Pretreatment Adherence to a Priori-Defined Dietary Patterns Is Associated with Decreased Nutrition Impact Symptom Burden in Head and Neck Cancer Survivors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Predictors: Pretreatment a Priori Diet Quality Index Scores
2.3. Covariates
2.4. Outcomes: NIS at 1-Year Postdiagnosis
2.5. Statistical Analysis
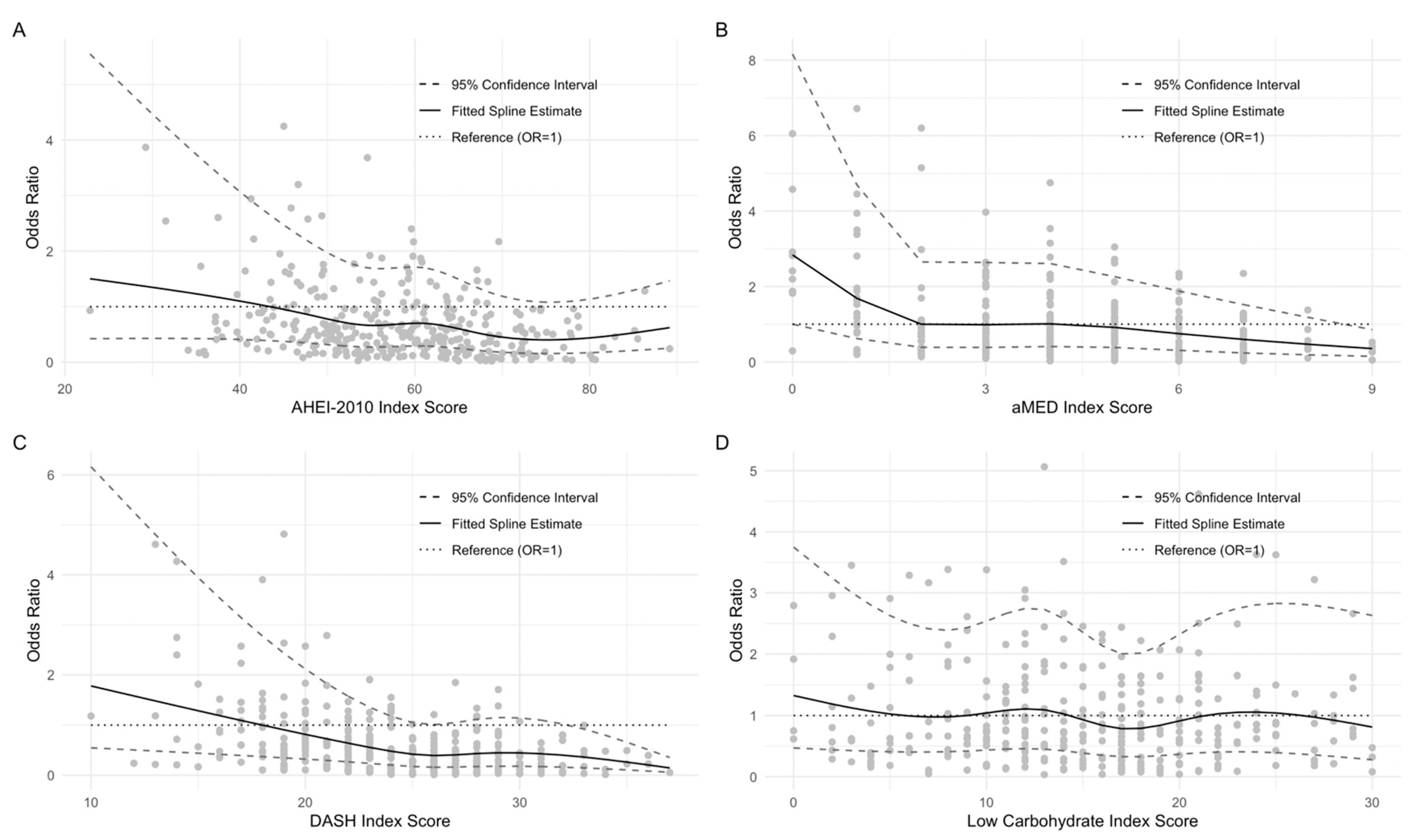
3. Results
3.1. Sample Characteristics
3.2. NIS Symptom Summary Scores and Potential Confounders
3.3. Diet Quality Scores
3.4. NIS Symptom Burden 1-Year Postdiagnosis
3.5. Analyses Using Single Nutrient Explanatory Variables
3.6. Subgroup and Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef] [Green Version]
- Crowder, S.L.; Douglas, K.G.; Yanina Pepino, M.; Sarma, K.P.; Arthur, A.E. Nutrition Impact Symptoms and Associated Outcomes in Post-Chemoradiotherapy Head and Neck Cancer Survivors: A Systematic Review. J. Cancer Surviv. 2018, 12, 479–494. [Google Scholar] [CrossRef]
- Rosenthal, D.I.; Mendoza, T.R.; Chambers, M.S.; Burkett, V.S.; Garden, A.S.; Hessell, A.C.; Lewin, J.S.; Ang, K.K.; Kies, M.S.; Gning, I.; et al. The M. D. Anderson Symptom Inventory-Head and Neck Module, a Patient-Reported Outcome Instrument, Accurately Predicts the Severity of Radiation-Induced Mucositis. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Gellrich, N.C.; Handschel, J.; Holtmann, H.; Krüskemper, G. Oral Cancer Malnutrition Impacts Weight and Quality of Life. Nutrients 2015, 7, 2145–2160. [Google Scholar] [CrossRef] [PubMed]
- Crowder, S.L.; Najam, N.; Sarma, K.P.; Fiese, B.H.; Arthur, A.E. Quality of Life, Coping Strategies, and Supportive Care Needs in Head and Neck Cancer Survivors: A Qualitative Study. Support. Care Cancer 2021, 29, 4349–4356. [Google Scholar] [CrossRef] [PubMed]
- Crowder, S.L.; Najam, N.; Sarma, K.P.; Fiese, B.H.; Arthur, A.E. Head and Neck Cancer Survivors’ Experiences with Chronic Nutrition Impact Symptom Burden after Radiation: A Qualitative Study. J. Acad. Nutr. Diet. 2020, 120, 1643–1653. [Google Scholar] [CrossRef]
- Ganzer, H.; Rothpletz-Puglia, P.; Byham-Gray, L.; Murphy, B.A.; Touger-Decker, R. The Eating Experience in Long-Term Survivors of Head and Neck Cancer: A Mixed-Methods Study. Support. Care Cancer 2015, 23, 3257–3268. [Google Scholar] [CrossRef]
- Crowder, S.L.; Sarma, K.P.; Mondul, A.M.; Chen, Y.T.; Li, Z.; Yanina Pepino, M.; Zarins, K.R.; Wolf, G.T.; Rozek, L.S.; Arthur, A.E. Pretreatment Dietary Patterns Are Associated with the Presence of Nutrition Impact Symptoms 1 Year after Diagnosis in Patients with Head and Neck Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1652–1659. [Google Scholar] [CrossRef]
- Gorenc, M.; Kozjek, N.R.; Strojan, P. Malnutrition and Cachexia in Patients with Head and Neck Cancer Treated with (Chemo)Radiotherapy. Rep. Pract. Oncol. Radiother. 2015, 20, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Berggren, K.L.; Restrepo Cruz, S.; Hixon, M.D.; Cowan, A.T.; Keysar, S.B.; Craig, S.; James, J.; Barry, M.; Ozbun, M.A.; Jimeno, A.; et al. MAPKAPK2 (MK2) Inhibition Mediates Radiation-Induced Inflammatory Cytokine Production and Tumor Growth in Head and Neck Squamous Cell Carcinoma. Oncogene 2019, 38, 7329–7341. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary Pattern Analysis: A New Direction in Nutritional Epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Arthur, A.E.; Goss, A.M.; Demark-Wahnefried, W.; Mondul, A.M.; Fontaine, K.R.; Chen, Y.T.; Carroll, W.R.; Spencer, S.A.; Rogers, L.Q.; Rozek, L.S.; et al. Higher Carbohydrate Intake Is Associated with Increased Risk of All-Cause and Disease-Specific Mortality in Head and Neck Cancer Patients: Results from a Prospective Cohort Study. Int. J. Cancer 2018, 143, 1105–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willett, W. Nutritional Epidemiology, 3rd ed.; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Willett, W.C.; Sampson, L.; Browne, M.L.; Stampfer, M.J.; Rosner, B.; Hennekens, C.H.; Speizer, F.E. THE USE OF A SELF-ADMINISTERED QUESTIONNAIRE TO ASSESS DIET FOUR YEARS IN THE PAST. Am. J. Epidemiol. 1988, 127, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Rimm, E.B.; Giovannucci, E.L.; Stampfer, M.J.; Colditz, G.A.; Litin, L.B.; Willett, W.C. Reproducibility and Validity of an Expanded Self-Administered Semiquantitative Food Frequency Questionnaire among Male Health Professionals. Am. J. Epidemiol. 1992, 135, 1114–1126. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Chiuve, S.E.; McCullough, M.L.; Rexrode, K.M.; Logroscino, G.; Hu, F.B. Adherence to a DASH-Style Diet and Risk of Coronary Heart Disease and Stroke in Women. Arch. Intern. Med. 2008, 168, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-Quality Scores and Plasma Concentrations of Markers of Inflammation and Endothelial Dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [CrossRef]
- Boucher, J.L. Mediterranean Eating Pattern. Diabetes Spectr. Publ. Am. Diabetes Assoc. 2017, 30, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative Dietary Indices Both Strongly Predict Risk of Chronic Disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Halton, T.L.; Willett, W.C.; Liu, S.; Manson, J.E.; Albert, C.M.; Rexrode, K.; Hu, F.B. Low-Carbohydrate-Diet Score and the Risk of Coronary Heart Disease in Women. N. Engl. J. Med. 2006, 355, 1991–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maino Vieytes, C.A.; Mondul, A.M.; Li, Z.; Zarins, K.R.; Wolf, G.T.; Rozek, L.S.; Arthur, A.E. Dietary Fiber, Whole Grains, and Head and Neck Cancer Prognosis: Findings from a Prospective Cohort Study. Nutrients 2019, 11, 2304. [Google Scholar] [CrossRef] [Green Version]
- Terrell, J.E.; Nanavati, K.A.; Esclamado, R.M.; Bishop, J.K.; Bradford, C.R.; Wolf, G.T. Head and Neck Cancer—Specific Quality of Life: Instrument Validation. JAMA Otolaryngol.-Head Neck Surg. 1997, 123, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Zahn, K.L.; Wong, G.; Bedrick, E.J.; Poston, D.G.; Schroeder, T.M.; Bauman, J.E. Relationship of Protein and Calorie Intake to the Severity of Oral Mucositis in Patients with Head and Neck Cancer Receiving Radiation Therapy. Head Neck 2012, 34, 655–662. [Google Scholar] [CrossRef]
- Bressan, V.; Stevanin, S.; Bianchi, M.; Aleo, G.; Bagnasco, A.; Sasso, L. The Effects of Swallowing Disorders, Dysgeusia, Oral Mucositis and Xerostomia on Nutritional Status, Oral Intake and Weight Loss in Head and Neck Cancer Patients: A Systematic Review. Cancer Treat. Rev. 2016, 45, 105–119. [Google Scholar] [CrossRef]
- Denaro, N.; Merlano, M.C.; Russi, E.G. Dysphagia in Head and Neck Cancer Patients: Pretreatment Evaluation, Predictive Factors, and Assessment during Radio-Chemotherapy, Recommendations. Clin. Exp. Otorhinolaryngol. 2013, 6, 117–126. [Google Scholar] [CrossRef]
- Ozkaya Akagunduz, O.; Eyigor, S.; Kirakli, E.; Tavlayan, E.; Erdogan Cetin, Z.; Kara, G.; Esassolak, M. Radiation-Associated Chronic Dysphagia Assessment by Flexible Endoscopic Evaluation of Swallowing (FEES) in Head and Neck Cancer Patients: Swallowing-Related Structures and Radiation Dose-Volume Effect. Ann. Otol. Rhinol. Laryngol. 2019, 128, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A Clinical Trial of the Effects of Dietary Patterns on Blood Pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, D.; Bennett, G.G.; Svetkey, L. The DASH Diet, 20 Years Later. JAMA 2017, 317, 1529. [Google Scholar] [CrossRef] [Green Version]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy Dietary Indices and Risk of Depressive Outcomes: A Systematic Review and Meta-Analysis of Observational Studies. Mol. Psychiatry 2019, 24, 965–986. [Google Scholar] [CrossRef] [Green Version]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean Diet Pyramid: A Cultural Model for Healthy Eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef]
- Viswanathan, H.; Wilson, J.A. Alcohol--the Neglected Risk Factor in Head and Neck Cancer. Clin. Otolaryngol. Allied Sci. 2004, 29, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Maier, H.; Dietz, A.; Gewelke, U.; Heller, W.D.; Weidauer, H. Tobacco and Alcohol and the Risk of Head and Neck Cancer. Clin. Investig. 1992, 70, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Potash, A.E.; Karnell, L.H.; Christensen, A.J.; Vander Weg, M.W.; Funk, G.F. Continued Alcohol Use in Patients with Head and Neck Cancer. Head Neck 2010, 32, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Shrotriya, S.; Deep, G.; Gu, M.; Kaur, M.; Jain, A.K.; Inturi, S.; Agarwal, R.; Agarwal, C. Generation of Reactive Oxygen Species by Grape Seed Extract Causes Irreparable DNA Damage Leading to G2/M Arrest and Apoptosis Selectively in Head and Neck Squamous Cell Carcinoma Cells. Carcinogenesis 2012, 33, 848–858. [Google Scholar] [CrossRef] [Green Version]
- Eastham, L.L.; Howard, C.M.; Balachandran, P.; Pasco, D.S.; Claudio, P.P. Eating Green: Shining Light on the Use of Dietary Phytochemicals as a Modern Approach in the Prevention and Treatment of Head and Neck Cancers. Curr. Top. Med. Chem. 2018, 18, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Bairati, I.; Meyer, F.; Gélinas, M.; Fortin, A.; Nabid, A.; Brochet, F.; Mercier, J.-P.; Têtu, B.; Harel, F.; Abdous, B.; et al. Randomized Trial of Antioxidant Vitamins to Prevent Acute Adverse Effects of Radiation Therapy in Head and Neck Cancer Patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 5805–5813. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.R.; Fleck, J.F.; Diehl, A.; Barletta, D.; Braga-Filho, A.; Barletta, A.; Ilha, L. Protective Effect of Alpha-Tocopherol in Head and Neck Cancer Radiation-Induced Mucositis: A Double-Blind Randomized Trial. Head Neck 2004, 26, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.E.; Peterson, K.E.; Shen, J.; Djuric, Z.; Taylor, J.M.G.; Hebert, J.R.; Duffy, S.A.; Peterson, L.A.; Bellile, E.L.; Whitfield, J.R.; et al. Diet and Proinflammatory Cytokine Levels in Head and Neck Squamous Cell Carcinoma. Cancer 2014, 120, 2704–2712. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | AHEI-2010 | aMED | DASH | Low-Carbohydrate | |||||
|---|---|---|---|---|---|---|---|---|---|
| Survivors # (%) | Q1 (n = 65) | Q5 (n = 64) | Q1 (n = 76) | Q5 (n = 46) | Q1 (n = 65) | Q5 (n = 59) | Q1 (n = 68) | Q5 (n = 57) | |
| Age (y) | |||||||||
| Mean (SD) | 60.4 (10.7) | 56.6 (10.9) | 62.1 (10.1) | 58.1 (11.1) | 59.8 (8.5) | 57.9 (10.2) | 62.3 (9.1) | 57.2 (9.8) | 62.3 (10.3) |
| Min/Max | 29/95 | 29/78 | 34/83 | 29/85 | 34/83 | 30/78 | 43/81 | 30/78 | 43/85 |
| Sex | |||||||||
| Male | 254 (78.6) | 52 (80.0) | 46 (71.9) | 65 (85.5) | 37 (80.4) | 57 (87.7) | 45 (76.3) | 51 (75.0) | 40 (70.2) |
| Female | 69 (21.4) | 13 (20.0) | 18 (28.1) | 11 (14.5) | 9 (19.6) | 8 (12.3) | 14 (23.7) | 17 (25.0) | 17 (29.8) |
| Education | |||||||||
| High school or less | 91 (28.2) | 25 (38.5) | 11 (17.2) | 24 (31.6) | 5 (10.9) | 31 (47.7) | 7 (11.9) | 20 (29.4) | 16 (28.1) |
| Some college or more | 232 (71.8) | 40 (61.5) | 53 (82.8) | 52 (68.4) | 41 (89.1) | 34 (52.3) | 52 (88.1) | 48 (70.6) | 41 (71.9) |
| Race/Ethnicity | |||||||||
| Non-Hispanic white | 310 (96.9) | 62 (95.4) | 62 (96.9) | 75 (98.7) | 44 (97.8) | 60 (93.8) | 55 (94.8) | 64 (94.1) | 55 (98.2) |
| Other | 7 (2.2) | 3 (4.6) | 1 (1.6) | 1 (1.3) | 1 (2.2) | 4 (6.2) | 2 (3.4) | 2 (2.9) | 1 (1.8) |
| Unknown | 3 (0.9) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.7) | 2 (2.9) | 0 (0.0) |
| BMI (kg/m2) | |||||||||
| Underweight and normal weight (<25) | 101 (31.3) | 21 (32.3) | 18 (28.1) | 18 (23.7) | 16 (34.8) | 23 (35.4) | 21 (35.6) | 21 (30.9) | 14 (24.6) |
| Overweight and obese (≥25) | 222 (68.7) | 44 (67.7) | 46 (71.9) | 58 (76.3) | 30 (65.2) | 42 (64.6) | 38 (64.4) | 47 (69.1) | 43 (75.4) |
| Site | |||||||||
| Larynx | 66 (20.4) | 12 (18.5) | 10 (15.6) | 21 (27.6) | 6 (13.0) | 19 (29.2) | 5 (8.5) | 9 (13.2) | 11 (19.3) |
| Oral cavity | 96 (29.7) | 22 (33.8) | 19 (29.7) | 23 (30.3) | 14 (30.4) | 16 (24.6) | 13 (22) | 20 (29.4) | 22 (38.6) |
| Oropharynx | 157 (48.6) | 30 (46.2) | 35 (54.7) | 32 (42.1) | 26 (56.5) | 28 (43.1) | 40 (67.8) | 39 (57.4) | 23 (40.4) |
| Hypopharynx | 4 (1.2) | 1 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (3.1) | 1 (1.7) | 0 (0.0) | 1 (1.8) |
| Stage | |||||||||
| 0, I, II | 104 (32.2) | 18 (27.7) | 20 (31.2) | 26 (34.2) | 13 (28.3) | 20 (30.8) | 14 (23.7) | 24 (35.3) | 18 (31.6) |
| III, IV | 219 (67.8) | 47 (72.3) | 44 (68.8) | 50 (65.8) | 33 (71.7) | 45 (69.2) | 45 (76.3) | 44 (64.7) | 39 (68.4) |
| HPV Status | |||||||||
| HPV-negative | 92 (28.5) | 20 (30.8) | 17 (26.6) | 25 (32.9) | 10 (21.7) | 17 (26.2) | 12 (20.3) | 17 (25.0) | 23 (40.4) |
| HPV-positive | 71 (22.0) | 8 (12.3) | 15 (23.4) | 16 (21.1) | 13 (28.3) | 11 (16.9) | 15 (25.4) | 20 (29.4) | 7 (12.3) |
| Unknown | 160 (49.5) | 37 (56.9) | 32 (50.0) | 35 (46.1) | 23 (50.0) | 37 (56.9) | 32 (54.2) | 31 (45.6) | 27 (47.4) |
| Treatment | |||||||||
| Surgery only | 74 (22.9) | 14 (21.5) | 20 (31.2) | 15 (19.7) | 9 (19.6) | 10 (15.4) | 8 (13.6) | 18 (26.5) | 17 (29.8) |
| Surgery + adjuvant radiation | 50 (15.5) | 12 (18.5) | 8 (12.5) | 14 (18.4) | 8 (17.4) | 11 (16.9) | 6 (10.2) | 4 (5.9) | 9 (15.8) |
| Radiation only | 27 (8.4) | 3 (4.6) | 3 (4.7) | 10 (13.2) | 5 (10.9) | 7 (10.8) | 5 (8.5) | 5 (7.4) | 3 (5.3) |
| Chemotherapy + radiation | 155 (48.0) | 29 (44.6) | 29 (45.3) | 33 (43.4) | 23 (50.0) | 32 (49.2) | 35 (59.3) | 35 (51.5) | 25 (43.9) |
| Chemotherapy only | 7 (2.2) | 2 (3.1) | 2 (3.1) | 3 (3.9) | 1 (2.2) | 2 (3.1) | 2 (3.4) | 2 (2.9) | 2 (3.5) |
| Palliative or unknown | 10 (3.1) | 5 (7.7) | 2 (3.1) | 1 (1.3) | 0 (0.0) | 3 (4.6) | 3 (5.1) | 4 (5.9) | 1 (1.8) |
| Smoking Status | |||||||||
| Current | 106 (32.8) | 34 (52.3) | 11 (17.2) | 33 (43.4) | 8 (17.4) | 37 (56.9) | 11 (18.6) | 24 (35.3) | 16 (28.1) |
| Former | 118 (36.5) | 11 (16.9) | 26 (40.6) | 23 (30.3) | 20 (43.5) | 16 (24.6) | 25 (42.4) | 23 (33.8) | 21 (36.8) |
| Never | 99 (30.7) | 20 (30.8) | 27 (42.2) | 20 (26.3) | 18 (39.1) | 12 (18.5) | 23 (39) | 21 (30.9) | 20 (35.1) |
| Drinking Status | |||||||||
| Current | 230 (71.2) | 49 (75.4) | 49 (76.6) | 57 (75.0) | 39 (84.8) | 50 (76.9) | 44 (74.6) | 48 (70.6) | 37 (64.9) |
| Former | 71 (22.0) | 11 (16.9) | 13 (20.3) | 15 (19.7) | 6 (13.0) | 14 (21.5) | 13 (22) | 18 (26.5) | 16 (28.1) |
| Never | 22 (6.8) | 5 (7.7) | 2 (3.1) | 4 (5.3) | 1 (2.2) | 1 (1.5) | 2 (3.4) | 2 (2.9) | 4 (7.0) |
| Characteristic | n | Mean NIS Summary Score (SD) | p a |
|---|---|---|---|
| Age (y) | |||
| ≥60 | 169 | 13.6 (6.0) | 0.09 |
| <60 | 154 | 14.7 (6.2) | |
| Sex | |||
| Male | 254 | 13.9 (5.8) | 0.18 |
| Female | 69 | 15 (7.2) | |
| Education | |||
| High school or less | 91 | 15.8 (6.4) | <0.01 ** |
| Some college or more | 232 | 13.5 (5.9) | |
| Race | |||
| Non-Hispanic white | 310 | 14.2 (6.2) | 0.72 |
| Other | 7 | 13.9 (6.4) | |
| Unknown | 3 | 13 (4.6) | |
| BMI (kg/m2) | |||
| Underweight and normal weight (<25) | 101 | 15 (6.6) | 0.08 |
| Overweight and obese (≥25) | 222 | 13.7 (5.9) | |
| Site | |||
| Larynx | 66 | 12.4 (5.7)† | <0.01 ** |
| Oral cavity | 96 | 13.8 (6.5) | |
| Oropharynx | 157 | 15.1 (6.0) † | |
| Hypopharynx | 4 | 13.2 (3.8) | |
| Stage | |||
| 0, I, II | 104 | 11.9 (5.7) | <0.01 ** |
| III, IV | 219 | 15.2 (6.0) | |
| HPV Status | |||
| HPV negative | 92 | 14.4 (6.5) | 0.44 |
| HPV positive | 71 | 14.5 (5.9) | |
| Unknown | 160 | 13.8 (6.0) | |
| Treatment | |||
| Surgery only | 74 | 11 (5.2) †‡¥ | <0.0001 ** |
| Surgery + adjuvant radiation | 50 | 16.3 (6.7) †ψ | |
| Radiation only | 27 | 11.2 (4.5) ψξω | |
| Chemotherapy + radiation | 155 | 15.1 (5.7) ‡ξ | |
| Chemotherapy only | 7 | 21 (8.0) ¥ω | |
| Palliative or unknown | 10 | 15.3 (5.4) | |
| Smoking Status | |||
| Current | 106 | 15.3 (6.5) | 0.58 |
| Former | 118 | 13.8 (6.0) | |
| Never | 99 | 13.3 (5.7) | |
| Drinking Status | |||
| Current | 230 | 13.8 (5.9) | 0.10 |
| Former | 71 | 15.3 (6.1) | |
| Never | 22 | 13.7 (7.8) |
| Index | Mean (SD) | Median | Minimum | Maximum | Theoretical (Max, Min) |
|---|---|---|---|---|---|
| AHEI-2010 | 57.99 (58) | 58.54 | 22.85 | 89.14 | (0, 110) |
| aMED | 4.08 (58) | 4 | 0 | 9 | (0, 9) |
| DASH | 23.98 (58) | 24 | 10 | 37 | (8, 40) |
| Low-Carbohydrate | 14.98 (58) | 15 | 0 | 30 | (0, 30) |
| Index/Symptom | Q1 | Q2 | Q3 | Q4 | Q5 | ptrend | pQ5-Q1 |
|---|---|---|---|---|---|---|---|
| AHEI-2010 | n = 65 | n = 65 | n = 64 | n = 65 | n = 64 | ||
| Trismus | 1.00 | 0.90 (0.39–2.06) | 0.93 (0.41–2.11) | 0.51 (0.21–1.17) | 0.65 (0.28–1.54) | 0.16 | 0.33 |
| Xerostomia | 1.00 | 1.07 (0.37–3.04) | 0.95 (0.32–2.76) | 0.50 (0.17–1.38) | 0.42 (0.14–1.21) | 0.04 * | 0.11 |
| Difficulty chewing | 1.00 | 0.93 (0.40–2.18) | 0.86 (0.36–2.01) | 0.38 (0.16–0.88) * | 0.55 (0.24–1.26) | 0.03 * | 0.16 |
| Dysphagia liquids | 1.00 | 0.59 (0.26–1.32) | 0.58 (0.26–1.28) | 0.48 (0.21–1.09) | 0.47 (0.19–1.09) | 0.07 | 0.08 |
| Dysphagia solids | 1.00 | 0.77 (0.34–1.77) | 0.77 (0.34–1.76) | 0.44 (0.19–0.99) * | 0.65 (0.28–1.50) | 0.15 | 0.32 |
| Taste | 1.00 | 0.76 (0.31–1.87) | 1.03 (0.41–2.63) | 0.40 (0.16–0.96) * | 0.49 (0.20–1.20) | 0.04 * | 0.12 |
| Mucositis | 1.00 | 1.45 (0.67–3.16) | 1.19 (0.55–2.58) | 0.64 (0.30–1.39) | 0.81 (0.37–1.78) | 0.18 | 0.61 |
| NIS summary score b | 1.00 | 0.85 (0.37–1.93) | 0.89 (0.39–2.03) | 0.44 (0.20–0.99) * | 0.65 (0.28–1.49) | 0.12 | 0.31 |
| aMED | n = 76 | n = 55 | n = 112 | n = 34 | n = 46 | ||
| Trismus | 1.00 | 0.76 (0.33–1.72) | 0.69 (0.34–1.39) | 0.30 (0.09–0.85) * | 0.84 (0.34–2.07) | 0.27 | 0.71 |
| Xerostomia | 1.00 | 0.69 (0.26–1.85) | 0.58 (0.24–1.39) | 0.28 (0.08–0.95) * | 0.30 (0.10–0.92) * | 0.01 * | 0.04 * |
| Difficulty chewing | 1.00 | 0.71 (0.31–1.65) | 0.58 (0.28–1.18) | 0.29 (0.11–0.76) * | 0.32 (0.13–0.79) * | <0.01 ** | 0.01 * |
| Dysphagia liquids | 1.00 | 0.40 (0.18–0.88) * | 0.37 (0.18–0.73) ** | 0.44 (0.17–1.13) | 0.13 (0.04–0.38) ** | <0.01 ** | <0.01 ** |
| Dysphagia solids | 1.00 | 0.27 (0.11–0.61) ** | 0.40 (0.19–0.81) * | 0.21 (0.07–0.56) ** | 0.34 (0.13–0.84) * | 0.02 * | 0.02 * |
| Taste | 1.00 | 0.44 (0.18–1.07) | 0.30 (0.14–0.65) ** | 0.57 (0.20–1.64) | 0.64 (0.24–1.72) | 0.60 | 0.37 |
| Mucositis | 1.00 | 0.69 (0.32–1.47) | 0.82 (0.42–1.56) | 1.23 (0.50–3.04) | 0.39 (0.16–0.91) * | 0.18 | 0.03 * |
| NIS summary score b | 1.00 | 0.36 (0.16–0.81) * | 0.35 (0.17–0.72) ** | 0.33 (0.12–0.86) * | 0.36 (0.14–0.88) * | 0.04 * | 0.03 * |
| DASH | n = 65 | n = 83 | n = 76 | n = 40 | n = 59 | ||
| Trismus | 1.00 | 0.62 (0.28–1.36) | 0.56 (0.25–1.24) | 0.50 (0.18–1.33) | 0.81 (0.34–1.93) | 0.48 | 0.63 |
| Xerostomia | 1.00 | 0.34 (0.12–0.92) * | 0.66 (0.23–1.89) | 0.27 (0.08–0.86) * | 0.27 (0.08–0.85) * | 0.04 * | 0.03 * |
| Difficulty chewing | 1.00 | 0.48 (0.21–1.07) | 0.40 (0.17–0.89) * | 0.50 (0.18–1.33) | 0.39 (0.16–0.95) * | 0.06 | 0.04 * |
| Dysphagia liquids | 1.00 | 0.48 (0.22–1.03) | 0.51 (0.23–1.10) | 0.49 (0.19–1.25) | 0.37 (0.15–0.90) * | 0.04 * | 0.03 * |
| Dysphagia solids | 1.00 | 0.46 (0.21–1.01) | 0.43 (0.19–0.95) * | 0.45 (0.17–1.18) | 0.54 (0.22–1.28) | 0.19 | 0.17 |
| Taste | 1.00 | 0.39 (0.15–0.93) * | 0.29 (0.11–0.71) ** | 0.16 (0.05–0.44) ** | 0.50 (0.18–1.37) | 0.04 * | 0.18 |
| Mucositis | 1.00 | 0.84 (0.41–1.74) | 0.54 (0.26–1.14) | 0.61 (0.24–1.48) | 0.61 (0.27–1.37) | 0.12 | 0.23 |
| NIS summary score b | 1.00 | 0.50 (0.22–1.11) | 0.31 (0.13–0.68) ** | 0.35 (0.13–0.92) * | 0.38 (0.15–0.91) * | 0.02 * | 0.03 * |
| Low-Carbohydrate | n = 68 | n = 69 | n = 72 | n = 57 | n = 57 | ||
| Trismus | 1.00 | 1.13 (0.52–2.46) | 1.12 (0.51–2.46) | 0.60 (0.25–1.41) | 0.68 (0.29–1.59) | 0.20 | 0.37 |
| Xerostomia | 1.00 | 2.64 (0.94–7.68) | 0.69 (0.26–1.81) | 1.57 (0.54–4.69) | 1.35 (0.47–3.93) | 0.94 | 0.58 |
| Difficulty chewing | 1.00 | 0.87 (0.40–1.91) | 1.17 (0.54–2.55) | 0.43 (0.18–0.97) * | 1.22 (0.53–2.84) | 0.85 | 0.65 |
| Dysphagia liquids | 1.00 | 2.03 (0.89–4.72) | 1.37 (0.60–3.19) | 0.92 (0.37–2.26) | 2.47 (1.06–5.91) * | 0.19 | 0.04 * |
| Dysphagia solids | 1.00 | 1.37 (0.63–2.98) | 1.21 (0.56–2.65) | 0.87 (0.39–1.96) | 1.25 (0.55–2.83) | 0.90 | 0.60 |
| Taste | 1.00 | 0.71 (0.30–1.66) | 0.58 (0.25–1.33) | 0.47 (0.19–1.16) | 0.59 (0.24–1.42) | 0.15 | 0.24 |
| Mucositis | 1.00 | 0.75 (0.36–1.57) | 0.68 (0.33–1.40) | 0.65 (0.30–1.39) | 0.87 (0.40–1.89) | 0.59 | 0.72 |
| NIS summary score b | 1.00 | 1.66 (0.77–3.65) | 1.24 (0.58–2.65) | 0.80 (0.36–1.77) | 1.25 (0.56–2.80) | 0.92 | 0.59 |
| Food Group | Q1 | Q2 | Q3 | Q4 | Q5 | ptrend | pQ5-Q1 |
|---|---|---|---|---|---|---|---|
| Legumes (servings/d) | 1.00 | 0.87 (0.44–1.72) | 0.79 (0.34–1.81) | 0.83 (0.38–1.83) | 0.69 (0.33–1.43) | 0.34 | 0.32 |
| Nuts (servings/d) | 1.00 | 0.68 (0.31–1.46) | 0.56 (0.25–1.23) | 0.61 (0.28–1.32) | 0.33 (0.15–0.72) ** | 0.01 * | 0.01 ** |
| Whole Grains (g/d) | 1.00 | 0.80 (0.36–1.74) | 0.73 (0.33–1.58) | 0.61 (0.27–1.37) | 0.61 (0.25–1.44) | 0.24 | 0.26 |
| Alcohol (g/d) | 1.00 | 0.75 (0.34–1.62) | 0.50 (0.23–1.06) | 0.90 (0.42–1.92) | 1.13 (0.52–2.48) | 0.24 | 0.75 |
| Red and Processed Meats (servings/d) | 1.00 | 1.94 (0.91–4.18) | 1.58 (0.75–3.32) | 1.87 (0.83–4.27) | 1.48 (0.63–3.47) | 0.54 | 0.37 |
| Total Fruit (servings/d) | 1.00 | 0.53 (0.23–1.19) | 0.27 (0.12–0.59) ** | 0.41 (0.18–0.91) * | 0.32 (0.14–0.74) ** | 0.03 * | 0.01 ** |
| Total Vegetables (servings/d) | 1.00 | 1.65 (0.76–3.67) | 1.41 (0.66–3.02) | 0.90 (0.42–1.95) | 1.32 (0.59–2.98) | 0.95 | 0.50 |
| UFA/SFA Ratio | 1.00 | 1.16 (0.53–2.55) | 0.63 (0.29–1.35) | 0.86 (0.39–1.87) | 0.66 (0.30–1.44) | 0.22 | 0.29 |
| Sugar-Sweetened Beverages (servings/d) | 1.00 | 1.33 (0.60–2.92) | 0.95 (0.44–2.04) | 0.92 (0.42–2.03) | 0.54 (0.23–1.24) | 0.05 * | 0.15 |
| Total Low-Fat Dairy (servings/d) | 1.00 | 0.81 (0.37–1.78) | 0.62 (0.29–1.31) | 0.65 (0.29–1.43) | 0.59 (0.27–1.28) | 0.27 | 0.18 |
| Total Sodium (mg/d) | 1.00 | 0.79 (0.35–1.73) | 0.99 (0.41–2.39) | 1.11 (0.41–3.03) | 0.65 (0.17–2.38) | 0.67 | 0.51 |
| n-3 Fatty Acids (g/d) | 1.00 | 1.05 (0.48–2.29) | 0.93 (0.43–2.01) | 0.75 (0.35–1.59) | 0.51 (0.23–1.09) | 0.04 * | 0.08 |
| Trans Fat (% of total kcal) | 1.00 | 0.46 (0.21–0.99) * | 0.68 (0.31–1.47) | 0.77 (0.35–1.68) | 1.10 (0.49–2.48) | 0.50 | 0.82 |
| Total Carbohydrate (g/d) | 1.00 | 1.18 (0.52–2.70) | 1.24 (0.49–3.15) | 1.45 (0.48–4.41) | 0.96 (0.22–4.24) | 0.98 | 0.96 |
| Total Protein (g/d) | 1.00 | 1.09 (0.47–2.50) | 0.94 (0.38–2.31) | 0.90 (0.32–2.57) | 0.47 (0.13–1.74) | 0.19 | 0.26 |
| Total Fat (g/d) | 1.00 | 0.96 (0.42–2.19) | 1.21 (0.50–2.95) | 1.03 (0.36–2.94) | 0.72 (0.18–2.88) | 0.70 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maino Vieytes, C.A.; Mondul, A.M.; Crowder, S.L.; Zarins, K.R.; Edwards, C.G.; Davis, E.C.; Wolf, G.T.; Rozek, L.S.; Arthur, A.E.; on behalf of the University of Michigan Head and Neck SPORE Program. Pretreatment Adherence to a Priori-Defined Dietary Patterns Is Associated with Decreased Nutrition Impact Symptom Burden in Head and Neck Cancer Survivors. Nutrients 2021, 13, 3149. https://doi.org/10.3390/nu13093149
Maino Vieytes CA, Mondul AM, Crowder SL, Zarins KR, Edwards CG, Davis EC, Wolf GT, Rozek LS, Arthur AE, on behalf of the University of Michigan Head and Neck SPORE Program. Pretreatment Adherence to a Priori-Defined Dietary Patterns Is Associated with Decreased Nutrition Impact Symptom Burden in Head and Neck Cancer Survivors. Nutrients. 2021; 13(9):3149. https://doi.org/10.3390/nu13093149
Chicago/Turabian StyleMaino Vieytes, Christian A., Alison M. Mondul, Sylvia L. Crowder, Katie R. Zarins, Caitlyn G. Edwards, Erin C. Davis, Gregory T. Wolf, Laura S. Rozek, Anna E. Arthur, and on behalf of the University of Michigan Head and Neck SPORE Program. 2021. "Pretreatment Adherence to a Priori-Defined Dietary Patterns Is Associated with Decreased Nutrition Impact Symptom Burden in Head and Neck Cancer Survivors" Nutrients 13, no. 9: 3149. https://doi.org/10.3390/nu13093149
APA StyleMaino Vieytes, C. A., Mondul, A. M., Crowder, S. L., Zarins, K. R., Edwards, C. G., Davis, E. C., Wolf, G. T., Rozek, L. S., Arthur, A. E., & on behalf of the University of Michigan Head and Neck SPORE Program. (2021). Pretreatment Adherence to a Priori-Defined Dietary Patterns Is Associated with Decreased Nutrition Impact Symptom Burden in Head and Neck Cancer Survivors. Nutrients, 13(9), 3149. https://doi.org/10.3390/nu13093149






