Abstract
Curcumin is one of the main polyphenolic compounds in the turmeric rhizome. It possesses antioxidant, anti-inflammatory, anti-cancer, anti-arthritis, anti-asthmatic, anti-microbial, anti-viral and anti-fungal properties. This review aims to provide an overview of the potential health benefits of curcumin to treat female reproductive disorders, including polycystic ovary syndrome (PCOS), ovarian failure and endometriosis. Comprehensive information on curcumin was retrieved from electronic databases, which were MEDLINE via EBSCOhost, Scopus and Google Scholar. The available evidence showed that curcumin reduced the high level of androgen in PCOS. Studies in rodents suggest that curcumin resulted in the disappearance of cysts and the appearance of healthy follicles and corpora lutea. Furthermore, animal studies showed curcumin improved the overall function of the ovary in ovarian diseases and reversed the disturbance in oxidative stress parameters. Meanwhile, in vitro and in vivo studies reported the positive effects of curcumin in alleviating endometriosis through anti-inflammatory, anti-proliferative, anti-angiogenic and pro-apoptotic mechanisms. Thus, curcumin possesses various effects on PCOS, ovarian diseases and endometriosis. Some studies found considerable therapeutic effects, whereas others found no effect. However, none of the investigations found curcumin to be harmful. Curcumin clinical trials in endometriosis and ovarian illness are still scarce; thus, future studies need to be conducted to confirm the safety and efficacy of curcumin before it could be offered as a complementary therapy agent.
1. Introduction
Turmeric or Curcuma longa is a rhizomatous herbal plant from the Zingiberaceae family. This plant is easily found throughout tropical Asia, including India and South China, Southeast Asia, Papua New Guinea and northern Australia [1]. In Asian countries, turmeric is widely used in food and medicine as a natural colouring and flavouring agent at a concentration range of 5–500 mg/kg [2]. Traditionally, turmeric has been used to treat digestive disorders, rheumatoid arthritis, conjunctivitis, liver ailment, urinary tract infection, smallpox, chickenpox, wounds and regulation of menstruation [3]. Turmeric has more than 300 biologically active components, such as polyphenols, sterols, diterpenes, sesquiterpenes, triterpenoids and alkaloids [3]. Turmeric rhizome contains a high number of polyphenols (bioflavonoids) called curcuminoids, which comprise curcumin (60–70%), demethoxycurcumin (20–30%) and bisdemethoxycurcumin (10–15%) [4]. The yellow colour of turmeric is due to the content of curcuminoids [5]. Considering its high content, curcumin is a well-studied compound. Numerous studies on curcumin have demonstrated its many benefits due to its antioxidant, anti-inflammatory, anti-cancer, anti-arthritis, anti-asthmatic, anti-microbial, anti-viral and anti-fungal properties [6,7,8,9,10,11,12,13].
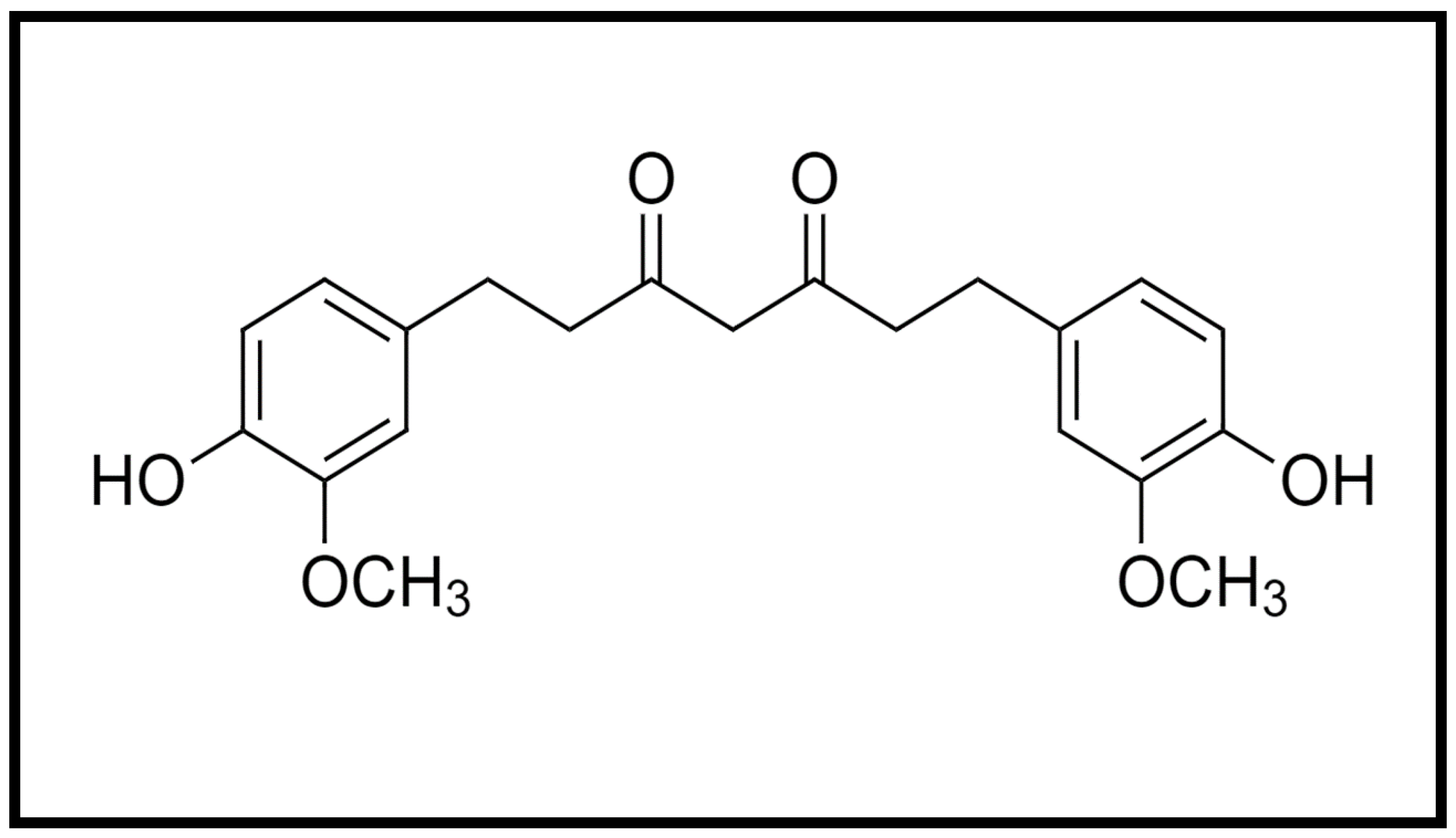
Curcumin is a hydrophobic molecule known as diferuloylmethane, with the chemical formula C21H20O6 and a molecular weight of 368.38 (Figure 1). Three reactive functional groups have been determined in curcumin: one diketone moiety and two phenolic groups. Among the biological and chemical reactions of curcumin are hydrogen donation reactions leading to oxidation of curcumin, reversible and irreversible nucleophilic addition reactions, hydrolysis, degradation and enzymatic reactions [4].
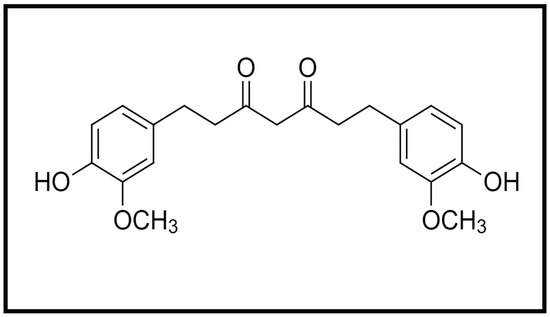
Figure 1.
Chemical structure of curcumin.
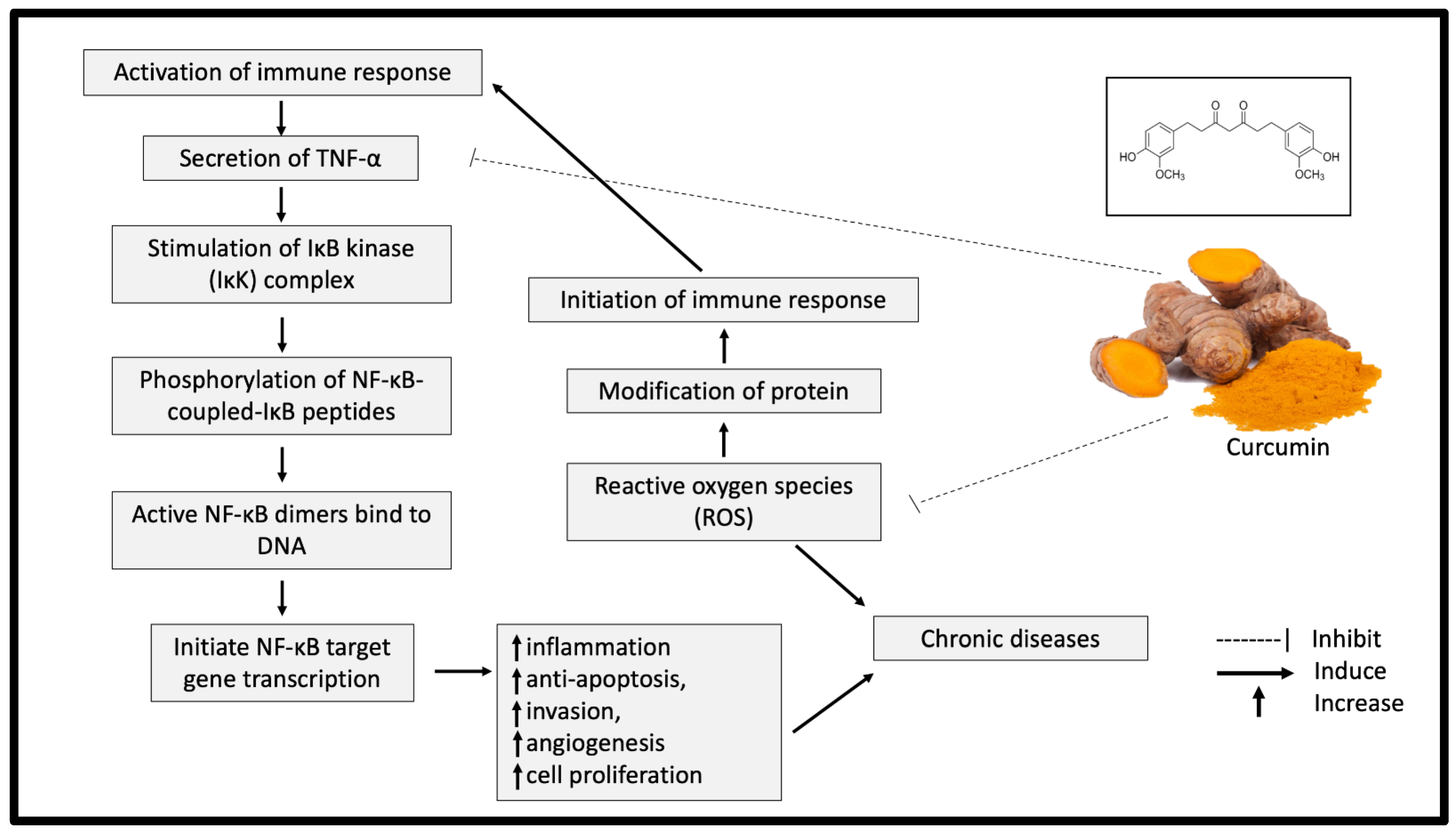
Curcumin exhibits significant antioxidant properties by breaking the chain reaction of free radical production. The ability of curcumin to capture hydrogen peroxide is also higher compared with the same concentration (20 mM) of commercial antioxidants, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) and vitamin E [14]. A meta-analysis study further supported this finding by indicating a significant reduction of oxidative stress markers, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH) and lipid peroxides after curcumin supplementation [15]. In addition, curcumin reduced the protein responses involved in inflammation processes, such as those of tumour necrosis factor alpha (TNF-α), interleukin-1 (IL-1), IL-2, IL-6, IL-8 and IL-12 [16]. TNF-α is a significant mediator of inflammation, which eventually leads to chronic diseases [17]. The presence of TNF-α activates nuclear factor (NF)-κB and amplifies the inflammatory responses [18]. Curcumin has been shown to inhibit the activation of TNF-α in the NF-κB pathway and neutralise the reactive oxygen species (ROS), causing oxidative stress (Figure 2) [18]. As oxidative stress and inflammation are implicated in most chronic diseases, curcumin supplementation could significantly offer various health benefits.
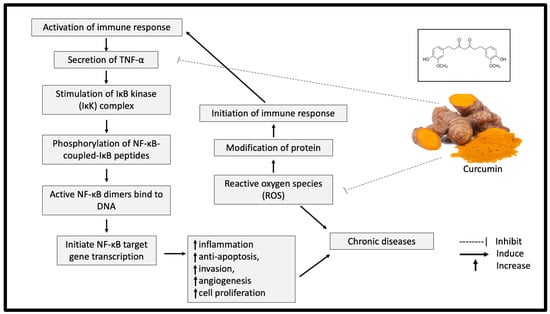
Figure 2.
Potential inhibitory effect of curcumin on inflammation and oxidative stress. TNF-α: tumor necrosis factor alpha; NF-κB: nuclear factor kappa B; IKK: IκB kinase.
Owing to its therapeutic potentials, various curcumin products have become available in the market in the form of tablets, capsules, drinks, ointments and cosmetics [19]. The broad use of curcumin in India, Japan, Thailand, China, Korea, Malaysia, Pakistan and the United States raises the safety level of curcumin consumption [20]. As a result, the US Food and Drug Administration (US FDA) has labelled curcumin as a “Generally Recognised As Safe” (GRAS) product [19]. Furthermore, the Joint United Nations and World Health Organization Expert Committee on Food Additives (JECFA) and European Food Safety Authority (EFSA) recommended the daily intake of 0–3 mg/kg body weight of curcumin [21]. In addition, curcumin supplementation in several clinical trials demonstrated good tolerability and safety profiles at doses between 4000 and 8000 mg/day [22].
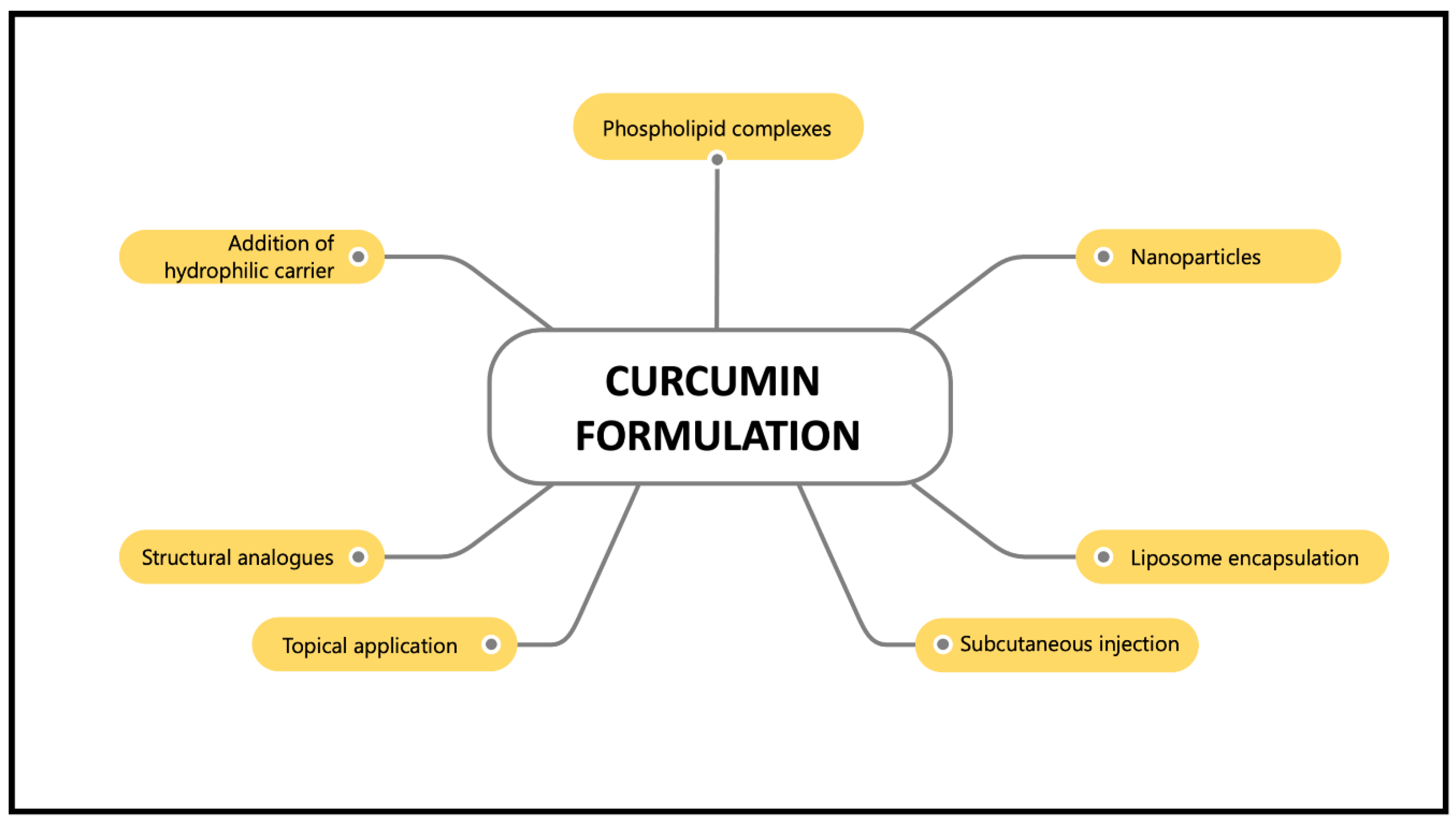
However, the main drawback of curcumin supplementation is its poor bioavailability, which is characterised by its poor absorption, rapid metabolism and rapid elimination [23]. Normally, curcumin is metabolised in the liver and intestines [24]. It is converted into water-soluble metabolites (glucuronides and sulphates) and excreted in the urine [23]. Oral administration of curcumin in rats showed 40% excretion in the faeces [25]. Thus, various curcumin nanoformulations, such as nanoparticles, liposome encapsulation, phospholipid complexes, structural analogues, subcutaneous injection, topical application and hydrophilic carrier addition have been introduced to maximise their bioavailability and activity and preventing curcumin from hydrolysis inactivation (Figure 3). These nanoformulations work by improving curcumin particle size, surface area, surface charge, hydrophobicity, as well as enhancing small intestine permeation [26]. Furthermore, curcumin nanoformulations have been reported to increase absorption more than 100-fold compared to the unformulated curcumin [27]. For example, curcumin has been formulated in a nano biocompatible form (nanocurcumin) to increase its water solubility and bioavailability. Co-administration of curcumin with piperine, an alkaloid in black pepper, enhances curcumin’s bioavailability by up to 2000% [28]. A synthesised nanoparticle form of curcumin, theracurmin, showed satisfactory plasma concentration after a single dose in healthy controls and cancer patients [29]. In addition, the encapsulation of curcumin in liposomes demonstrated inhibitory effects on endometrial cancer by inducing cell apoptosis, inhibiting cell proliferation and inhibiting invasive and metastatic bioactivities [30]. Due to the variety of effective formulations available nowadays, problems regarding the poor bioavailability of curcumin should no longer be the primary concern. Hence, these curcumin nanoformulations have made a significant contribution to the pharmaceutical industry and have been demonstrated to be beneficial in treating various human diseases [26].
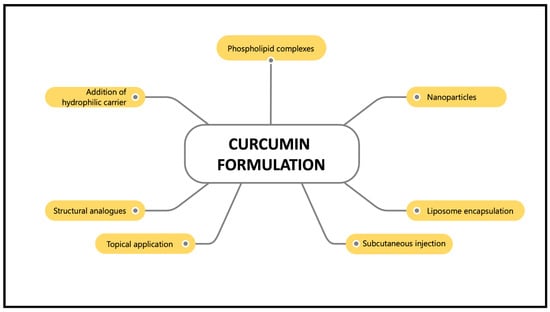
Figure 3.
Various curcumin formulations that enhance its bioavailability.
Reviews on the therapeutic potentials of curcumin on female reproductive disorders, including polycystic ovary syndrome (PCOS), ovarian failure and endometriosis, remain scarce. Therefore, this review aims to summarise the potential health benefits of curcumin on female reproductive disorders.
2. Methods of Review
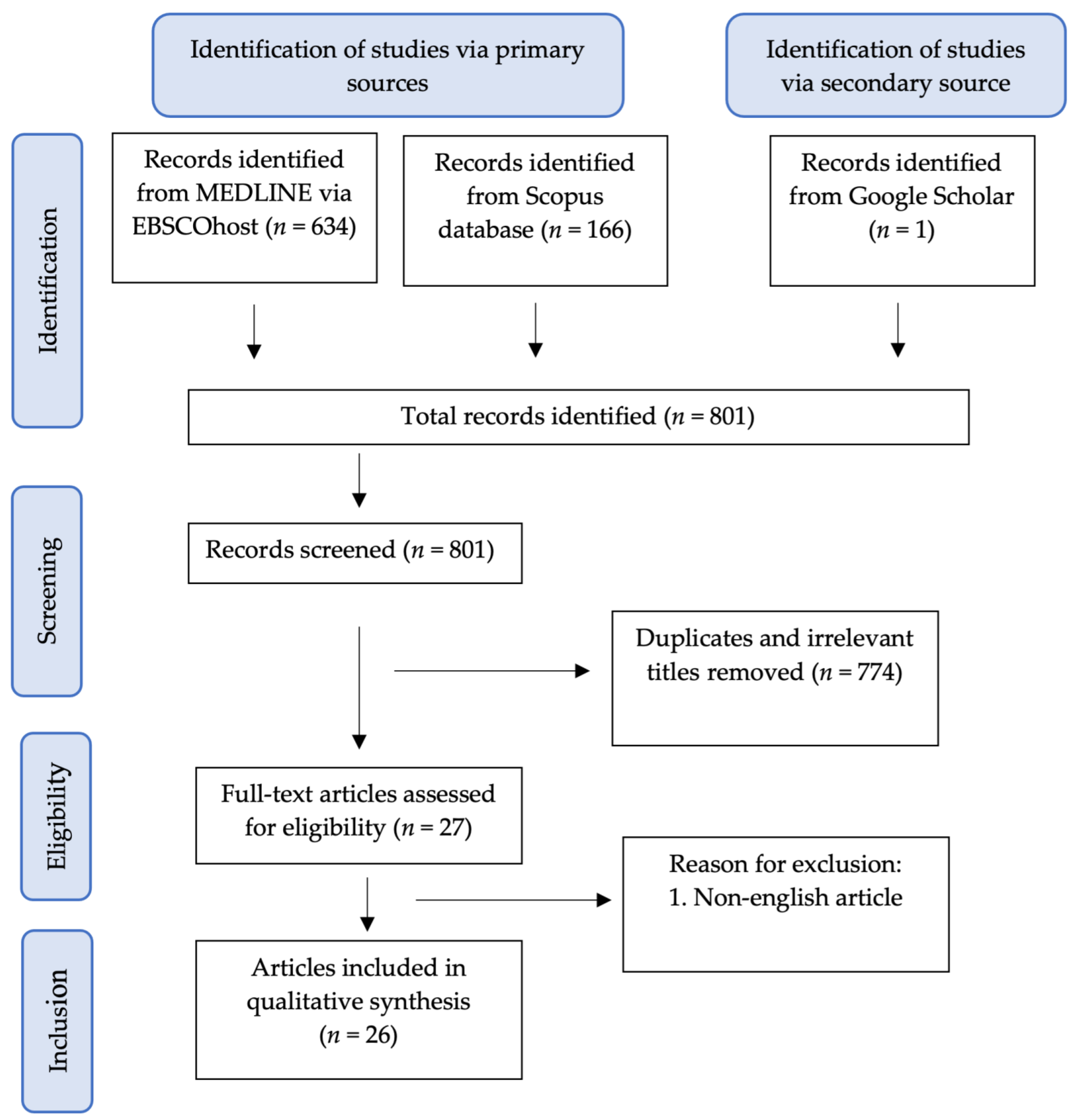
A literature search was undertaken to identify relevant articles related to the therapeutic potentials of curcumin in female reproductive disorders. Peer-reviewed and full-text English articles published from 2000 to March 2021 were gathered in electronic databases, including MEDLINE via EBSCOhost, Scopus and Google Scholar. The following keywords were used: (1) Curcumin and (2) PCOS or Endometriosis or Ovarian Disease. Only studies involving curcumin treatment on PCOS, endometriosis and ovarian disease were included for this review. The literature search was summarised in the flowchart in Figure 4.
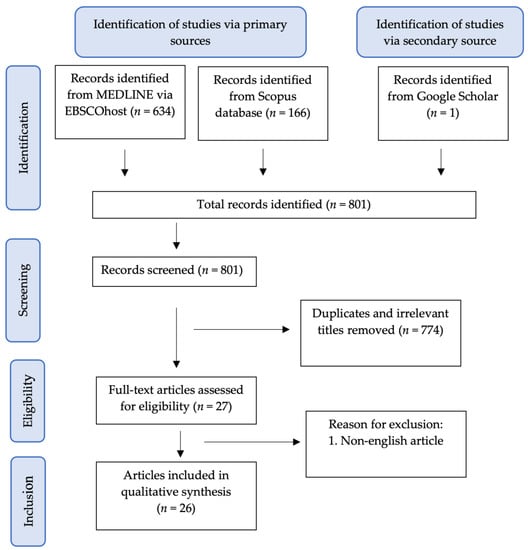
Figure 4.
Flowchart of the article selection process.
The selection of articles involved two phases. In the first phase, the titles and abstracts were screened, and any articles that did not match the inclusion criteria were excluded. In the second phase, the remaining articles were retrieved and screened thoroughly by all authors. Any differences in opinion were resolved by the discussion between the authors.
The following data were recorded from the studies: the type and age of used samples, the treatment given to the subjects, the type of analysed parameters and the method of analysis, and the results and conclusion of the studies.
3. Effect of Curcumin on Polycystic Ovary Syndrome (PCOS)
PCOS is a complex disease; it is a combination of endocrine, reproductive, metabolic and psychological disorders [31]. PCOS affects around 6–20% of women’s reproductive age, depending on the diagnostic criteria [32]. The pathophysiology of PCOS has not been fully clarified, and currently, only symptomatic treatments have been administered to patients with PCOS [33,34]. Numerous herbal remedies have been tested on women with PCOS and PCOS-induced animal models [35,36,37]. These remedies include green tea, welsh onion root and Chinese herbal medicine [38,39,40]. Curcumin has been studied for its potential in PCOS treatment. We found four clinical trials (Table 1) and five in vivo studies (Table 2) that investigated the effects of curcumin on women with PCOS and PCOS-induced animals. All these studies reported different degrees of curcumin benefits to PCOS. However, no adverse effect has been reported.

Table 1.
Summary of the effects of curcumin on PCOS in humans.

Table 2.
Summary of the effects of curcumin on PCOS in animals.
One of the main features of PCOS is the elevated level of androgens [50]. Curcumin has been shown to reduce the high level of androgen in PCOS in three studies. A randomised controlled clinical trial involving 67 women with PCOS treated with 500 mg curcumin powder for 12 weeks three times daily showed that the level of dehydroepiandrosterone was decreased compared with the placebo group [42]. Meanwhile, two animal studies reported that the elevated testosterone level in PCOS-induced rats was reduced after 100 and 200 mg/kg curcumin and 50 and 100 mg/kg nano curcumin treatments [47,49]. Apart from disrupted androgen levels, PCOS also affected women’s LH/FSH ratio balance [51]. However, a randomised controlled clinical trial showed that curcumin has no effects on LH, FSH and oestrogen levels in PCOS women [42]. According to the researchers, these might be due to the different health statuses of study participants and different doses and treatment durations used in different studies [42]. In animal studies, 50 mg/kg nano curcumin and 200 mg/kg curcumin treatment reversed the reduction in oestradiol and progesterone levels in PCOS-induced rats [47,49]. Curcumin has oestrogenic properties, which might explain its effects on hormone levels [52].
Insulin resistance is one of the pathophysiologies of PCOS [53]. Nearly half of the women diagnosed with PCOS have insulin resistance, which causes hyperinsulinemia [50]. Curcumin has various effects on insulin levels and insulin resistance in women with PCOS. Three clinical trials investigated the effect of curcumin on insulin resistance in women with PCOS. However, only one study proved the benefit of curcumin compared with placebo. In a randomised control trial, women with PCOS were administered with 500 mg/day curcumin for 12 weeks, which reduced the serum insulin, homeostatic model assessment of insulin resistance (HOMA-IR) and fasting glucose levels, as well as increased quantitative insulin sensitivity check index (QUICKI) [41]. Another randomised trial on women with PCOS who were given curcumin at 500 mg three times daily for 12 weeks only showed a reduction in fasting blood glucose, but no effect was found on fasting insulin, HOMA-IR and QUICKI [42]. A randomised study involving overweight or obese women with PCOS reported that administration with 500 mg curcumin twice daily decreased serum insulin level and increased QUICKI after curcumin administration for six weeks. However, comparing the curcumin-treated and placebo groups, no difference was found in serum insulin and QUICKI [43]. The inconsistent findings of curcumin effects on insulin and glucose levels in PCOS women might be due to the participants’ varying ages, health statuses and different inclusion criteria. For example, one study included impaired glucose tolerance as an inclusion criterion [42], while another excluded diabetic participants [43]. Hence, more studies should be conducted to clarify the discrepancy in curcumin’s effect on insulin resistance in PCOS. Meanwhile, a study on PCOS-induced rats reported that treatment with 50, 100 and 200 mg/kg nano curcumin increased the expression of pancreatic PI3K/AKT/mTOR protein levels, which are the pathways reportedly associated with defective insulin level and insulin resistance [47,54]. This finding might explain the possible mechanism of curcumin on altering the insulin pathway.
Obesity is a risk factor for the development of PCOS [55,56]. It is estimated that 38–88% of women with PCOS are overweight or obese [43]. A modest weight loss of 5–10% results in clinically meaningful improvements in PCOS features [55]. In two clinical trials, Jamilian et al. [41] found a significant reduction in body weight and BMI in curcumin-treated women with PCOS, but Heshmati et al. [42] found no changes in BMI and waist circumference. In both trials, women with PCOS of almost the same range of BMI were involved; the trials lasted for 12 weeks, and the same amount of curcumin was used (500 mg). However, Heshmati administered curcumin three times a day, whereas Jamilian et al. only administered curcumin once a day. Thus, a disparity exists in the weight-loss potential, implying that more research is needed.
Curcumin has been tested for its lipid profile improvement properties in animals and humans. In a clinical trial, 500 mg/day curcumin reduced total cholesterol, low-density lipoprotein (LDL)-cholesterol and total-/HDL cholesterol ratio and increased high-density lipoprotein (HDL)-cholesterol levels in women with PCOS [52]. However, another clinical trial did not find any difference in cholesterol, triglyceride, LDL and HDL levels after curcumin treatment in women with PCOS [43]. In two studies that used PCOS-induced rats, 50 mg/kg of nano curcumin and 100 mg/kg of curcumin improved triglyceride, total cholesterol and LDL and HDL cholesterol levels [47,49]. Further studies into curcumin mechanism of action in lowering lipid might explain the discrepancies that exist.
The therapeutic potentials of curcumin are attributed to its anti-inflammatory properties [57]. In two animal studies, 100, 200, 300 and 400 mg/kg curcumin treatment on PCOS-induced rats reduced interleukin-6 and C-reactive protein [45,48]. Nanocurcumin treatment also decreased TNF-α levels in PCOS-induced rats [44]. However, in a clinical trial on women with PCOS, curcumin treatment did not affect high-sensitivity C-reactive protein (hs-CRP) [43].
PCOS is the leading cause of anovulatory infertility [31]. Curcumin has the potential of improving ovarian function in PCOS, as shown in animal studies. In a study by Abuelezz et al., treatment with nano curcumin showed thickened granulosa cells and oocyte appearance in PCOS-induced rats [47]. Another study reported a significant reduction in thickness of theca layer and increased corpus luteum diameter in the curcumin-treated group compared with the PCOS group [48]. Moreover, curcumin treatment displayed results comparable with those of clomiphene citrate, the first-line therapy for anovulatory PCOS, which resulted in the disappearance of cysts and the appearance of healthy follicles and corpora lutea [49]. However, another study found that 5.4 mg/100 g curcumin in nanoparticle form reduced the total number of primary, secondary, antral and primordial follicles compared with the PCOS rat group [46]. These contradictory discoveries of curcumin’s effects on ovarian follicles are expected due to different methods used in inducing PCOS animal models as well as the different forms of curcumin used.
Oxidative stress is involved in the pathophysiology of PCOS [58]. A systematic review and meta-analysis of oxidative stress markers involving 4933 women with PCOS and 3671 controls found homocysteine, malondialdehyde (MDA), dimethylarginine, SOD, glutathione and araoxonase-1 levels were abnormal in women with PCOS [59]. Curcumin possesses excellent antioxidant potential [60,61]. A randomised controlled clinical trial on 72 women with PCOS reported that supplementation 1500 mg curcumin three times daily for three months resulted in the increased activity of the GSH enzyme and peroxisome proliferator-activated receptor γ coactivator 1α [44]. However, the same study did not find any difference in gene expression of sirtuin-1 and the activity of the SOD enzyme [44]. In animal studies, nano curcumin at 50, 100 and 200 mg/kg doses decreased the MDA level and increased GSH and SOD activities in PCOS-induced rats [47]. Another study in PCOS induced rats showed that 100 and 200 mg/kg curcumin increased SOD and CAT activities, whereas the TBARS level decreased and the GSH level increased only at 200 mg/kg curcumin [49].
Curcumin has been shown to inhibit apoptosis in some diseases while promoting it in others [62,63,64]. Nanoparticle curcumin treatment reportedly decreased Bcl-2-associated X protein (BAX) and caspase3 (CASP3) protein expression and increased the levels of B-cell lymphoma 2 (Bcl2) expression. This study also showed reduced apoptosis in the granulosa cells of PCOS-induced rats after curcumin treatment [46].
Many discrepancies remain in the effect of curcumin on PCOS, especially in clinical trials. However, the animal and human studies discussed above have provided an excellent foundation for curcumin’s potentials as PCOS complementary treatment; thus, further investigation is warranted to endorse the use of curcumin in the future.
4. Effect of Curcumin on Ovarian Diseases
Curcumin has beneficial effects on ovary-related diseases that could be achieved through its anti-inflammation, anti-apoptosis and antioxidant properties. Table 3 outlines the effects of curcumin on ovarian disorders. In one study, mice with D-galactose induced premature ovarian failure were treated with 100 mg/kg/day curcumin intraperitoneally for 42 days [65]. In this study, curcumin has been shown to inhibit d-galactose-induced oxidative stress, apoptosis and ovarian injury. Increased SOD and decreased MDA levels, as well as reduced SOD2 and CAT mRNA expression levels, were found in the curcumin-treated group. In addition, this study also showed that curcumin increases NF-E2-related factor-2 (Nrf2) and HO-1 protein expression levels, which are the proteins involved in the mechanism of ROS removal [65]. Nrf2 can bind to antioxidant response elements (AREs) in the promoter region of Nrf2 target genes, which used HO-1 sequential enzymatic processes to eliminate ROS [66].

Table 3.
Summary of the effects of curcumin on ovarian diseases.
In another study, a rat model of ovarian ischemia–reperfusion injury was administered with 200 mg/kg curcumin intraperitoneally, simultaneously with reperfusion. It was found that curcumin administration did not alter nitric oxide (NO), NO synthase (NOS), xanthine oxidase (XO), total antioxidant status (TAS) and total oxidant status (TOS) [68]. Interestingly, in another rat model of ovarian ischemia–reperfusion injury, 1 mg/kg nano curcumin improved the oxidative status parameters but not 100 mg/kg. These results included significantly higher values of superoxide dismutase, total glutathione, GSH, glutathione reductase and glutathione S-transferase and substantially lower values of nitric oxide synthase, MDA, myeloperoxidase and 8-hydroxy-2 deoxyguanine levels [69]. The findings from these two studies showed that nanocurcumin at very low concentrations (1 mg/kg) produced significant improvements compared to the native curcumin (100 mg/kg and 200 mg/kg curcumin), which might be attributed to its high bioavailability and longer half-life.
In a more specific oxidative stress study, Kunming mice were injected with 8 mg/kg sodium arsenite to induced ovarian oxidative stress and treated with 100, 150 or 200 mg/kg curcumin once per day for 21 days. Curcumin reversed the disturbance in oxidative stress parameters (ROS, MDA and SOD, except for GSH) induced by sodium arsenite. Furthermore, in this study, p66Shc expression, upregulated under oxidative stress, was significantly lowered by curcumin treatment [71]. The p66Shc protein reportedly involves signalling pathways that regulate the cellular response to oxidative stress and cell lifespan. Thus, when the expression of p66Shc is upregulated, the generation of ROS increases and the oxidative damage to cells becomes severe [72].
Curcumin improves the overall function of the ovary in ovarian diseases. Curcumin administration at 100 mg/kg/day to premature ovarian failure mice increased the number of primordial follicles and increased AMH expression levels, which reflected the size of the ovarian follicle pool [65,73]. Meanwhile, administration of curcumin at 100, 150 and 200 mg/kg once per day reduced the sodium arsenite-induced increment in the atretic follicle [71]. In the mice model of autoimmune disease of the ovaries, 100 μg/g curcumin treatment four times a week alleviated the reduced level of oocytes in metaphases I and II [70].
The anti-apoptotic effect of curcumin on the ovary has been reported in several studies. Curcumin (100 mg/kg/day) administration reduced apoptosis of granulosa cells and reduced cleaved caspase-3 and -9 protein expression levels in mice with D-galactose-induced premature ovarian failure [65]. An in vitro study on porcine ovarian granulosa cells demonstrated that curcumin reportedly reduced PCNA and its mRNA, as well as increased both Bax and its mRNA and reduced cell viability [67]. However, in the mice model of autoimmune ovary disease, 100 μg/g curcumin did not alter the number of apoptotic cells in the thymus, spleen and lymph node but reduced the necrosis in these cells [70].
5. Effect of Curcumin on Endometriosis
Endometriosis is a chronic gynaecological disorder representing the implantation of endometrial glands and stroma outside the uterine cavity [74]. It affects adolescents and reproductive-aged women and is commonly associated with infertility, dyspareunia, dysmenorrhea and chronic pelvic pain [75]. The pathogenesis of endometriosis has not been fully understood. To date, nearly all current treatment options for endometriosis suppress endometrial function and are not curative. Combined oral contraceptives and progestins are commonly prescribed as first-line therapy to alleviate pain symptoms. However, if the first-line therapies are ineffective, contraindicated or not tolerated, gonadotropin-releasing hormone-agonists are prescribed. In case of resistance to other treatments, an aromatase inhibitor is prescribed [76]. Presently, curcumin was found to have anti-endometriosis, antioxidant and anti-inflammatory properties [77].
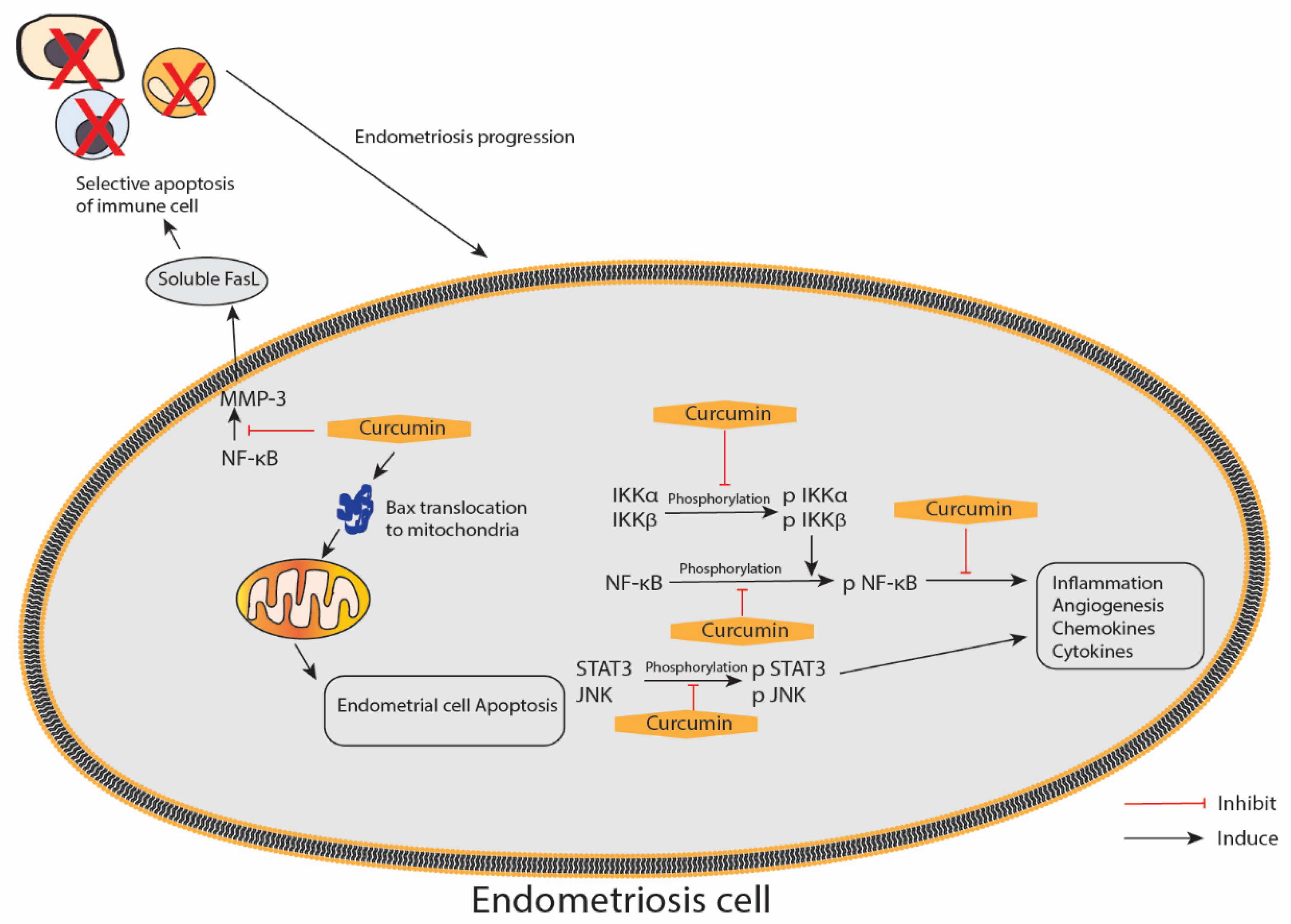
In this review, we found six studies that utilised the animal model of endometriosis, which involved the transplantation of endometrium derived from the animal endometrium, and five in vitro studies that explored the effect of curcumin against endometriosis (Table 4: animal (in vivo) studies; Table 5: in vitro studies). All these studies reported different degrees of curcumin’s advantage on endometriosis in terms of therapeutic potential. In addition, the studies reported the positive effects of curcumin on the endometrium, particularly those exerted through anti-inflammatory, anti-proliferative, anti-angiogenic and pro-apoptotic mechanisms. Figure 5 illustrates the proposed effect of curcumin on the inflammatory and apoptotic pathways in endometriosis.

Table 4.
Summary of the effects of curcumin on endometriosis in animals.

Table 5.
Summary of the effects of curcumin on endometriosis in in vitro studies.
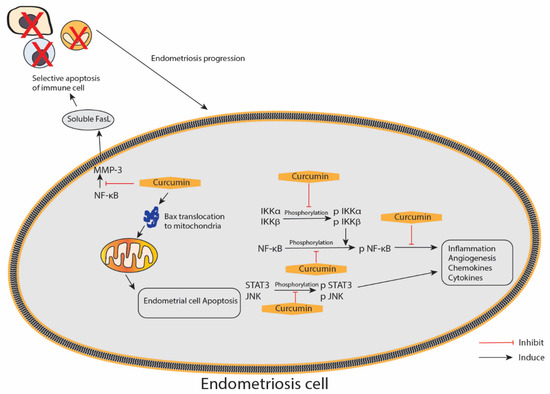
Figure 5.
An illustration of the proposed curcumin effect on the inflammatory and apoptotic pathways in endometriosis. IKKα/β—inhibitor of nuclear factor κ-B kinase subunit α/β; JNK—c-Jun N-terminal kinases; NF-κB—nuclear factor κ-light-chain-enhancer of activated B; STAT3—signal transducer and activator of transcription 3; p—phosphorylated form; MMP-3—matrix metalloproteinase 3; FasL—Fas ligands; arrow represents a promotion, and blunt arrow represents inhibition.
The anti-inflammatory effects of curcumin are mediated through interference with the expression or activation of multiple key signalling molecules, including nuclear factor-κB [87]. In a study conducted by Kim et al., curcumin effectively suppressed ICAM-1 and VCAM-1 gene and protein expressions and the secretions of IL-6, IL-8 and MCP-1 by inhibiting the activation of NF-κB induced by TNF-α, which is a central proinflammatory cytokine in the endometriotic disease process, in human ectopic human endometriotic stromal cells [82]. Furthermore, CAM-1 and VCAM-1 protein expressions in TNF-α-activated endometriotic stromal cells after curcumin treatment measured by immunofluorescence microscopic and Western blot analyses were in accordance with the ICAM-1 and VCAM-1 expression patterns, as shown by qRT-PCR analysis, thereby indicating that both cell surface and cytoplasmic proteins, as well as the mRNA expressions of ICAM-1 and VCAM-1 in endometriotic stromal cells induced by TNF-α, were inhibited by curcumin [82].
Eutopic endometrial cells function differently in women with endometriosis compared with disease-free women with normal endometrium. The cells are resistant to apoptosis and have other selective advantages for survival outside the uterine cavity, leading to their implantation and invasion of the peritoneum and other ectopic sites [88]. A study conducted by Chowdhury et al. reported increased chemokines and cytokines produced in eutopic endometrial tissue from women with endometriosis might increase angiogenesis and proliferation. The study used a primary cell culture of human endometriotic stromal cells from women with endometriosis (EESCs) and normal endometrial stromal cells (NESCs) as subjects, which were then treated with curcumin at different doses of 1, 5, 10, 20 and 40 μg/mL for 24, 48 and 72 h. The various secretions of chemokines and cytokines, i.e., IL-6, IL-8, IP-10, G-CSF, MCP-1 and RANTES, were highly expressed in EESCs. However, IL-10 and IL-12 expression levels did not differ between EESCs and NESCs. After the treatment with curcumin, a significant inhibition towards the secretion of IL-6, IL-8, IP-10, G-CSF, MCP-1 and RANTES in EESCs after 48 h was observed. In contrast, curcumin significantly promoted IL-10 and IL-12 secretions in EESCs after 48 h. IL-17 was utterly absent in the media after treatment with curcumin for 24 h. The Western blot result showed that curcumin inhibited the phosphorylation of IKKα, IKKβ and NF-κB in EESCs. Moreover, it significantly inhibited the phosphorylation of JNK and STAT3 in EESCs. JNK expression also significantly decreased after curcumin treatment. Hence, curcumin has therapeutic potential and nullifies the abnormal activation of chemokines and cytokines, IKKα/β, NF-κB, STAT3 and JNK signalling pathways to reduce inflammation, which is associated with endometriosis [83].
Angiogenesis is essential in the pathogenesis of endometriosis, as it establishes a new blood supply critical for developing endometriotic lesions [89]. VEGF is a signalling protein produced by cells; it stimulates angiogenesis, vasculogenesis and vascular permeability [90]. McLaren et al. demonstrated that the VEGF levels increased in the peritoneal fluid of patients with endometriosis [91]. Angiogenesis is essential in developing endometriosis and is regulated by a variety of pro-angiogenic genes and signalling molecules, including VEGF. Curcumin exhibited an anti-angiogenic effect in two studies, namely, one animal study and one in vitro study. Hong Cao et al. reported that after the treatment with 20 and 50 μmol/L curcumin in vitro, the proportion of VEGF positive expression decreased compared with the 0 μmol/L groups, and accordingly, the fluorescence intensities of VEGF staining decreased [86]. Another study reported that eutopic and ectopic endometrium expression of VEGF protein in the model rat group was higher than that in the normal group, according to the results of Western blot analysis. VEGF protein expression in the ectopic endometrium of the EMS rats was also higher than in the eutopic endometrium. After curcumin treatment, VEGF protein expression of the ectopic endometrium decreased with increasing doses of curcumin (50, 100 and 150 mg) [79].
Apoptosis is the process of programmed cell death that occurs in multicellular organisms [92,93]. Curcumin may be used in cancer treatment due to its ability to induce apoptosis and inhibit angiogenesis [66,94]. However, the role of curcumin in human endometriotic and endometrial stromal cells remains unclear. Therefore, the study by Cao et al. aimed to determine the apoptotic potential of curcumin in human endometriosis. Treatment with 50 μmol/l curcumin resulted in 4.7% early apoptosis and 28.4% late apoptosis in endometriotic stromal cells, as well as 2.8% early apoptosis and 21.4% late apoptosis in endometrial stromal cells. The data showed that curcumin reduced cell survival in human endometriotic and endometrial stromal cells in vitro [86]. A study by Jana et al. reported that curcumin treatment at 12, 24 and 48mg/kg/daily for three days induced Cyt-c release and caspase-9 expression, suggesting the involvement of mitochondrial pathway in stimulating apoptosis [72]. Curcumin also increased the size of mitochondria, decreased the expression of Cyt-c and increased the expression of Bax within the mitochondria. They also found that curcumin regresses endometriosis via mitochondria-mediated apoptosis by p53-dependent and -independent pathways [74]. Hence, curcumin has been shown to exhibit the pro-apoptotic property in endometriosis.
Although the exact mechanism underlying the development of endometriosis remains unclear, evidence suggests the crucial role of oestrogen in establishing and regulating this disease [95]. Oestradiol (E2) is an essential promoter of the growth of both eutopic and ectopic endometrium. The primary source of E2 is the ovary, and E2 was recently found to be an effective regulator of endometriosis. An in vitro study conducted by Ying Zhang et al. utilised primary cell culture of endometriotic stromal cells, normal endometrial stromal cells, endometriotic epithelial cells and normal endometrial epithelial cells; results revealed that the E2 value of the endometriotic epithelial cells was higher than that of the endometriotic stromal cells. However, the expression of E2 in normal endometrial stromal and epithelial cells was extremely low. WST-8 result showed that ectopic endometriotic stromal cells had a higher growth rate than endometrial stromal cells. After curcumin treatment (10, 30 and 50 μM), the number of endometriotic stromal cells was reduced, and cell growth slowed compared with the 0 μmol/L groups. E2 level was lower after treatment with curcumin, especially in the 30 and 50 μmol/L groups compared with the 0 μmol/L groups. This study showed that curcumin could inhibit the proliferation of endometrial cells by reducing the E2 value [85].
Thus, the available evidence showed that curcumin exerts a beneficial effect against endometriosis. However, further studies are required to fully elucidate the molecular mechanisms underlying the effects of curcumin and investigate its potential to treat endometriosis in humans.
6. Conclusions
Curcumin has many different effects on PCOS, ovarian diseases and endometriosis. Some studies found considerable therapeutic effects, whereas others found no effect. However, none of the investigations found curcumin to be harmful. Curcumin has been evaluated in clinical trials involving women with PCOS. The results of these trials may serve as the foundation for further exploration of the curcumin potential as a complementary therapy to the existing treatment plan. Additionally, there is a lack of human clinical trials to validate the effects of curcumin on endometriosis and ovarian diseases; hence, it warrants further investigation. Meanwhile, research on curcumin has grown extensively with various curcumin supplements that have become available in the market in the form of tablets, capsules and drinks. However, the use of curcumin supplements in the prevention and complementary treatment of female reproductive diseases is still limited. Thus, future studies should be conducted to assess its efficacy in managing female reproductive diseases and prove the safety of these supplements.
Author Contributions
Conceptualisation, D.A.M.K., N.S., A.N.M.Y., M.I.A.M.K. and M.H.M.; methodology, M.I.A.M.K., M.H.M. and D.A.M.K.; writing—original draft preparation, D.A.M.K., N.S. and A.N.M.Y.; illustration, D.A.M.K., N.S. and A.N.M.Y.; writing—review and editing, M.H.M. and M.I.A.M.K.; supervision, M.H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universiti Kebangsaan Malaysia (Faculty of Medicine Fundamental Grant; grant number FF-2020-241).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dosoky, N.S.; Setzer, W.N. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Aggarwal, B.B. Turmeric, the Golden Spice: From Traditional Medicine to Modern Medicine. In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group, LLC.: Boca Raton, FL, USA, 2011. [Google Scholar]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akram, M.; Afzal, A.; Khan, U.; Abdul, H.; Mohiuddin, E.; Asif, M. Curcuma longa and Curcumin: A review article. Rom. J. Biol.-Plant Biol. 2010, 55, 65–70. [Google Scholar]
- Tabrizi, R.; Vakili, S.; Akbari, M.; Mirhosseini, N.; Lankarani, K.B.; Rahimi, M.; Mobini, M.; Jafarnejad, S.; Vahedpoor, Z.; Asemi, Z. The effects of curcumin-containing supplements on biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 253–262. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Balaguer, M.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Improving antioxidant and antimicrobial properties of curcumin by means of encapsulation in gelatin through electrohydrodynamic atomization. Food Hydrocoll. 2017, 70, 313–320. [Google Scholar] [CrossRef]
- Amalraj, A.; Varma, K.; Jacob, J.; Divya, C.; Kunnumakkara, A.B.; Stohs, S.J.; Gopi, S. A Novel Highly Bioavailable Curcumin Formulation Improves Symptoms and Diagnostic Indicators in Rheumatoid Arthritis Patients: A Randomized, Double-Blind, Placebo-Controlled, Two-Dose, Three-Arm, and Parallel-Group Study. J. Med. Food 2017, 20, 1022–1030. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, S.; Zhou, L.; Yu, F.; Ding, H.; Li, P.; Zhou, M.; Wang, K. Potential Mechanisms of Action of Curcumin for Cancer Prevention: Focus on Cellular Signaling Pathways and miRNAs. Int. J. Biol. Sci. 2019, 15, 1200–1214. [Google Scholar] [CrossRef] [Green Version]
- Ng, Z.Y.; Wong, J.Y.; Panneerselvam, J.; Madheswaran, T.; Kumar, P.; Pillay, V.; Hsu, A.; Hansbro, N.; Bebawy, M.; Wark, P.; et al. Assessing the potential of liposomes loaded with curcumin as a therapeutic intervention in asthma. Colloids Surf. B Biointerfaces 2018, 172, 51–59. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Farha, A.K.; Kim, G.; Gul, K.; Gan, R.-Y.; Corke, H. Antimicrobial and anticancer applications and related mechanisms of curcumin-mediated photodynamic treatments. Trends Food Sci. Technol. 2020, 97, 341–354. [Google Scholar] [CrossRef]
- Song, L.; Zhang, F.; Yu, J.; Wei, C.; Han, Q.; Meng, X. Antifungal effect and possible mechanism of curcumin mediated photodynamic technology against Penicillium expansum. Postharvest Biol. Technol. 2020, 167, 111234. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Mudnakudu-Nagaraju, K.K.; Shivamallu, C.; Subramaniam, K.J.T.; Radhakrishnan, A.; Bhojraj, S.; Kuppusamy, G. Antiviral and immunomodulatory activity of curcumin: A case for prophylactic therapy for COVID-19. Heliyon 2021, 7, e06350. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Serban, M.-C.; Ursoniu, S.; Banach, M. Effect of curcuminoids on oxidative stress: A systematic review and meta-analysis of randomized controlled trials. J. Funct. Foods 2015, 18, 898–909. [Google Scholar] [CrossRef]
- Anthwal, A.; Thakur, B.K.; Rawat, M.S.; Rawat, D.S.; Tyagi, A.K.; Aggarwal, B.B. Synthesis, characterization and in vitro anticancer activity of C-5 curcumin analogues with potential to inhibit TNF-α-induced NF-κB activation. BioMed Res. Int. 2014, 2014, 524161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathore, S.; Siddiqui, M.; Sharma, P.; Devi, S.; Nagar, J.; Khalid, M. Curcumin: A Review for Health Benefits. Int. J. Sci. Res. (IJSR) 2020, 7, 273–290. [Google Scholar]
- González-Ramos, R.; Van Langendonckt, A.; Defrère, S.; Lousse, J.C.; Colette, S.; Devoto, L.; Donnez, J. Involvement of the nuclear factor-κB pathway in the pathogenesis of endometriosis. Fertil. Steril. 2010, 94, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [Green Version]
- Vallée, A.; Lecarpentier, Y. Curcumin and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in cancer chemoprevention: Molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch. Pharm. 2010, 343, 489–499. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanai, M.; Imaizumi, A.; Otsuka, Y.; Sasaki, H.; Hashiguchi, M.; Tsujiko, K.; Matsumoto, S.; Ishiguro, H.; Chiba, T. Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother. Pharmacol. 2012, 69, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Gong, Z.; Zhou, S.; Yang, S.; Wang, D.; Chen, X.; Wu, J.; Liu, L.; Zhong, S.; Zhao, J.; et al. Liposomal Curcumin Targeting Endometrial Cancer Through the NF-κB Pathway. Cell. Physiol. Biochem. 2018, 48, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270. [Google Scholar] [CrossRef]
- Khadilkar, S.S. Can Polycystic Ovarian Syndrome be cured? Unfolding the Concept of Secondary Polycystic Ovarian Syndrome! J. Obstet. Gynaecol. India 2019, 69, 297–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennett, C.C.; Simon, J. The role of polycystic ovary syndrome in reproductive and metabolic health: Overview and approaches for treatment. Diabetes Spectr. 2015, 28, 116–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, C.-Y.; Cho, I.-H.; Park, K.S. Therapeutic Effects and Mechanisms of Herbal Medicines for Treating Polycystic Ovary Syndrome: A Review. Front. Pharmacol. 2020, 11, 1192. [Google Scholar] [CrossRef]
- Arentz, S.; Smith, C.A.; Abbott, J.; Bensoussan, A. Nutritional supplements and herbal medicines for women with polycystic ovary syndrome; a systematic review and meta-analysis. BMC Complement. Altern. Med. 2017, 17, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moini Jazani, A.; Nasimi Doost Azgomi, H.; Nasimi Doost Azgomi, A.; Nasimi Doost Azgomi, R. A comprehensive review of clinical studies with herbal medicine on polycystic ovary syndrome (PCOS). Daru 2019, 27, 863–877. [Google Scholar] [CrossRef]
- Kamal, D.A.M.; Salamt, N.; Zaid, S.S.M.; Mokhtar, M.H. Beneficial Effects of Green Tea Catechins on Female Reproductive Disorders: A Review. Molecules 2021, 26, 2675. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Yang, H.; Lee, S.R.; Kwon, S.W.; Hong, E.J.; Lee, H.W. Welsh Onion Root (Allium fistulosum) Restores Ovarian Functions from Letrozole Induced-Polycystic Ovary Syndrome. Nutrients 2018, 10, 1430. [Google Scholar] [CrossRef] [Green Version]
- Ong, M.; Peng, J.; Jin, X.; Qu, X. Chinese Herbal Medicine for the Optimal Management of Polycystic Ovary Syndrome. Am. J. Chin. Med. 2017, 45, 405–422. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Foroozanfard, F.; Kavossian, E.; Aghadavod, E.; Shafabakhsh, R.; Hoseini, A.; Asemi, Z. Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 2020, 36, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Moini, A.; Sepidarkish, M.; Morvaridzadeh, M.; Salehi, M.; Palmowski, A.; Mojtahedi, M.F.; Shidfar, F. Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Phytomed. Int. J. Phytother. Phytopharm. 2021, 80, 153395. [Google Scholar] [CrossRef] [PubMed]
- Sohaei, S.; Amani, R.; Tarrahi, M.J.; Ghasemi-Tehrani, H. The effects of curcumin supplementation on glycemic status, lipid profile and hs-CRP levels in overweight/obese women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled clinical trial. Complementary Ther. Med. 2019, 47, 102201. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Golab, F.; Morvaridzadeh, M.; Potter, E.; Akbari-Fakhrabadi, M.; Farsi, F.; Tanbakooei, S.; Shidfar, F. The effects of curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor γ coactivator 1α gene expression in polycystic ovarian syndrome (PCOS) patients: A randomized placebo-controlled clinical trial. Diabetes Metab. Syndr. 2020, 14, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Karimzadeh Bardei, L.; Hojati, V.; Ghorbani, A.G.; Nabiuni, M. Anti-Inflammatory Effects of Curcumin on Insulin Resistance Index, Levels of Interleukin-6, C-Reactive Protein, and Liver Histology in Polycystic Ovary Syndrome-Induced Rats. Cell J. 2017, 19, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Fatemi Abhari, S.M.; Khanbabaei, R.; Hayati Roodbari, N.; Parivar, K.; Yaghmaei, P. Curcumin-loaded super-paramagnetic iron oxide nanoparticle affects on apoptotic factors expression and histological changes in a prepubertal mouse model of polycystic ovary syndrome-induced by dehydroepiandrosterone—A molecular and stereological study. Life Sci. 2020, 249, 117515. [Google Scholar] [CrossRef] [PubMed]
- Abuelezz, N.Z.; Shabana, M.E.; Abdel-Mageed, H.M.; Rashed, L.; Morcos, G.N.B. Nanocurcumin alleviates insulin resistance and pancreatic deficits in polycystic ovary syndrome rats: Insights on PI3K/AkT/mTOR and TNF-α modulations. Life Sci. 2020, 256, 118003. [Google Scholar] [CrossRef]
- Mohammadi, S.; Kayedpoor, P.; Karimzadeh-Bardei, L.; Nabiuni, M. The Effect of Curcumin on TNF-α, IL-6 and CRP Expression in a Model of Polycystic Ovary Syndrome as an Inflammation State. J. Reprod. Infertil. 2017, 18, 352–360. [Google Scholar] [PubMed]
- Reddy, P.S.; Begum, N.; Mutha, S.; Bakshi, V. Beneficial effect of Curcumin in Letrozole induced polycystic ovary syndrome. Asian Pac. J. Reprod. 2016, 5, 116–122. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef] [PubMed]
- Burt Solorzano, C.M.; Beller, J.P.; Abshire, M.Y.; Collins, J.S.; McCartney, C.R.; Marshall, J.C. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids 2012, 77, 332–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmeier, B.E.; Mirisola, V.; Romeo, F.; Generoso, L.; Esposito, A.; Dell’Eva, R.; Blengio, F.; Killian, P.H.; Albini, A.; Pfeffer, U. Reference Profile Correlation Reveals Estrogen-like Trancriptional Activity of Curcumin. Cell. Physiol. Biochem. 2010, 26, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wang, S.; Zhang, Z.; Lin, Q.; Liu, Y.; Xiao, Y.; Xiao, K.; Wang, Z. Defective insulin signaling and the protective effects of dimethyldiguanide during follicular development in the ovaries of polycystic ovary syndrome. Mol. Med. Rep. 2017, 16, 8164–8170. [Google Scholar] [CrossRef] [Green Version]
- Barber, T.M.; Hanson, P.; Weickert, M.O.; Franks, S. Obesity and Polycystic Ovary Syndrome: Implications for Pathogenesis and Novel Management Strategies. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119874042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCartney, C.R.; Marshall, J.C. Polycystic Ovary Syndrome. N. Engl. J. Med. 2016, 375, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Farhood, B.; Mortezaee, K.; Goradel, N.H.; Khanlarkhani, N.; Salehi, E.; Nashtaei, M.S.; Najafi, M.; Sahebkar, A. Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J. Cell. Physiol. 2019, 234, 5728–5740. [Google Scholar] [CrossRef] [PubMed]
- Khashchenko, E.; Vysokikh, M.; Uvarova, E.; Krechetova, L.; Vtorushina, V.; Ivanets, T.; Volodina, M.; Tarasova, N.; Sukhanova, I.; Sukhikh, G. Activation of Systemic Inflammation and Oxidative Stress in Adolescent Girls with Polycystic Ovary Syndrome in Combination with Metabolic Disorders and Excessive Body Weight. J. Clin. Med. 2020, 9, 1399. [Google Scholar] [CrossRef]
- Murri, M.; Luque-Ramírez, M.; Insenser, M.; Ojeda-Ojeda, M.; Escobar-Morreale, H.F. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): A systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 268–288. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant Potential of Curcumin—A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef]
- Tanvir, E.M.; Hossen, M.S.; Hossain, M.F.; Afroz, R.; Gan, S.H.; Khalil, M.I.; Karim, N. Antioxidant Properties of Popular Turmeric (Curcuma longa) Varieties from Bangladesh. J. Food Qual. 2017, 2017, 8471785. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Cao, X.; Hu, X.; Li, S.; Wang, J. The anti-apoptotic, antioxidant and anti-inflammatory effects of curcumin on acrylamide-induced neurotoxicity in rats. BMC Pharmacol. Toxicol. 2020, 21, 62. [Google Scholar] [CrossRef]
- Loganes, C.; Lega, S.; Bramuzzo, M.; Vecchi Brumatti, L.; Piscianz, E.; Valencic, E.; Tommasini, A.; Marcuzzi, A. Curcumin Anti-Apoptotic Action in a Model of Intestinal Epithelial Inflammatory Damage. Nutrients 2017, 9, 578. [Google Scholar] [CrossRef] [Green Version]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.; Dai, Y.; Fu, H.; Zheng, Y.; Bao, D.; Yin, Y.; Chen, Q.; Nie, X.; Hao, Q.; Hou, D.; et al. Curcumin exerts a protective effect against premature ovarian failure in mice. J. Mol. Endocrinol. 2018, 60, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of Cell Protection by Heme Oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [Green Version]
- Kádasi, A.; Maruniaková, N.; Štochmaľová, A.; Bauer, M.; Grossmann, R.; Harrath, A.H.; Kolesárová, A.; Sirotkin, A.V. Direct effect of curcumin on porcine ovarian cell functions. Anim. Reprod. Sci. 2017, 182, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Eser, A.; Hizli, D.; Haltas, H.; Namuslu, M.; Kosus, A.; Kosus, N.; Kafali, H. Effects of curcumin on ovarian ischemia-reperfusion injury in a rat model. Biomed. Rep. 2015, 3, 807–813. [Google Scholar] [CrossRef] [Green Version]
- Behroozi-Lak, T.; Ebrahimpour, M.; Zarei, L.; Pourjabali, M.; Farhad, N.; Mohaddesi, H. Systemic administration of curcumin nanoparticles protects ischemia-reperfusion injury in ovaries: An animal model study. Rev. Assoc. Med. Bras. 2018, 64, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Alekseyeva, I.N.; Makogon, N.V.; Bryzgina, T.M.; Voznesenskaya, T.Y.; Sukhina, V.S. Effects of NF-κB blocker curcumin on oogenesis and immunocompetent organ cells in immune ovarian injury in mice. Bull. Exp. Biol. Med. 2011, 151, 432–435. [Google Scholar] [CrossRef]
- Wang, X.N.; Zhang, C.J.; Diao, H.L.; Zhang, Y. Protective Effects of Curcumin against Sodium Arsenite-induced Ovarian Oxidative Injury in a Mouse Model. Chin. Med. J. 2017, 130, 1026–1032. [Google Scholar] [CrossRef]
- Betts, D.H.; Bain, N.T.; Madan, P. The p66Shc Adaptor Protein Controls Oxidative Stress Response in Early Bovine Embryos. PLoS ONE 2014, 9, e86978. [Google Scholar] [CrossRef] [Green Version]
- Kevenaar, M.E.; Meerasahib, M.F.; Kramer, P.; van de Lang-Born, B.M.N.; de Jong, F.H.; Groome, N.P.; Themmen, A.P.N.; Visser, J.A. Serum Anti-Müllerian Hormone Levels Reflect the Size of the Primordial Follicle Pool in Mice. Endocrinology 2006, 147, 3228–3234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jana, S.; Paul, S.; Swarnakar, S. Curcumin as anti-endometriotic agent: Implication of MMP-3 and intrinsic apoptotic pathway. Biochem. Pharmacol. 2012, 83, 797–804. [Google Scholar] [CrossRef]
- Culley, L.; Law, C.; Hudson, N.; Denny, E.; Mitchell, H.; Baumgarten, M.; Raine-Fenning, N. The social and psychological impact of endometriosis on women’s lives: A critical narrative review. Hum. Reprod. Update 2013, 19, 625–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrero, S.; Evangelisti, G.; Barra, F. Current and emerging treatment options for endometriosis. Expert Opin. Pharm. 2018, 19, 1109–1125. [Google Scholar] [CrossRef]
- Jelodar, G.; Azimifar, A. Evaluation of serum cancer antigen 125, resistin, leptin, homocysteine, and total antioxidant capacity in rat model of endometriosis treated with Curcumin. Physiol. Rep. 2019, 7, e14016. [Google Scholar] [CrossRef] [Green Version]
- Swarnakar, S.; Paul, S. Curcumin arrests endometriosis by downregulation of matrix metalloproteinase-9 activity. Indian J. Biochem. Biophys. 2009, 46, 59–65. [Google Scholar] [PubMed]
- Zhang, Y.; Cao, H.; Hu, Y.Y.; Wang, H.; Zhang, C.J. Inhibitory effect of curcumin on angiogenesis in ectopic endometrium of rats with experimental endometriosis. Int. J. Mol. Med. 2011, 27, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Kizilay, G.; Uz, Y.H.; Seren, G.; Ulucam, E.; Yilmaz, A.; Cukur, Z.; Kayisli, U.A. In vivo effects of curcumin and deferoxamine in experimental endometriosis. Adv. Clin. Exp. Med. 2017, 26, 207–213. [Google Scholar] [CrossRef]
- Jana, S.; Rudra, D.S.; Paul, S.; Snehasikta, S. Curcumin delays endometriosis development by inhibiting MMP-2 activity. Indian J. Biochem. Biophys. 2012, 49, 342–348. [Google Scholar]
- Kim, K.H.; Lee, E.N.; Park, J.K.; Lee, J.R.; Kim, J.H.; Choi, H.J.; Kim, B.S.; Lee, H.W.; Lee, K.S.; Yoon, S. Curcumin attenuates TNF-α-induced expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and proinflammatory cytokines in human endometriotic stromal cells. Phytother. Res. 2012, 26, 1037–1047. [Google Scholar] [CrossRef]
- Chowdhury, I.; Banerjee, S.; Driss, A.; Xu, W.; Mehrabi, S.; Nezhat, C.; Sidell, N.; Taylor, R.N.; Thompson, W.E. Curcumin attenuates proangiogenic and proinflammatory factors in human eutopic endometrial stromal cells through the NF-κB signaling pathway. J. Cell. Physiol. 2019, 234, 6298–6312. [Google Scholar] [CrossRef] [Green Version]
- Hendarto, H.; Yohanes Ardianta Widyanugraha, M.; Widjiati, W. Curcumin improves growth factors expression of bovine cumulus-oocyte complexes cultured in peritoneal fluid of women with endometriosis. Int. J. Reprod. Biomed. 2018, 16, 775–782. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, H.; Yu, Z.; Peng, H.Y.; Zhang, C.J. Curcumin inhibits endometriosis endometrial cells by reducing estradiol production. Iran J. Reprod. Med. 2013, 11, 415–422. [Google Scholar]
- Cao, H.; Wei, Y.X.; Zhou, Q.; Zhang, Y.; Guo, X.P.; Zhang, J. Inhibitory effect of curcumin in human endometriosis endometrial cells via downregulation of vascular endothelial growth factor. Mol. Med. Rep. 2017, 16, 5611–5617. [Google Scholar] [CrossRef] [Green Version]
- Bharti, A.C.; Donato, N.; Singh, S.; Aggarwal, B.B. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood 2003, 101, 1053–1062. [Google Scholar] [CrossRef]
- Liu, H.; Lang, J.H. Is abnormal eutopic endometrium the cause of endometriosis? The role of eutopic endometrium in pathogenesis of endometriosis. Med. Sci. Monit. 2011, 17, Ra92–Ra99. [Google Scholar] [CrossRef] [Green Version]
- Laschke, M.W.; Menger, M.D. Basic mechanisms of vascularization in endometriosis and their clinical implications. Hum. Reprod. Update 2018, 24, 207–224. [Google Scholar] [CrossRef]
- Meyer, M.; Clauss, M.; Lepple-Wienhues, A.; Waltenberger, J.; Augustin, H.G.; Ziche, M.; Lanz, C.; Büttner, M.; Rziha, H.J.; Dehio, C. A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. EMBO J 1999, 18, 363–374. [Google Scholar] [CrossRef] [Green Version]
- McLaren, J.; Prentice, A.; Charnock-Jones, D.S.; Millican, S.A.; Müller, K.H.; Sharkey, A.M.; Smith, S.K. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J. Clin. Investig. 1996, 98, 482–489. [Google Scholar] [CrossRef]
- Kaczanowski, S. Apoptosis: Its origin, history, maintenance and the medical implications for cancer and aging. Phys Biol. 2016, 13, 031001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutscher, L.M.; Shaham, S. Non-apoptotic cell death in animal development. Cell Death Differ. 2017, 24, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Giudice, L.C.; Lessey, B.A.; Abrao, M.S.; Kotarski, J.; Archer, D.F.; Diamond, M.P.; Surrey, E.; Johnson, N.P.; Watts, N.B.; et al. Treatment of Endometriosis-Associated Pain with Elagolix, an Oral GnRH Antagonist. N. Engl. J. Med. 2017, 377, 28–40. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).