 443–448. [Google Scholar] [CrossRef]
- Ascensão, A.; Rebelo, A.; Oliveira, E.; Marques, F.; Pereira, L.; Magalhães, J. Biochemical impact of a soccer match—Analysis of oxidative stress and muscle damage markers throughout recovery. Clin. Biochem. 2008, 41, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-kinase- and exercise-related muscle damage implications for muscle performance and recovery. J Nutr Metab. 2012, 2012, 960363. [Google Scholar] [CrossRef] [PubMed]
- Safran, M.R.; Seaber, A.V.; Garrett, W.E., Jr. Warm-up and muscular injury prevention. An update. Sports Med. 1989, 8, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.Y.; Tain, Y.L.; Yu, H.R.; Huang, L.T. The effects of resveratrol in the treatment of metabolic syndrome. Int. J. Mol Sci. 2019, 20, 535. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Carpéné, C.; Mercader, J. Resveratrol, Metabolic Syndrome, and Gut Microbiota. Nutrients 2018, 10, 1651. [Google Scholar] [CrossRef]
- Zortea, K.; Franco, V.C.; Francesconi, L.P.; Cereser, K.M.; Lobato, M.I.R.; Belmonte-de-Abreu, P.S. Resveratrol supplementation in schizophrenia patients: A randomized clinical trial evaluating serum glucose and cardiovascular risk factors. Nutrients 2016, 8, 73. [Google Scholar] [CrossRef]
- Sadi, G.; Şahin, G.; Bostancı, A. Modulation of renal insulin signaling pathway and antioxidant enzymes with streptozotocin-induced diabetes: Effects of resveratrol. Medicina (Kaunas) 2018, 55, 3. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, L.; Cui, J.; Huoc, Z.; Xue, J.; Cui, H.; Mao, Q.; Yang, R. Resveratrol inhibits NF-kB signaling through suppression of p65 and IkappaB kinase activities. Pharmazie 2013, 68, 689–694. [Google Scholar] [PubMed]
- Vanamala, J.; Reddivari, L.; Radhakrishnan, S.; Tarver, C. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer 2010, 10, 238. [Google Scholar] [CrossRef]
- De Sá Coutinho, D.; Pacheco, M.T.; Frozza, R.L.; Bernardi, A. Anti-inflammatory effects of resveratrol: Mechanistic insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef]
- Baltaci, S.B.; Mogulkoc, R.; Baltaci, A.K. Resveratrol and exercise. Biomed. Rep. 2016, 5, 525–530. [Google Scholar] [CrossRef]
- Wu, R.E.; Huang, W.C.; Liao, C.C.; Chang, Y.K.; Kan, N.W.; Huang, C.C. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 2013, 18, 4689–4702. [Google Scholar] [CrossRef]
- Kan, N.W.; Lee, M.C.; Tung, Y.T.; Chiu, C.C.; Huang, C.C.; Huang, W.C. The synergistic effects of resveratrol combined with resistant training on exercise performance and physiological adaption. Nutrients 2018, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.J.; Ho, C.S.; Lee, M.C.; Ho, C.S.; Huang, C.C.; Kan, N.W. Protective effects of resveratrol supplementation on contusion induced muscle injury. Int. J. Med. Sci. 2020, 17, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Jhang, W.L.; Lee, C.C.; Kan, N.W.; Hsu, Y.J.; Ho, C.S.; Chang, C.H.; Cheng, Y.C.; Lin, J.S.; Huang, C.C. The effect of kefir supplementation on improving human endurance exercise performance and antifatigue. Metabolites 2021, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Hsu, Y.J.; Ho, C.S.; Chang, C.H.; Liu, C.W.; Huang, C.C.; Chiang, W.D. Evaluation of the Efficacy of Supplementation with Planox® Lemon Verbena Extract in Improving Oxidative Stress and Muscle Damage: A Randomized Double-Blind Controlled Trial. Int. J. Med. Sci. 2021, 18, 2641–2652. [Google Scholar] [CrossRef]
- Gathercole, R.J.; Sporer, B.C.; Stellingwerff, T.; Sleivert, G.G. Comparison of the capacity of different jump and sprint field tests to detect neuromuscular fatigue. J. Strength Cond Res. 2015, 29, 2522–2531. [Google Scholar] [CrossRef]
- Gathercole, R.; Sporer, B.; Stellingwerff, T.; Sleivert, G. Alternative countermovement-jump analysis to quantify acute neuromuscular fatigue. Int. J. Sports Physiol. Perform. 2015, 10, 84–92. [Google Scholar] [CrossRef]
- Durkalec-Michalski, K.; Nowaczyk, P.M.; Adrian, J.; Kamińska, J.; Podgórski, T. The influence of progressive-chronic and acute sodium bicarbonate supplementation on anaerobic power and specific performance in team sports: A randomized, double-blind, placebo-controlled crossover study. Nutr. Metab. 2020, 17, 38. [Google Scholar] [CrossRef]
- Hodgson, D.D.; Lima, C.D.; Low, J.L.; Behm, D.G. Four Weeks of roller massage training did not impact range of motion, pain pressure threshold, voluntary contractile properties or jump performance. Int. J. Sports Phys. Ther. 2018, 13, 835–845. [Google Scholar] [CrossRef]
- Serdar, C.C.; Cihan, M.; Yücel, D.; Serdar, M.A. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem Med. 2021, 31, 010502. [Google Scholar] [CrossRef]
- Jakeman, J.R.; Lambrick, D.M.; Wooley, B.; Babraj, J.A.; Faulkner, J.A. Effect of an acute dose of omega-3 fish oil following exercise-induced muscle damage. Eur. J. Appl. Physiol. 2017, 117, 575–582. [Google Scholar] [CrossRef]
- Marián, V.; Katarína, L.; Dávid, O.; Matúš, K.; Simon, W. Improved maximum strength, vertical jump and sprint performance after 8 weeks of jump squat training with individualized loads. J. Sports Sci. Med. 2016, 15, 492–500. [Google Scholar]
- Kenny, I.C.; Ó Cairealláin, A.; Comyns, T.M. Validation of an electronic jump mat to assess stretch-shortening cycle function. J. Strength Cond Res. 2012, 26, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Romero-Moraleda, B.; González-García, J.; Cuéllar-Rayo, Á.; Balsalobre-Fernández, C.; Muñoz-García, D.; Morencos, E. Effects of Vibration and Non-Vibration Foam Rolling on Recovery after Exercise with Induced Muscle Damage. J. Sports Sci. Med. 2019, 18, 172–180. [Google Scholar] [PubMed]
- Morgan, P.T.; Wollman, P.M.; Jackman, S.R.; Bowtell, J.L. Flavanol-rich cacao mucilage juice enhances recovery of power but not strength from intensive exercise in healthy, young men. Sports 2018, 6, 159. [Google Scholar] [CrossRef]
- Willems, M.E.; Hale, T.; Wilkinson, C.S. Effect of manual massage on muscle-specific soreness and single leg jump performance after downhill treadmill walking. Med. Sportiva. 2009, 13, 61–66. [Google Scholar] [CrossRef]
- Abad Colil, F.; Ramírez Campillo, R.; Álvarez, C.; Castro, M.; Silva, S.F.; Izquierdo Redín, M. Effects of beta-hydroxy-beta-methylbutyrate supplementation on physical performance of young players during an intensified soccer-training period: A short report. Hum. Mov. 2017, 18, 91–96. [Google Scholar] [CrossRef]
- Boullosa, D.; Abreu, L.; de Conceição, F.A.; Rodríguez, Y.C.; Jimenez-Reyes, P. The influence of training background on different rate of force development calculations during countermovement jump. Kinesiology 2018, 50, 90–95. [Google Scholar]
- Stien, N.; Saeterbakken, A.H.; Hermans, E.; Vereide, V.A.; Olsen, E.; Andersen, V. Comparison of climbing-specific strength and endurance between lead and boulder climbers. PLoS ONE 2019, 14, e0222529. [Google Scholar] [CrossRef]
- Nieman, D.C.; Zwetsloot, K.A.; Simonson, A.J.; Hoyle, A.T.; Wang, X.; Nelson, H.K.; Lefranc-Millot, C.; Guérin-Deremaux, L. Effects of whey and pea protein supplementation on post-eccentric exercise muscle damage: A randomized trial. Nutrients 2020, 12, 2382. [Google Scholar] [CrossRef]
- Tayech, A.; Mejri, M.A.; Chaouachi, M.; Chaabene, H.; Hambli, M.; Brughelli, M.; Behm, D.G.; Chaouachi, A. Taekwondo anaerobic intermittent kick test: Discriminant validity and an update with the gold-standard wingate test. J. Hum. Kinet. 2020, 71, 229–242. [Google Scholar] [CrossRef]
- Calbet, J.A.; De Paz, J.A.; Garatachea, N.; Cabeza de Vaca, S.; Chavarren, J. Anaerobic energy provision does not limit Wingate exercise performance in endurance-trained cyclists. J. Appl. Physiol. (1985) 2003, 94, 668–676. [Google Scholar] [CrossRef]
- Parolin, M.L.; Chesley, A.; Matsos, M.P.; Spriet, L.L.; Jones, N.L.; Heigenhauser, G.J. Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. Am. J. Physiol. 1999, 277, E890–E900. [Google Scholar] [CrossRef]
- Frescas, D.; Valenti, L.; Accili, D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J. Biol. Chem. 2005, 280, 20589–20595. [Google Scholar] [CrossRef] [PubMed]
- Pelfort, X.; Torres-Claramunt, R.; Sánchez-Soler, J.F.; Hinarejos, P.; Leal-Blanquet, J.; Valverde, D.; Monllau, J.C. Pressure algometry is a useful tool to quantify pain in the medial part of the knee: An intra- and inter-reliability study in healthy subjects. Orthop. Traumatol. Surg. Res. 2015, 101, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Pereira Panza, V.S.; Diefenthaeler, F.; da Silva, E.L. Benefits of dietary phytochemical supplementation on eccentric exercise-induced muscle damage: Is including antioxidants enough? Nutrition 2015, 31, 1072–1082. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [PubMed]
- Zabriskie, H.A.; Blumkaitis, J.C.; Moon, J.M.; Currier, B.S.; Stefan, R.; Ratliff, K.; Harty, P.S.; Stecker, R.A.; Rudnicka, K.; Jäger, R.; et al. Yeast Beta-Glucan Supplementation Downregulates Markers of Systemic Inflammation after Heated Treadmill Exercise. Nutrients 2020, 12, 1144. [Google Scholar] [CrossRef]
- Laupheimer, M.W.; Perry, M.; Benton, S.; Malliaras, P.; Maffulli, N. Resveratrol exerts no effect on inflammatory response and delayed onset muscle soreness after a marathon in male athletes.: A randomised, double-blind, placebo-controlled pilot feasibility study. Transl. Med. UniSa 2014, 10, 38–42. [Google Scholar] [PubMed]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef]
- Heckel, Z.; Atlasz, T.; Tékus, É.; Kőszegi, T.; Laczkó, J.; Váczi, M. Monitoring exercise-induced muscle damage indicators and myoelectric activity during two weeks of knee extensor exercise training in young and old men. PLoS ONE 2019, 14, e0224866. [Google Scholar] [CrossRef]
- Arakawa, K.; Hosono, A.; Shibata, K.; Ghadimi, R.; Fuku, M.; Goto, C.; Imaeda, N.; Tokudome, Y.; Hoshino, H.; Marumoto, M.; et al. Changes in blood biochemical markers before, during, and after a 2-day ultramarathon. Open Access J. Sports Med. 2016, 7, 43–50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xiao, N.N. Effects of resveratrol supplementation on oxidative damage and lipid peroxidation induced by strenuous exercise in rats. Biomol. Ther. 2015, 23, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef] [PubMed]
- Soleas, G.J.; Angelini, M.; Grass, L.; Diamandis, E.P.; Goldberg, D.M. Absorption of trans-resveratrol in rats. Methods Enzymol. 2001, 335, 145–154. [Google Scholar]
- Biasutto, L.; Marotta, E.; Garbisa, S.; Zoratti, M.; Paradisi, C. Determination of quercetin and resveratrol in whole blood—Implications for bioavailability studies. Molecules 2010, 15, 6570–6579. [Google Scholar] [CrossRef] [PubMed]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef]

| Parameters | Group | Pre | 2 h | 24 h |
|---|---|---|---|---|
| WBC (cells/mcL) | Placebo | 6433 ± 239 a | 6887 ± 334 a | 5723 ± 284 a |
| RES-500 | 6518 ± 289 a | 6945 ± 413 a | 5775 ± 350 a | |
| RES-1000 | 6597 ± 427 a | 6943 ± 546 a | 5683 ± 491 a | |
| Neutrophils (%) | Placebo | 69.6 ± 3.8 a | 65.5 ± 2.4 a | 54.2 ± 1.6 a,* |
| RES-500 | 69.7 ± 3.8 a | 64.6 ± 1.2 a | 54.6 ± 2.2 a,* | |
| RES-1000 | 69.8 ± 2.4 a | 65.1 ± 1.6 a | 54.5 ± 2.3 a,* | |
| Lymphocytes (%) | Placebo | 25.3 ± 1.7 a | 26.0 ± 2.0 a | 36.4 ± 1.5 a,* |
| RES-500 | 24.9 ± 2.9 a | 26.7 ± 1.1 a | 36.1 ± 2.2 a,* | |
| RES-1000 | 25.9 ± 1.8 a | 26.3 ± 1.4 a | 36.1 ± 2.2 a,* | |
| Monocytes (%) | Placebo | 4.3 ± 0.8 a | 5.0 ± 0.3 a | 5.4 ± 0.1 a |
| RES-500 | 4.1 ± 0.8 a | 5.4 ± 0.4 a | 5.4 ± 0.4 a | |
| RES-1000 | 4.8 ± 0.8 a | 5.6 ± 0.3 a | 5.6 ± 0.3 a | |
| Eosinophils (%) | Placebo | 0.8 ± 0.2 a | 0.7 ± 0.1 a | 0.8 ± 0.1 a |
| RES-500 | 0.8 ± 0.2 a | 0.7 ± 0.1 a | 0.8 ± 0.1 a | |
| RES-1000 | 0.8 ± 0.3 a | 0.6 ± 0.1 a | 0.7 ± 0.1 a | |
| Basophils (%) | Placebo | 2.1 ± 0.6 a | 2.7 ± 0.3 a | 1.5 ± 0.1 a |
| RES-500 | 2.5 ± 0.4 a | 2.5 ± 0.2 a | 1.6 ± 0.2 a | |
| RES-1000 | 2.4 ± 0.3 a | 2.6 ± 0.2 a | 1.6 ± 0.2 a | |
| NLR (%) | Placebo | 2.6 ± 0.2 a | 2.7 ± 0.3 a | 1.5 ± 0.1 a,* |
| RES-500 | 2.7 ± 0.3 a | 2.5 ± 0.2 a | 1.6 ± 0.2 a,* | |
| RES-1000 | 2.8 ± 0.2 a | 2.6 ± 0.2 a | 1.6 ± 0.2 a,* |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-C.; Lee, M.-C.; Ho, C.-S.; Hsu, Y.-J.; Ho, C.-C.; Kan, N.-W. Protective and Recovery Effects of Resveratrol Supplementation on Exercise Performance and Muscle Damage following Acute Plyometric Exercise. Nutrients 2021, 13, 3217. https://doi.org/10.3390/nu13093217
Huang C-C, Lee M-C, Ho C-S, Hsu Y-J, Ho C-C, Kan N-W. Protective and Recovery Effects of Resveratrol Supplementation on Exercise Performance and Muscle Damage following Acute Plyometric Exercise. Nutrients. 2021; 13(9):3217. https://doi.org/10.3390/nu13093217
Chicago/Turabian StyleHuang, Chi-Chang, Mon-Chien Lee, Chin-Shan Ho, Yi-Ju Hsu, Chien-Chang Ho, and Nai-Wen Kan. 2021. "Protective and Recovery Effects of Resveratrol Supplementation on Exercise Performance and Muscle Damage following Acute Plyometric Exercise" Nutrients 13, no. 9: 3217. https://doi.org/10.3390/nu13093217
APA StyleHuang, C.-C., Lee, M.-C., Ho, C.-S., Hsu, Y.-J., Ho, C.-C., & Kan, N.-W. (2021). Protective and Recovery Effects of Resveratrol Supplementation on Exercise Performance and Muscle Damage following Acute Plyometric Exercise. Nutrients, 13(9), 3217. https://doi.org/10.3390/nu13093217








