Narrative Review of Low-Intake Dehydration in Older Adults
Abstract
1. Introduction
2. Materials and Methods
3. Results
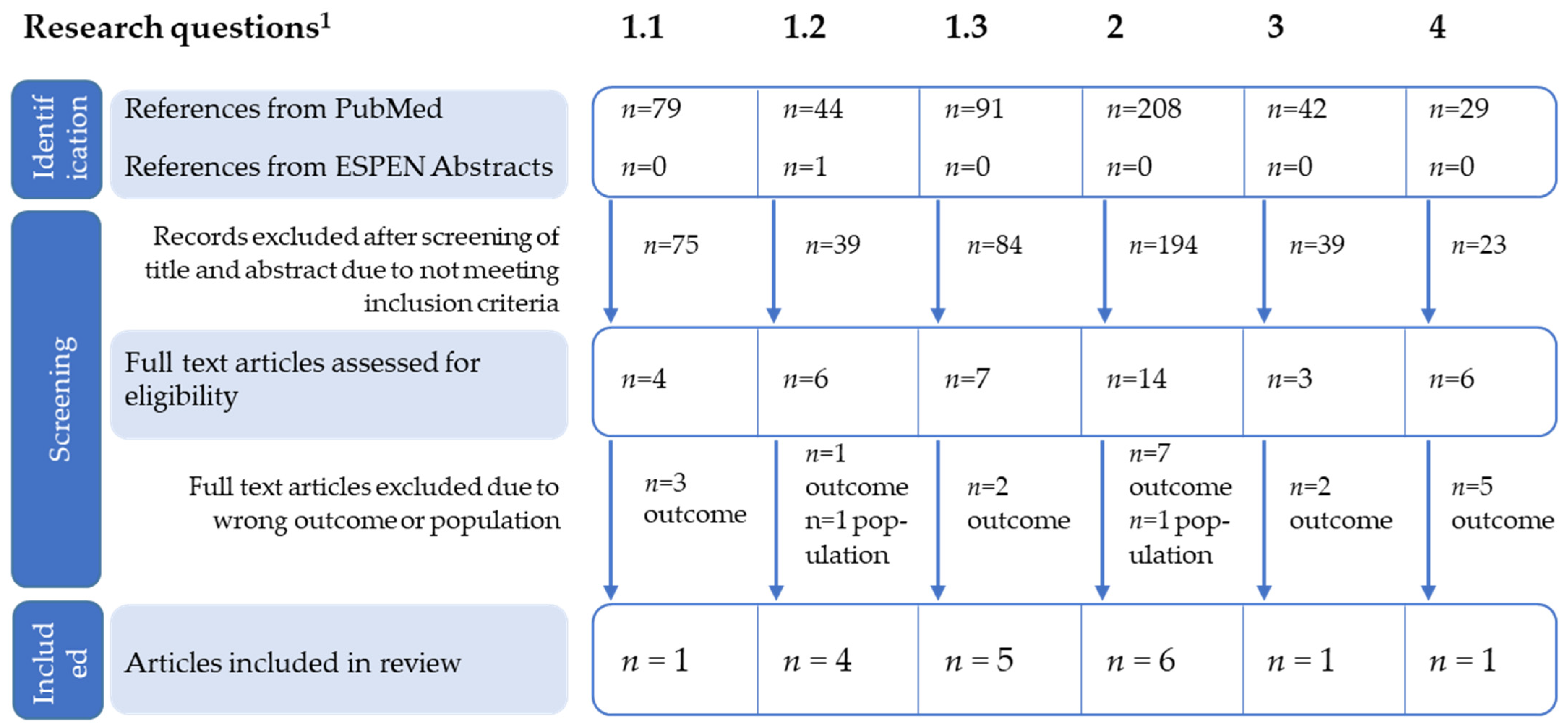
3.1. Literature Search
3.2. How Should Low-Intake Dehydration Be Identified in Older Persons?
- Osmolality
- Osmolarity
- Clinical signs and bioelectrical Impedance (BIA)
3.3. What Interventions May Help to Support Older Persons to Drink Well and Prevent Low-Intake Dehydration?
- Extending drinking opportunities comprised three interventions:
- Pre-breakfast drinks: providing drinks to residents moved to the dining room prior to breakfast at one nursing home.
- Drinks after meals: residents were offered hot drinks after lunch and dinner at another nursing home.
- Protected Drinks Time (PDT): all residents were served a drink and where needed, provided with assistance to drink during the mid-afternoon drinks round at both nursing homes.
- The choice of beverages was increased by developing a Drinks Menu. The menu provided support for residents when choosing a drink and encouraged staff to offer more than one drink. The Drinks Menu was combined with PDT and introduced in both homes.
3.4. How Much Should Older People Drink Each Day?
3.5. What Should Older People Drink Each Day?
3.6. Level of Evidence
4. Discussion
4.1. How Should Low-Intake Dehydration Be Identified in Older Persons?
- A prospective, interventional study that targets parameters of normal hydration (e.g., plasma osmolarities 280–300 mOsm/kg) and determines whether this translates to health and health economics co-benefits.
- The causality of the association between plasma osmolarity thresholds and adverse outcomes needs to be tested through interventional studies.
- The development of a suitable device for the routine, bedside assessment of plasma osmolality.
4.2. What Interventions May Help to Support Older Persons to Drink Well and Prevent Low-Intake Dehydration?
4.3. How Much Should Older People Drink Each Day?
4.4. What Should Older People Drink Each Day?
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lacey, J.; Corbett, J.; Forni, L.; Hooper, L.; Hughes, F.; Minto, G.; Moss, C.; Price, S.; Whyte, G.; Woodcock, T.; et al. A multidisciplinary consensus on dehydration: Definitions, diagnostic methods and clinical implications. Ann. Med. 2019, 51, 232–251. [Google Scholar] [CrossRef] [PubMed]
- Paulis, S.J.; Everink, I.H.; Halfens, R.J.; Lohrmann, C.; Schols, J.M.G.A. Prevalence and Risk Factors of Dehydration Among Nursing Home Residents: A Systematic Review. J. Am. Med. Dir. Assoc. 2018, 19, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Bunn, D.K.; Downing, A.; Jimoh, F.O.; Groves, J.; Free, C.; Cowap, V.; Potter, J.F.; Hunter, P.; Shepstone, L. Which Frail Older People Are Dehydrated? The UK DRIE Study. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2016, 71, 1341–1347. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.J.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef] [PubMed]
- Streicher, M.; Wirth, R.; Schindler, K.; Sieber, C.C.; Hiesmayr, M.; Volkert, D. Dysphagia in Nursing Homes—Results from the NutritionDay Project. J. Am. Med. Dir. Assoc. 2018, 19, 141–147.e2. [Google Scholar] [CrossRef]
- Painter, V.; Le Couteur, D.; Waite, L.M. Texture-modified food and fluids in dementia and residential aged care facilities. Clin. Interv. Aging 2017, 12, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Mentes, J. Oral Hydration in Older Adults: Greater. Awareness is needed in preventing, recognizing, and treating dehydrationm. AJN. Am. J. Nurs. 2006, 106, 40–49. [Google Scholar] [CrossRef]
- Hooper, L.; Abdelhamid, A.; Attreed, N.J.; Campbell, W.W.; Channell, A.M.; Chassagne, P.; Culp, K.R.; Fletcher, S.J.; Fortes, M.B.; Fuller, N.; et al. Clinical symptoms, signs and tests for identification of impending and current water-loss dehydration in older people. Cochrane Database Syst. Rev. 2015, 2015, CD009647. [Google Scholar] [CrossRef]
- Edmonds, C.J.; Foglia, E.; Booth, P.; Fu, C.H.; Gardner, M. Dehydration in older people: A systematic review of the effects of dehydration on health outcomes, healthcare costs and cognitive performance. Arch. Gerontol. Geriatr. 2021, 95, 104380. [Google Scholar] [CrossRef]
- Paulis, S.J.C.; Everink, I.H.J.; Halfens, R.J.G.; Lohrmann, C.; Wirnsberger, R.R.; Gordon, A.L.; Schols, J.M.G.A. Diagnosing dehydration in the nursing home: International consensus based on a modified Delphi study. Eur. Geriatr. Med. 2020, 11, 393–402. [Google Scholar] [CrossRef]
- Munk, T.; Bech, C.B.; Klausen, T.W.; Rønholt, F.; Suetta, C.; Knudsen, A.W. Accuracy of the calculated serum osmolarity to screen for hyperosmolar dehydration in older hospitalised medical patients. Clin. Nutr. ESPEN 2021, 43, 415–419. [Google Scholar] [CrossRef]
- Wojszel, Z.B. Impending Low Intake Dehydration at Admission to A Geriatric Ward- Prevalence and Correlates in a Cross-Sectional Study. Nutrients 2020, 12, 398. [Google Scholar] [CrossRef]
- Mantantzis, K.; Drewelies, J.; Duezel, S.; Steinhagen-Thiessen, E.; Demuth, I.; Wagner, G.G.; Lindenberger, U.; Gerstorf, D. Dehydration predicts longitudinal decline in cognitive functioning and well-being among older adults. Psychol. Aging 2020, 35, 517–528. [Google Scholar] [CrossRef]
- Bunn, D.K.; Hooper, L. Signs and Symptoms of Low-Intake Dehydration Do Not Work in Older Care Home Residents—DRIE Diagnostic Accuracy Study. J. Am. Med. Dir. Assoc. 2019, 20, 963–970. [Google Scholar] [CrossRef]
- Johnson, P.; Hahn, R.G. Signs of Dehydration in Nursing Home Residents. J. Am. Med. Dir. Assoc. 2018, 19. [Google Scholar] [CrossRef]
- Akdeniz, M.; Boeing, H.; Müller-Werdan, U.; Aykac, V.; Steffen, A.; Schell, M.; Blume-Peytavi, U.; Kottner, J. Effect of Fluid Intake on Hydration Status and Skin Barrier Characteristics in Geriatric Patients: An Explorative Study. Ski. Pharmacol. Physiol. 2018, 31, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ekman, L.; Johnson, P.; Hahn, R. Signs of Dehydration after Hip Fracture Surgery: An Observational Descriptive Study. Medicina 2020, 56, 361. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.; Hodgson, P.; Thompson, J.; Bainbridge, L.; Johnson, A.; Storey, P. Hydration Interventions for older people living in residential and nursing care homes: Overview of the literature. Br. Med. Bull. 2019, 131, 71–79. [Google Scholar] [CrossRef]
- Jimoh, O.F.; Brown, T.J.; Bunn, D.; Hooper, L. Beverage Intake and Drinking Patterns—Clues to Support Older People Living in Long-Term Care to Drink Well: DRIE and FISE Studies. Nutrients 2019, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Marra, M.V.; Simmons, S.F.; Shotwell, M.S.; Hudson, A.L.; Hollingsworth, E.K.; Long, E.; Kuertz, B.; Silver, H.J.; Kuertz, B. Elevated Serum Osmolality and Total Water Deficit Indicate Impaired Hydration Status in Residents of Long-Term Care Facilities Regardless of Low or High Body Mass Index. J. Acad. Nutr. Diet. 2016, 116, 828–836.e2. [Google Scholar] [CrossRef] [PubMed]
- Bak, A.; Wilson, J.; Tsiami, A.; Loveday, H. Drinking vessel preferences in older nursing home residents: Optimal design and potential for increasing fluid intake. Br. J. Nurs. 2018, 27, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Bak, A.; Tingle, A.; Greene, C.; Tsiami, A.; Canning, D.; Myron, R.; Loveday, H. Improving hydration of care home residents by increasing choice and opportunity to drink: A quality improvement study. Clin. Nutr. 2019, 38, 1820–1827. [Google Scholar] [CrossRef]
- Masot, O.; Miranda, J.; Santamaría, A.; Pueyo, E.P.; Pascual, A.; Botigué, T. Fluid Intake Recommendation Considering the Physiological Adaptations of Adults Over 65 Years: A Critical Review. Nutrients 2020, 12, 3383. [Google Scholar] [CrossRef] [PubMed]
- Polhuis, K.C.M.M.; Wijnen, A.H.C.; Sierksma, A.; Calame, W.; Tieland, M. The Diuretic Action of Weak and Strong Alcoholic Beverages in Elderly Men: A Randomized Diet-Controlled Crossover Trial. Nutrients 2017, 9, 660. [Google Scholar] [CrossRef]
- Bak, A.; Tsiami, A.; Greene, C. Methods of Assessment of Hydration Status and their Usefulness in Detecting Dehydration in the Elderly. Curr. Res. Nutr. Food Sci. J. 2017, 5, 43–54. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- Oates, L.L.; Price, C.I. Clinical assessments and care interventions to promote oral hydration amongst older patients: A narrative systematic review. BMC Nurs. 2017, 16, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abdelhamid, A.; Bunn, D.K.; Copley, M.; Cowap, V.; Dickinson, A.; Gray, L.; Howe, A.; Killett, A.; Lee, H.; Li, F.; et al. Effectiveness of interventions to directly support food and drink intake in people with dementia: Systematic review and meta-analysis. BMC Geriatr. 2016, 16, 26. [Google Scholar] [CrossRef]
- Botigué, T.; Masot, O.; Miranda, J.; Nuin, C.; Viladrosa, M.; Lavedán, A.; Zwakhalen, S. Prevalence and Risk Factors Associated with Low Fluid Intake in Institutionalized Older Residents. J. Am. Med. Dir. Assoc. 2019, 20, 317–322. [Google Scholar] [CrossRef]
- Wotton, K.; Crannitch, K.; Munt, R. Prevalence, risk factors and strategies to prevent dehydration in older adults. Contemp. Nurse 2008, 31, 44–56. [Google Scholar] [CrossRef]
- Bunn, D.; Hooper, L.; Welch, A. Dehydration and Malnutrition in Residential Care: Recommendations for Strategies for Improving Practice Derived from a Scoping Review of Existing Policies and Guidelines. Geriatrics 2018, 3, 77. [Google Scholar] [CrossRef] [PubMed]
- Boockvar, K.S.; Judon, K.M.; Eimicke, J.P.; Teresi, J.A.; Inouye, S.K. Hospital Elder Life Program in Long-Term Care (HELP-LTC): A Cluster Randomized Controlled Trial. J. Am. Geriatr. Soc. 2020, 68, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Mentes, J.C.; Tripp-Reimer, T. Barriers and Facilitators in Nursing Home Intervention Research. West. J. Nurs. Res. 2002, 24, 918–936. [Google Scholar] [CrossRef]
- Bhanu, C.; Avgerinou, C.; Kharicha, K.; Bauernfreund, Y.; Croker, H.; Liljas, A.; Rea, J.; Kirby-Barr, M.; Hopkins, J.; Walters, K. ‘I’ve never drunk very much water and I still don’t, and I see no reason to do so’: A qualitative study of the views of community-dwelling older people and carers on hydration in later life. Age Ageing 2019, 49, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Lazenby-Paterson, T. Thickened liquids: Do they still have a place in the dysphagia toolkit? Curr. Opin. Otolaryngol. Head Neck Surg. 2020, 28, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Lea, E.J.; Goldberg, L.R.; Price, A.D.; Tierney, L.T.; McInerney, F. Staff awareness of food and fluid care needs for older people with dementia in residential care: A qualitative study. J. Clin. Nurs. 2017, 26, 5169–5178. [Google Scholar] [CrossRef]
- Nell, D.; Neville, S.; Bellew, R.; O’Leary, C.; Beck, K.L. Factors affecting optimal nutrition and hydration for people living in specialised dementia care units: A qualitative study of staff caregivers’ perceptions. Australas. J. Ageing 2016, 35, E1–E6. [Google Scholar] [CrossRef] [PubMed]
- Törmä, J.; Pingel, R.; Cederholm, T.; Saletti, A.; Winblad, U. Is it possible to influence ability, willingness and understanding among nursing home care staff to implement nutritional guidelines? A comparison of a facilitated and an educational strategy. Int. J. Older People Nurs. 2021, 16, e12367. [Google Scholar] [CrossRef]
- Bunn, D.; Jimoh, F.; Wilsher, S.H.; Hooper, L. Increasing Fluid Intake and Reducing Dehydration Risk in Older People Living in Long-Term Care: A Systematic Review. J. Am. Med. Dir. Assoc. 2015, 16, 101–113. [Google Scholar] [CrossRef]
- Steven, A.; Wilson, G.; Young-Murphy, L.; Bevc, S.; Wong, A.; Danilovich, M. The Implementation of an Innovative Hydration Monitoring App in Care Home Settings: A Qualitative Study. JMIR mHealth uHealth 2019, 7, e9892. [Google Scholar] [CrossRef]
- Korsgaard, D.; Bjøner, T.; Nilsson, N.C. Where would you like to eat? A formative evaluation of mixed-reality solitary meals in virtual environments for older adults with mobility impairments who live alone. Food Res. Int. 2019, 117, 30–39. [Google Scholar] [CrossRef]
- Eichhorn, C.; Plecher, D.A.; Lurz, M.; Leipold, N.; Böhm, M.; Krcmar, H.; Ott, A.; Volkert, D.; Hiyama, A.; Klinker, G. The Innovative Reminder in Senior-Focused Technology (THIRST)—Evaluation of Serious Games and Gadgets for Alzheimer Patients. In Proceedings of the 5th International Conference, ITAP 2019, Held as Part of the 21st HCI International Conference, HCII 2019, Orlando, FL, USA, 26–31 July 2019. Proceedings, Part II. [Google Scholar]
- Thomas, D.R.; Cote, T.R.; Lawhorne, L.; Levenson, S.A.; Rubenstein, L.; Smith, D.A.; Stefanacci, R.G.; Tangalos, E.G.; Morley, J.E. Dehydration Council. Understanding Clinical Dehydration and Its Treatment. J. Am. Med. Dir. Assoc. 2008, 9, 292–301. [Google Scholar] [CrossRef] [PubMed]
| Research Question (RQ) | Recommendation § | Grade of Recommendation * | |
|---|---|---|---|
| 1 | How should low-intake dehydration be identified in older persons? (RQ 1.1, 1.2, 1.3) | An action threshold of directly measured serum osmolality > 300 mOsm/kg should be used to identify low-intake dehydration in older adults | B |
| Where directly measured osmolality is not available then the osmolarity equation (osmolarity = 1.86 × (Na+ + K+) + 1.15 × glucose + urea + 14 (all measured in mmol/L) with an action threshold of >295 mmol/L) should be used to screen for low-intake dehydration in older persons | B | ||
| Simple signs and tests commonly used to assess low-intake dehydration such as skin turgor, mouth dryness, weight change, urine color or specific gravity, shall NOT be used to assess hydration status in older adults. | A | ||
| Bioelectrical impedance shall NOT be used to assess hydration status in older adults as it has not been shown to be usefully diagnostic | A | ||
| 2 | What interventions may help to support older persons to drink well and prevent low-intake dehydration? | To prevent dehydration in older persons living in residential care, institutions should implement multicomponent strategies across their institutions for all residents | B |
| These strategies should include high availability of drinks, varied choice of drinks, frequent offering of drinks, staff awareness of the need for adequate fluid intake, staff support for drinking and staff support in taking older adults to the toilet quickly and when they need it. | B | ||
| Strategies to support adequate fluid intake should be developed including older persons themselves, staff, management, and policymakers | A | ||
| 3 | How much should older people drink each day? | Older women should be offered at least 1.6 L of drinks each day, while older men should be offered at least 2.0 L of drinks each day unless there is a clinical condition that requires different approach | B |
| 4 | What should older people drink each day? | A range of appropriate (i.e., hydrating) drinks should be offered to older people according to their preferences | B |
| RQ 1 | Publication | Study Type if Applicable: Population | Relevant Findings | Level of Evidence 2 | Consistency with ESPEN Guideline |
|---|---|---|---|---|---|
| 1.1 | Lacey et al. 2019[1] | Expert opinion n = 12, experts of varying specialties |
| 4 | Yes |
| 1.2 | Lacey et al. 2019[1] | See above |
| 4 | Yes |
| Munk et al. 2021 [11] | Diagnostic accuracy study n = 90, older adults from emergency medical department |
| 2+ | Yes | |
| Woijszel et al. 2020 [12] | Cohort study n = 358, hospitalized older adults |
| 2+ | Yes | |
| Mantantzis et al. 2020 [13] | Cohort study n = 1047, community-dwelling older adults |
| 2+ | Yes | |
| 1.3 | Bunn and Hooper 2019 [14] | Diagnostic accuracy study n = 188, care home residents |
| 2+ | Yes |
| Lacey et al. 2019 [1] | See above |
| 4 | Yes | |
| Johnson & Hahn 2018 [15] | Cohort study n = 60, nursing home residents |
| 2− | Yes | |
| Akdeniz et al. 2018 [16] | Cohort study n = 40, hospitalized older adults |
| 2+ | Yes | |
| Ekman et al. 2020 [17] | Cohort study n = 38, rehabilitating older adults after hip surgery |
| 2− | No | |
| 2 | Cook et al. 2019 [18] | Literature review care home residents |
| 4 | Yes |
| Painter et al. 2017 [6] | Literature review dementia and aged care facilities |
| 4 | Yes | |
| Jimoh et al. 2019 [19] | Cohort study n = 22, long-term care residents |
| 2- | Yes | |
| Marra et al. 2016 [20] | Cohort study n = 247, long-term-care residents |
| 2+ | yes | |
| Bak et al. 2018 [21] | Pre-post study Two wards, nursing home residents |
| n.a. | Yes | |
| Wilson et al. 2019 [22] | Pre-post study Two care homes, Care home residents |
| n.a. | Yes | |
| 3 | Masot et al. [23] | Literature review Older adults at different care levels |
| 4 | Yes |
| 4 | Polhuis et al. [24] | Randomized Trial n = 20, elderly community-dwelling men |
| 1++ | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, A.M.; Seemer, J.; Knudsen, A.W.; Munk, T. Narrative Review of Low-Intake Dehydration in Older Adults. Nutrients 2021, 13, 3142. https://doi.org/10.3390/nu13093142
Beck AM, Seemer J, Knudsen AW, Munk T. Narrative Review of Low-Intake Dehydration in Older Adults. Nutrients. 2021; 13(9):3142. https://doi.org/10.3390/nu13093142
Chicago/Turabian StyleBeck, Anne Marie, Johanna Seemer, Anne Wilkens Knudsen, and Tina Munk. 2021. "Narrative Review of Low-Intake Dehydration in Older Adults" Nutrients 13, no. 9: 3142. https://doi.org/10.3390/nu13093142
APA StyleBeck, A. M., Seemer, J., Knudsen, A. W., & Munk, T. (2021). Narrative Review of Low-Intake Dehydration in Older Adults. Nutrients, 13(9), 3142. https://doi.org/10.3390/nu13093142





