The Effects of Aerobic-Resistance Training and Broccoli Supplementation on Plasma Dectin-1 and Insulin Resistance in Males with Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
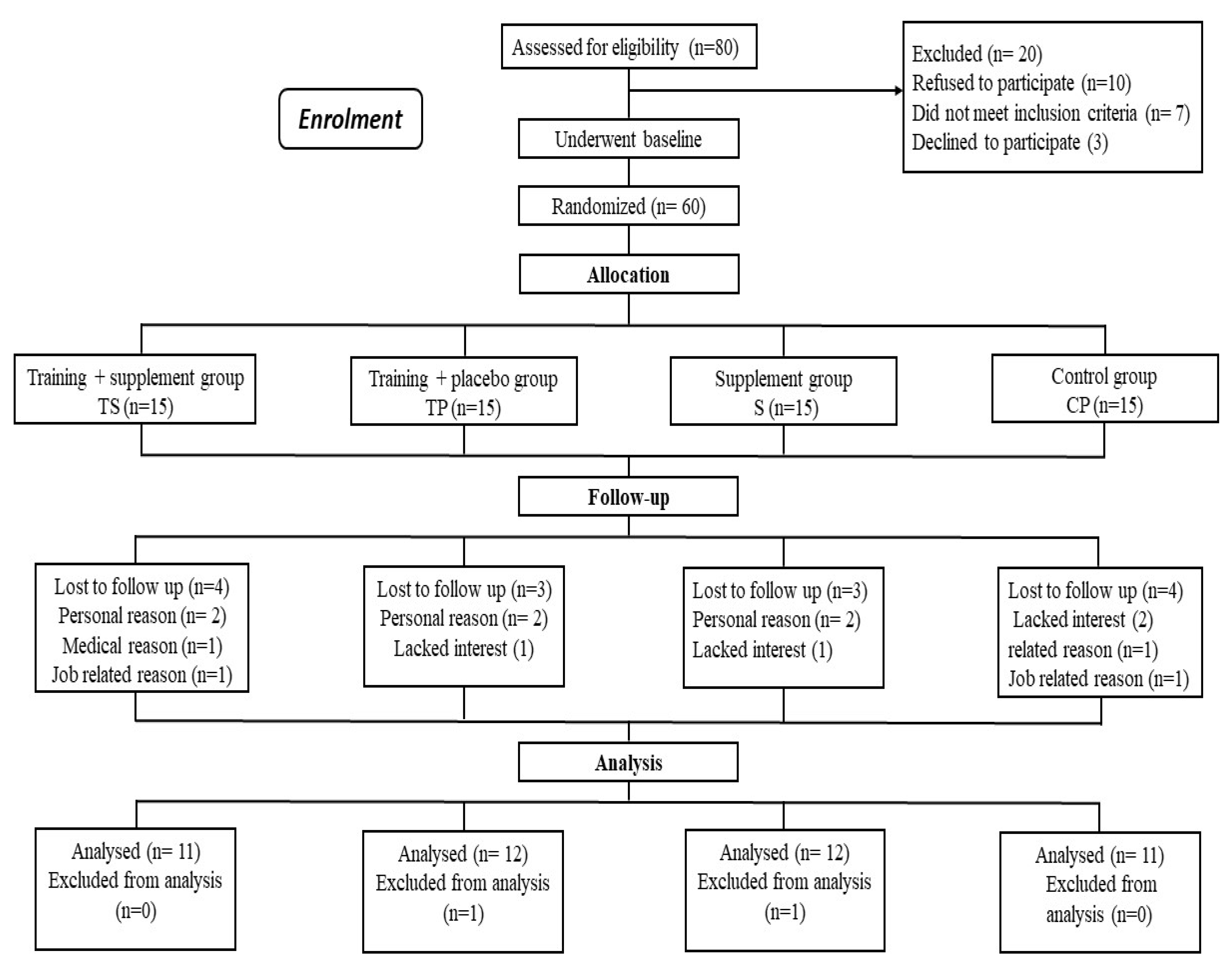
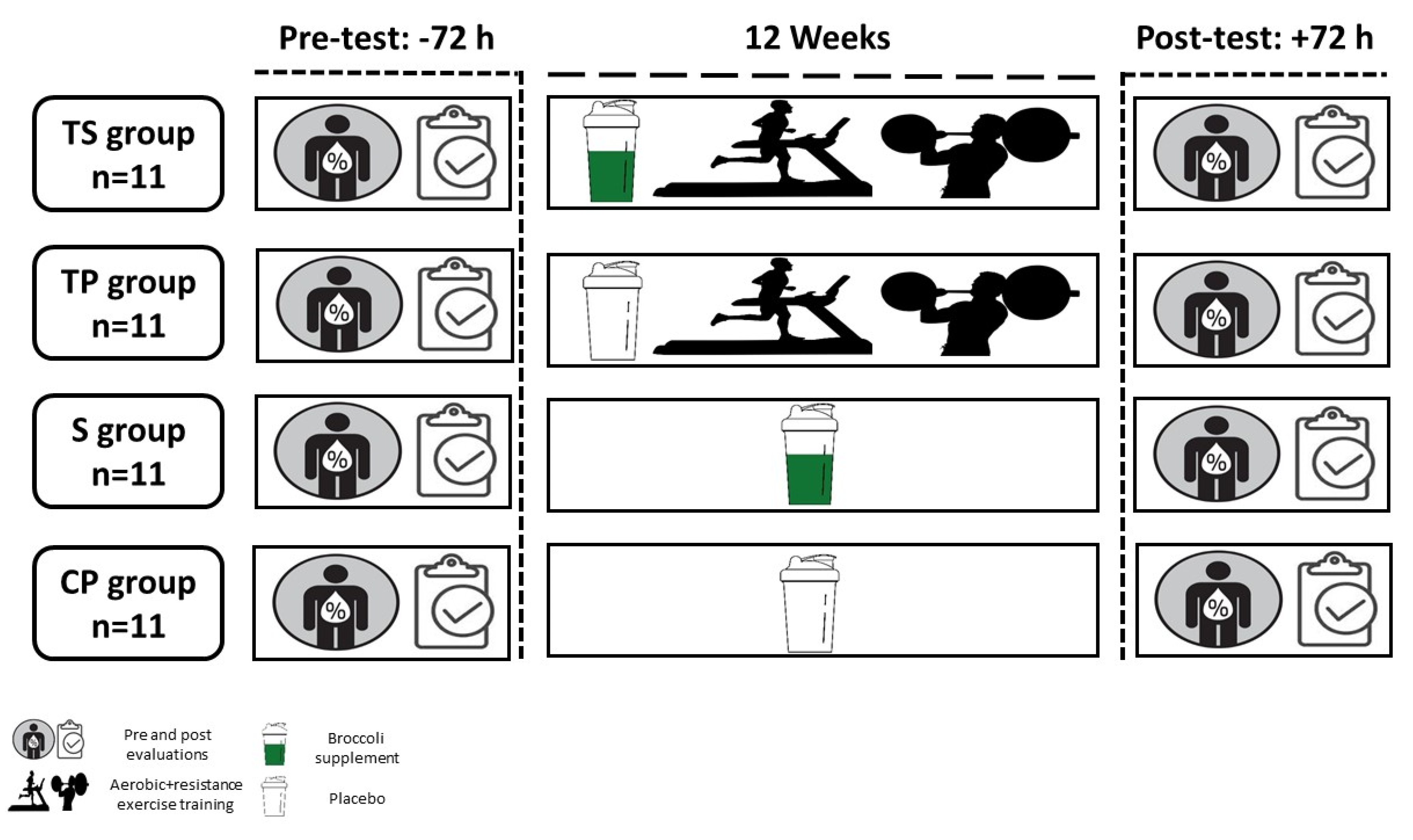
2.2. Experimental Design
2.3. Cardio-Respiratory Fitness, Body Composition and One-Repetition Maximum Assessments
2.4. Exercise Training Program
2.5. Nutrient Intake and Dietary Analysis
2.6. Supplementation Protocol
2.7. Biochemical Analysis
2.8. Statistical Analysis
3. Results
3.1. Dietary Analysis
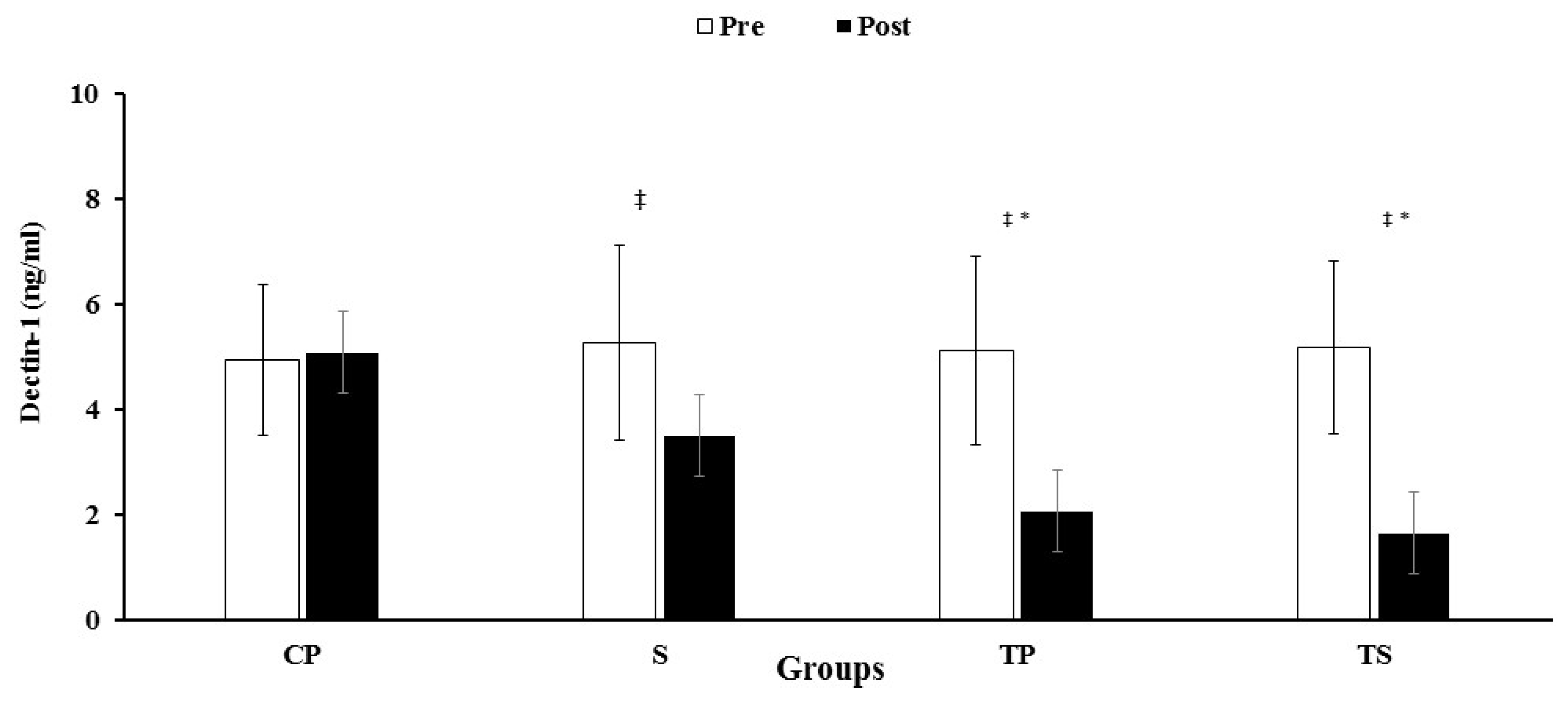
3.2. Dectin-1, CRP, IL-6 and TNF-α
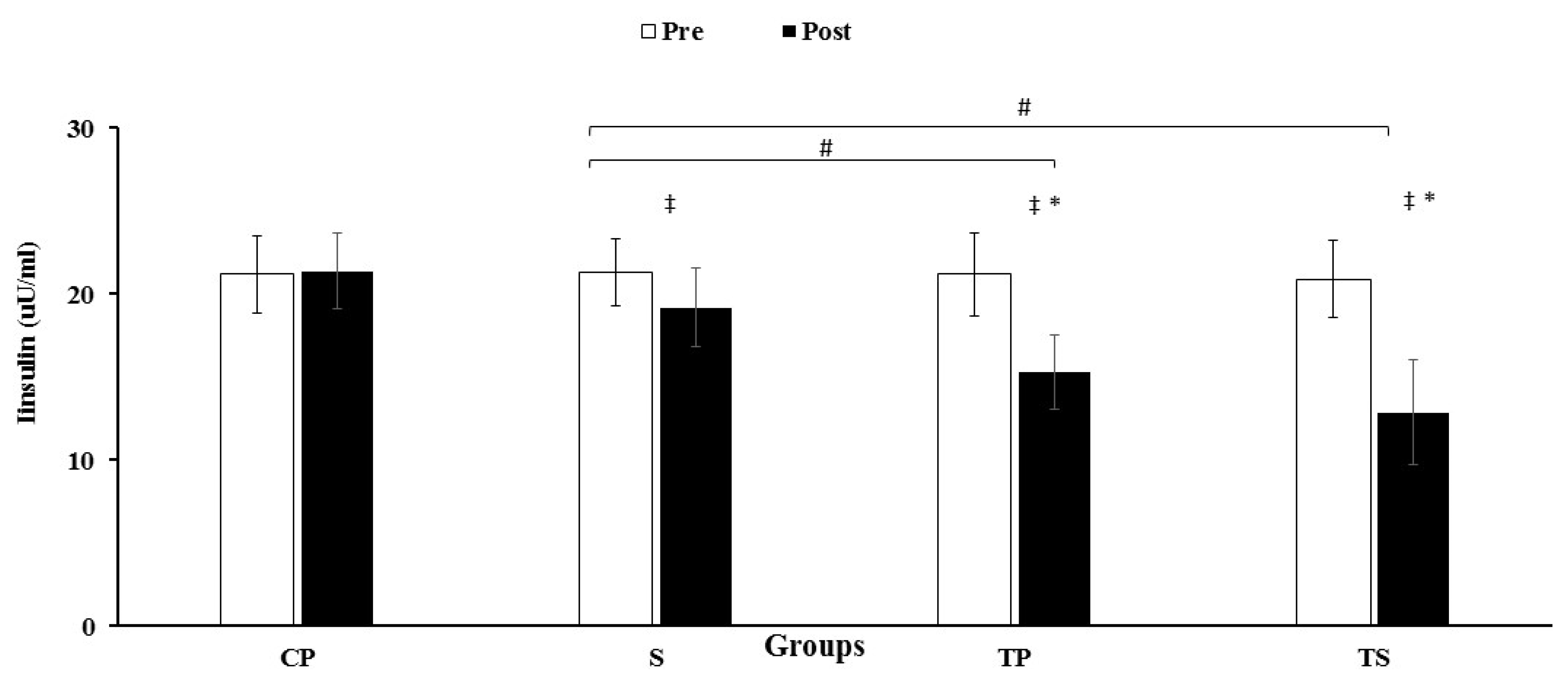
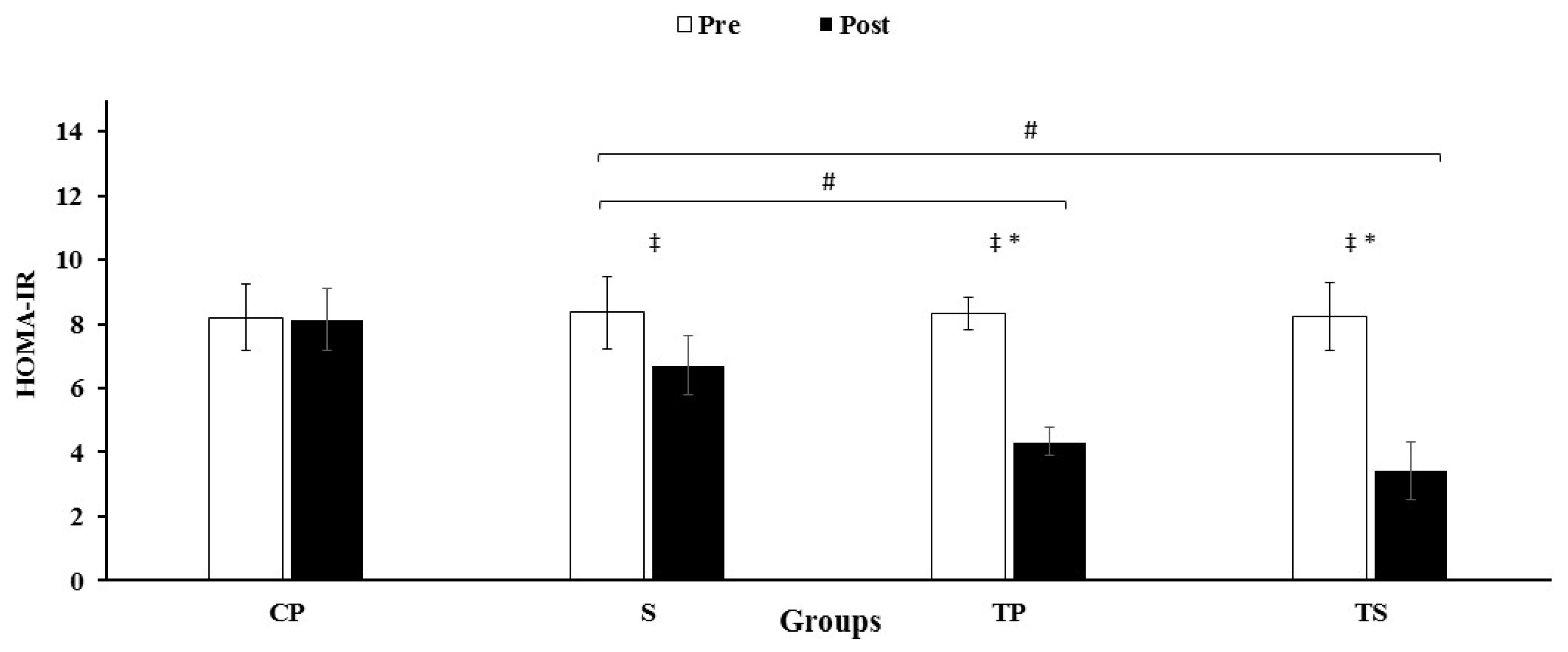
3.3. Insulin, HOMA-IR and Glucose
3.4. Lipid Profiles
3.5. Body Composition Variables and VO2peak
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Statement
References
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [Green Version]
- Joseph, J.J.; Echouffo-Tcheugui, J.B.; Golden, S.H.; Chen, H.; Jenny, N.S.; Carnethon, M.R.; Jacobs, D.; Burke, G.L.; Vaidya, D.; Ouyang, P.; et al. Physical activity, sedentary behaviors and the incidence of type 2 diabetes mellitus: The Multi-Ethnic Study of Atherosclerosis (MESA). BMJ Open Diabetes Res. Care 2016, 4, e000185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira Otto, M.C.; Padhye, N.S.; Bertoni, A.G.; Jacobs, D.R., Jr.; Mozaffarian, D. Everything in moderation-dietary diversity and quality, central obesity and risk of diabetes. PLoS ONE 2015, 10, e0141341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004, 25, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Espinosa, N.; García-Hernández, M.H.; Reynaga-Hernández, E.; Cortés-García, J.D.; Corral-Fernández, N.E.; Rodríguez-Rivera, J.G.; Bravo-Ramírez, A.; González-Amaro, R.; Portales-Pérez, D. Abnormal expression and function of Dectin-1 receptor in type 2 diabetes mellitus patients with poor glycemic control (HbA1c > 8%). Metabolism 2012, 61, 1538–1546. [Google Scholar] [CrossRef]
- Rosas, M.; Liddiard, K.; Kimberg, M.; Faro-Trindade, I.; McDonald, J.U.; Williams, D.L.; Brown, G.; Taylor, P. The induction of inflammation by dectin-1 in vivo is dependent on myeloid cell programming and the progression of phagocytosis. J. Immunol. 2008, 181, 3549–3557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Underhill, D.M.; Rossnagle, E.; Lowell, C.A.; Simmons, R.M. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 2005, 106, 2543–2550. [Google Scholar] [CrossRef] [Green Version]
- Castoldi, A.; Andrade-Oliveira, V.; Aguiar, C.F.; Amano, M.T.; Lee, J.; Miyagi, M.T.; Latância, M.T.; Braga, T.; da Silva, M.B.; Ignácio, A.; et al. Dectin-1 activation exacerbates obesity and insulin resistance in the absence of MyD88. Cell Rep. 2017, 19, 2272–2288. [Google Scholar] [CrossRef] [Green Version]
- Nestle, M. Broccoli sprouts as inducers of carcinogen-detoxifying enzyme systems: Clinical, dietary, and policy implications. Proc. Natl. Acad. Sci. USA 1997, 94, 11149–11151. [Google Scholar] [CrossRef] [Green Version]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Potential efficacy of broccoli sprouts as a unique supplement for management of type 2 diabetes and its complications. J. Med. Food 2013, 16, 375–382. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Hosseinpanah, F.; Keyzad, A.; Azizi, F. Effects of broccoli sprout with high sulforaphane concentration on inflammatory markers in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. J. Funct. Foods 2012, 4, 837–841. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Hosseinpanah, F.; Hedayati, M.; Hosseinpour-Niazi, S.; Azizi, F. Broccoli sprouts reduce oxidative stress in type 2 diabetes: A randomized double-blind clinical trial. Eur. J. Clin. Nutr. 2011, 65, 972–977. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Suzuki, K. The integrative role of sulforaphane in preventing inflammation, oxidative stress and fatigue: A review of a potential protective phytochemical. Antioxidants 2020, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Komine, S.; Warabi, E.; Akiyama, K.; Ishii, A.; Ishige, K.; Mizokami, Y.; Kuga, K.; Horie, M.; Miwa, Y.; et al. Nuclear factor (erythroid derived 2)-like 2 activation increases exercise endurance capacity via redox modulation in skeletal muscles. Sci. Rep. 2017, 7, 1–11. [Google Scholar]
- Malaguti, M.; Angeloni, C.; Garatachea, N.; Baldini, M.; Leoncini, E.; Collado, P.S.; Teti, G.; Falconi, M.; González-Gallego, J.; Hrelia, S.; et al. Sulforaphane treatment protects skeletal muscle against damage induced by exhaustive exercise in rats. J. Appl. Physiol. 2009, 107, 1028–1036. [Google Scholar] [CrossRef] [Green Version]
- Cardenia, V.; Rodriguez-Estrada, M.T.; Lorenzini, A.; Bandini, E.; Angeloni, C.; Hrelia, S.; Malaguti, M. Effect of broccoli extract enriched diet on liver cholesterol oxidation in rats subjected to exhaustive exercise. J. Steroid Biochem. Mol. Biol. 2017, 169, 137–144. [Google Scholar] [CrossRef]
- Saeidi, A.; Haghighi, M.M.; Kolahdouzi, S.; Daraei, A.; Abderrahmane, A.B.; Essop, M.F.; Laher, I.; Hackney, A.C.; Zouhal, H. The effects of physical activity on adipokines in individuals with overweight/obesity across the lifespan: A narrative review. Obes. Rev. 2021, 22, e13090. [Google Scholar] [CrossRef]
- Harty, P.S.; Cottet, M.L.; Malloy, J.K.; Kerksick, C.M. Nutritional and supplementation strategies to prevent and attenuate exercise-induced muscle damage: A brief review. Sports Med. Open 2019, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cradock, K.A.; ÓLaighin, G.; Finucane, F.M.; Gainforth, H.L.; Quinlan, L.R.; Ginis, K.A.M. Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Jorge, M.L.M.P.; de Oliveira, V.N.; Resende, N.M.; Paraiso, L.F.; Calixto, A.; Diniz, A.L.D.; Resende, E.S.; Ropelle, E.; Carvalheira, J.; Espindola, F.S.; et al. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism 2011, 60, 1244–1252. [Google Scholar] [CrossRef]
- Annibalini, G.; Lucertini, F.; Agostini, D.; Vallorani, L.; Gioacchini, A.; Barbieri, E.; Guescini, M.; Casadei, L.; Passalia, A.; Del Sal, M.; et al. Concurrent aerobic and resistance training has anti-inflammatory effects and increases both plasma and leukocyte levels of IGF-1 in late middle-aged type 2 diabetic patients. Oxidative Med. Cell. Longev. 2017, 2017, 3937842. [Google Scholar] [CrossRef]
- Lucotti, P.; Monti, L.D.; Setola, E.; Galluccio, E.; Gatti, R.; Bosi, E.; Piatti, P. Aerobic and resistance training effects compared to aerobic training alone in obese type 2 diabetic patients on diet treatment. Diabetes Res. Clin. Pract. 2011, 94, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Francois, M.E.; Pistawka, K.J.; Halperin, F.A.; Little, J.P. Cardiovascular benefits of combined interval training and post-exercise nutrition in type 2 diabetes. J. Diabetes Complicat. 2018, 32, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Peltonen, M.; Koenig, W.; Sütfels, K.; Lindström, J.; Martin, S.; Ilanne-Parikka, P.; Eriksson, J.G.; Aunola, S.; Keinänen-Kiukaanniemi, S.; et al. Anti-inflammatory effect of lifestyle changes in the Finnish Diabetes Prevention Study. Diabetologia 2009, 52, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathanson, V. Revising the declaration of Helsinki. BMJ Publ. Group 2013, 346, f2837. [Google Scholar] [CrossRef] [Green Version]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [PubMed]
- Brzycki, M. Strength testing—Predicting a one-rep max from reps-to-fatigue. J. Phys. Educ. Recreat. Danc. 1993, 64, 88–90. [Google Scholar] [CrossRef]
- Mendes, R.; Sousa, N.; Almeida, A.; Subtil, P.; Guedes-Marques, F.; Reis, V.M.; Themudo-Barata, J.L. Exercise prescription for patients with type 2 diabetes—A synthesis of international recommendations: Narrative review. Br. J. Sports Med. 2016, 50, 1379–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Ruffino, J.; Davies, N.; Morris, K.; Ludgate, M.; Zhang, L.; Webb, R.; Thomas, A.W. Moderate-intensity exercise alters markers of alternative activation in circulating monocytes in females: A putative role for PPARγ. Eur. J. Appl. Physiol. 2016, 116, 1671–1682. [Google Scholar] [CrossRef] [Green Version]
- Conti, F.F.; Brito, J.D.O.; Bernardes, N.; Dias, D.D.S.; Malfitano, C.; Morris, M.; Llesuy, S.F.; Irigoyen, M.-C.; De Angelis, K. Positive effect of combined exercise training in a model of metabolic syndrome and menopause: Autonomic, inflammatory, and oxidative stress evaluations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1532–R1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Buchanan, T.A.; Spicer, D.V.; Tripathy, D.; Bernstein, L.; Mortimer, J. Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: A randomized controlled trial. J. Clin. Oncol. 2018, 36, 875–883. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Protective effects of sulforaphane on exercise-induced organ damage via inducing antioxidant defense responses. Antioxidants 2020, 9, 136. [Google Scholar] [CrossRef] [Green Version]
- López-Chillón, M.T.; Carazo-Díaz, C.; Prieto-Merino, D.; Zafrilla, P.; Moreno, D.A.; Villaño, D. Effects of long-term consumption of broccoli sprouts on inflammatory markers in overweight subjects. Clin. Nutr. 2019, 38, 745–752. [Google Scholar] [CrossRef]
- Yu, C.; He, Q.; Zheng, J.; Li, L.Y.; Hou, Y.H.; Song, F.Z. Sulforaphane improves outcomes and slows cerebral ischemic/reperfusion injury via inhibition of NLRP3 inflammasome activation in rats. Int. Immunopharmacol. 2017, 45, 74–78. [Google Scholar] [CrossRef]
- Magalhães, J.P.; Santos, D.A.; Correia, I.R.; Hetherington-Rauth, M.; Ribeiro, R.; Raposo, J.F.; Matos, A.; Bicho, M.D.; Sardinha, L.B. Impact of combined training with different exercise intensities on inflammatory and lipid markers in type 2 diabetes: A secondary analysis from a 1-year randomized controlled trial. Cardiovasc. Diabetol. 2020, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cuff, D.J.; Meneilly, G.S.; Martin, A.; Ignaszewski, A.; Tildesley, H.D.; Frohlich, J.J. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care 2003, 26, 2977–2982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zouhal, H.; Zare-Kookandeh, N.; Haghighi, M.M.; Daraei, A.; de Sousa, M.; Soltani, M.; Ben Abderrahman, A.; Tijani, J.M.; Hackney, A.C.; Laher, I.; et al. Physical activity and adipokine levels in individuals with type 2 diabetes: A literature review and practical applications. Rev. Endocr. Metab. Disord. 2021. [Google Scholar] [CrossRef] [PubMed]
- Brooks, N.; Layne, J.E.; Gordon, P.L.; Roubenoff, R.; Nelson, M.E.; Castaneda-Sceppa, C. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int. J. Med. Sci. 2007, 4, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Carey, A.; Febbraio, M. Interleukin-6 and insulin sensitivity: Friend or foe? Diabetologia 2004, 47, 1135–1142. [Google Scholar] [CrossRef] [Green Version]
- Kubaszek, A.; Pihlajamäki, J.; Komarovski, V.; Lindi, V.; Lindström, J.; Eriksson, J.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; et al. Promoter polymorphisms of the TNF-α (G-308A) and IL-6 (C-174G) genes predict the conversion from impaired glucose tolerance to type 2 diabetes: The Finnish Diabetes Prevention Study. Diabetes 2003, 52, 1872–1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsiotra, P.; Tsigos, C.; Raptis, S. TNFα and leptin inhibit basal and glucose-stimulated insulin secretion and gene transcription in the HIT-T15 pancreatic cells. Int. J. Obes. 2001, 25, 1018–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golbidi, S.; Laher, I. Exercise induced adipokine changes and the metabolic syndrome. J. Diabetes Res. 2014, 2014, 726861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes 2003, 52, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Ashraf, M.H.N.; Facci, M.; Wang, R.; Paterson, P.G.; Ferrie, A.; Juurlink, B.H.J. Dietary approach to attenuate oxidative stress, hypertension, and inflammation in the cardiovascular system. Proc. Natl. Acad. Sci. USA 2004, 101, 7094–7099. [Google Scholar] [CrossRef] [Green Version]
- Kursawe, R.; Eszlinger, M.; Narayan, D.; Liu, T.; Bazuine, M.; Cali, A.M.; D’Adamo, E.; Shaw, M.; Pierpont, B.; Shulman, G.I.; et al. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: Association with insulin resistance and hepatic steatosis. Diabetes 2010, 59, 2288–2296. [Google Scholar] [CrossRef] [Green Version]
- Armah, C.N.; Derdemezis, C.; Traka, M.H.; Dainty, J.R.; Doleman, J.F.; Saha, S.; Leung, W.; Potter, J.F.; Lovegrove, J.A.; Mithen, R.F.; et al. Diet rich in high glucoraphanin broccoli reduces plasma LDL cholesterol: Evidence from randomised controlled trials. Mol. Nutr. Food Res. 2015, 59, 918–926. [Google Scholar] [CrossRef]
- Gordon, B.; Chen, S.; Durstine, J.L. The effects of exercise training on the traditional lipid profile and beyond. Curr. Sports Med. Rep. 2014, 13, 253–259. [Google Scholar] [CrossRef]
- Saeidi, A.; Seifi-Ski-Shahr, F.; Soltani, M.; Daraei, A.; Shirvani, H.; Laher, I.; Hackney, A.C.; Johnson, K.E.; Basati, G.; Zouhal, H. Resistance training, gremlin 1 and macrophage migration inhibitory factor in obese men: A randomised trial. Arch. Physiol. Biochem. 2020, 28, 1–9. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M. Comparison of effects of diet versus exercise weight loss regimens on LDL and HDL particle size in obese adults. Lipids Health Dis. 2011, 10, 1–5. [Google Scholar] [CrossRef] [Green Version]
| Weeks | Aerobic Training | Resistance Training | |||||
|---|---|---|---|---|---|---|---|
| Duration (min/Sessions) | Intensity (VO2peak) | Sessions Per Week | Sets | Repetitions | 1-RM | Sessions Per Week | |
| 1 to 2 | 10 | 60% | 3 | 3 | 12–15 | 60–65% | 3 |
| 2 to 4 | 15 | 65% | 3 | 3 | 12–15 | 60–65% | 3 |
| 4 to 6 | 20 | 70% | 3 | 3 | 8–10 | 70% | 3 |
| 6 to 8 | 25 | 70% | 3 | 3 | 8–10 | 70% | 3 |
| 8 to 12 | 30 | 70% | 3 | 3 | 8–10 | 70% | 3 |
| CP | S | TP | TS | |||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Energy (kcal/d) | 2364.5 ± 164.6 | 2383.1 ± 106.6 | 2370.1 ± 162.9 | 2385.6 ± 157.0 | 2362.1 ± 214.2 | 2340.3 ± 213.4 | 2393.6 ± 215.5 | 2358.0 ± 193.2 |
| CHO (g/d) | 295.1 ± 24.0 | 305.1 ± 36.1 | 298.0 ± 33.1 | 304.6 ± 34.8 | 289.0 ±31.5 | 291.6 ± 29.5 | 292.7 ± 31.9 | 295.0 ± 32.4 |
| Fat (g/d) | 79.8 ± 16.8 | 77.0 ± 15.7 | 79.6 ± 15.8 | 76.9 ± 14.2 | 81.4± 16.0 | 78.9 ± 14.5 | 83.8 ± 17.0 | 79.1 ± 14.6 |
| Protein (g/d) | 116.3 ± 16.9 | 117.3 ± 17 | 115.3 ± 19.2 | 118.7 ± 19.2 | 118.2 ± 17.4 | 115.9 ± 18.7 | 117.0 ± 18.9 | 116.3 ± 17.8 |
| CP | S | TP | TS | |||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Glucose (mmol/L) | 8.71 ± 0.73 | 8.58 ± 0.65 | 8.78 ± 0.75 | 7.89 ± 0.75 ‡ | 8.79 ± 0.71 | 6.38 ± 0.47 ‡*# | 8.86 ± 0.71 | 5.94 ± 0.54 ‡*# |
| HDL-C (mmol/L) | 0.84 ± 0.05 | 0.84 ± 0.03 | 0.94 ± 0.04 | 0.99 ± 0.04 ‡* | 0.92 ± 0.06 | 1.16 ± 0.09 ‡*# | 0.90 ± 0.06 | 1.16 ± 0.06 ‡*# |
| LDL (mmol/L) | 4.11 ± 0.12 | 4.08 ± 0.12 | 4.06 ± 0.13 | 3.98 ± 0.20 ‡ | 4.11 ± 0.12 | 3.74 ± 0.13 ‡* | 4.20 ± 0.12 | 3.46 ± 0.21 ‡*# |
| TC (mmol/L) | 6.20 ± 0.25 | 6.23 ± 0.26 | 6.07 ± 0.26 | 5.99 ± 0.28 ‡ | 6.05 ± 0.24 | 5.43 ± 0.23 ‡*# | 6.12 ± 0.28 | 5.32 ± 0.23 ‡*# |
| TG (mmol/L) | 6.20 ± 0.023 | 6.20 ± 0.28 | 2.23 ± 0.28 | 6.10 ± 0.28 ‡ | 6.12 ± 0.29 | 5.64 ± 0.36 ‡* | 6.23 ± 0.29 | 5.43 ± 0.34 ‡*# |
| hs-CRP (ng/mL) | 4.20 ± 1.02 | 4.25 ± 1.04 | 4.51 ± 1.84 | 3.75 ± 1.62 ‡ | 4.53 ± 1.16 | 3.05 ± 1.01 ‡ | 4.22 ± 1.21 | 2.48 ± 0.56 ‡ |
| IL-6 (pg/mL) | 4.89 ± 0.90 | 4.96 ± 1.09 | 4.90 ± 1.59 | 4.59 ± 1.34 ‡ | 4.98 ± 1.05 | 2.85 ± 1.01 ‡* | 5.22 ± 1.21 | 2.64 ± 2.01 ‡* |
| TNF-α (pg/mL) | 14.60 ± 3.18 | 14.78 ± 3.29 | 14.97 ± 4.32 | 13.45 ± 3.89 ‡ | 14.55 ± 4.36 | 11.53 ± 3.56 ‡ | 14.97 ± 3.80 | 7.78 ± 2.63 ‡* |
| Weight (kg) | 86.36 ± 4.56 | 86.72 ± 3.90 | 85.00 ± 3.31 | 85.18 ± 3.57 | 86.09 ± 4.41 | 79.90 ± 3.53 ‡* | 86.63 ± 4.90 | 79.09 ± 3.78 ‡* |
| FFM (kg) | 28.36 ± 1.56 | 28.27 ± 1.84 | 29.63 ± 2.06 | 29.45 ± 2.29 | 28.90 ± 1.44 | 32.27 ± 2.05 ‡* | 28.72 ± 1.73 | 32.09 ± 2.30 ‡* |
| Fat (%) | 29.54 ± 1.12 | 29.63 ± 2.50 | 29.81 ± 2.04 | 29.45 ± 1.91 | 29.54 ± 1.75 | 25.90 ± 1.81 ‡*# | 30.18 ± 1.83 | 25.09 ± 2.21 ‡*# |
| BMI (kg/m2) | 28.34 ± 1.13 | 28.46 ± 0.96 | 27.38 ± 1.34 | 27.43 ± 1.33 | 28.51 ± 0.84 | 27.29 ± 0.74 ‡ | 28.06 ± 0.82 | 26.17 ± 0.78 ‡* |
| VO2peak (mL/kg/min) | 28.09 ± 1.97 | 27.63 ± 2.29 | 28.27 ± 2.45 | 28.36 ± 2.33 | 28.81 ± 3.45 | 32.90 ± 3.91 ‡*# | 28.09 ± 2.42 | 33.36± 0.69 ‡*# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeidi, A.; Soltani, M.; Daraei, A.; Nohbaradar, H.; Haghighi, M.M.; Khosravi, N.; Johnson, K.E.; Laher, I.; Hackney, A.C.; VanDusseldorp, T.A.; et al. The Effects of Aerobic-Resistance Training and Broccoli Supplementation on Plasma Dectin-1 and Insulin Resistance in Males with Type 2 Diabetes. Nutrients 2021, 13, 3144. https://doi.org/10.3390/nu13093144
Saeidi A, Soltani M, Daraei A, Nohbaradar H, Haghighi MM, Khosravi N, Johnson KE, Laher I, Hackney AC, VanDusseldorp TA, et al. The Effects of Aerobic-Resistance Training and Broccoli Supplementation on Plasma Dectin-1 and Insulin Resistance in Males with Type 2 Diabetes. Nutrients. 2021; 13(9):3144. https://doi.org/10.3390/nu13093144
Chicago/Turabian StyleSaeidi, Ayoub, Mohammad Soltani, Ali Daraei, Hanieh Nohbaradar, Marjan Mosalman Haghighi, Nikoo Khosravi, Kelly E. Johnson, Ismail Laher, Anthony C. Hackney, Trisha A. VanDusseldorp, and et al. 2021. "The Effects of Aerobic-Resistance Training and Broccoli Supplementation on Plasma Dectin-1 and Insulin Resistance in Males with Type 2 Diabetes" Nutrients 13, no. 9: 3144. https://doi.org/10.3390/nu13093144
APA StyleSaeidi, A., Soltani, M., Daraei, A., Nohbaradar, H., Haghighi, M. M., Khosravi, N., Johnson, K. E., Laher, I., Hackney, A. C., VanDusseldorp, T. A., & Zouhal, H. (2021). The Effects of Aerobic-Resistance Training and Broccoli Supplementation on Plasma Dectin-1 and Insulin Resistance in Males with Type 2 Diabetes. Nutrients, 13(9), 3144. https://doi.org/10.3390/nu13093144








