Cord Serum Calcitriol Inversely Correlates with Maternal Blood Pressure in Urinary Tract Infection-Affected Pregnancies: Sex-Dependent Immune Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Sample Collection and Quantification of VD-Metabolites in Cord Serum
2.3. Real Time PCR Amplifications
2.4. Statistical Analysis
3. Results
3.1. The Umbilical Cord Serum Concentrations of VD-Metabolites Were Significantly Higher in UTI Samples
3.2. Comparative Studies of Placental Cathelicidin and CYP27B1 mRNA Expression
3.3. Correlation Studies between Expression of Placental Factors, Cord Serum VD-Metabolites, and Newborn/Maternal Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y. Vasoactivators and Placental Vasoactivity. In Vascular Biology of the Placenta; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Irani, R.A.; Xia, Y. The functional role of the renin-angiotensin system in pregnancy and preeclampsia. Placenta 2008, 29, 763–771. [Google Scholar] [CrossRef]
- Duvekot, J.J.; Peeters, L.L. Maternal cardiovascular hemodynamic adaptation to pregnancy. Obstet. Gynecol. Surv. 1994, 49, S1–S14. [Google Scholar] [CrossRef]
- Sanghavi, M.; Rutherford, J.D. Cardiovascular physiology of pregnancy. Circulation 2014, 130, 1003–1008. [Google Scholar] [CrossRef]
- Duvekot, J.J.; Peeters, L.L. Renal hemodynamics and volume homeostasis in pregnancy. Obstet. Gynecol. Surv. 1994, 49, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Minshall, R.D.; Pavcnik, D.; Browne, D.L.; Hermsmeyer, K. Nongenomic vasodilator action of progesterone on primate coronary arteries. J. Appl. Physiol. 2002, 92, 701–708. [Google Scholar] [CrossRef]
- Pringle, K.G.; Tadros, M.A.; Callister, R.J.; Lumbers, E.R. The expression and localization of the human placental prorenin/renin-angiotensin system throughout pregnancy: Roles in trophoblast invasion and angiogenesis? Placenta 2011, 32, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, E.; Ishida, J.; Sugiyama, F.; Horiguchi, H.; Murakami, K.; Fukamizu, A. Hypertension induced in pregnant mice by placental renin and maternal angiotensinogen. Science 1996, 274, 995–998. [Google Scholar] [CrossRef]
- Brar, H.S.; Do, Y.S.; Tam, H.B.; Valenzuela, G.J.; Murray, R.D.; Longo, L.D.; Yonekura, M.L.; Hsueh, W.A. Uteroplacental unit as a source of elevated circulating prorenin levels in normal pregnancy. Am. J. Obstet. Gynecol. 1986, 155, 1223–1226. [Google Scholar] [CrossRef]
- Blum, M.; Weisman, Y.; Turgeman, S.; Cabili, S.; Wollman, Y.; Peer, G.; Stern, N.; Silverberg, D.; Schwartz, D.; Iaina, A. Pregnancy decreases immunoreactive parathyroid hormone level in rats with chronic renal failure. Clin. Sci. 1999, 96, 427–430. [Google Scholar] [CrossRef]
- Gray, T.K.; Lester, G.E.; Lorenc, R.S. Evidence for extra-renal 1 alpha-hydroxylation of 25-hydroxyvitamin D3 in pregnancy. Science 1979, 204, 1311–1313. [Google Scholar] [CrossRef]
- Park, H.; Wood, M.R.; Malysheva, O.V.; Jones, S.; Mehta, S.; Brannon, P.M.; Caudill, M.A. Placental vitamin D metabolism and its associations with circulating vitamin D metabolites in pregnant women. Am. J. Clin. Nutr. 2017, 106, 1439–1448. [Google Scholar] [CrossRef]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef]
- Lind, L.; Hanni, A.; Lithell, H.; Hvarfner, A.; Sorensen, O.H.; Ljunghall, S. Vitamin D is related to blood pressure and other cardiovascular risk factors in middle-aged men. Am. J. Hypertens. 1995, 8, 894–901. [Google Scholar] [CrossRef]
- Lind, L.; Wengle, B.; Wide, L.; Ljunghall, S. Reduction of blood pressure during long-term treatment with active vitamin D (alphacalcidol) is dependent on plasma renin activity and calcium status. A double-blind, placebo-controlled study. Am. J. Hypertens. 1989, 2, 20–25. [Google Scholar] [CrossRef]
- Pfeifer, M.; Begerow, B.; Minne, H.W.; Nachtigall, D.; Hansen, C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J. Clin. Endocrinol. Metab. 2001, 86, 1633–1637. [Google Scholar] [CrossRef][Green Version]
- Adela, R.; Borkar, R.M.; Mishra, N.; Bhandi, M.M.; Vishwakarma, G.; Varma, B.A.; Ragampeta, S.; Banerjee, S.K. Lower Serum Vitamin D Metabolite Levels in Relation to Circulating Cytokines/Chemokines and Metabolic Hormones in Pregnant Women with Hypertensive Disorders. Front. Immunol. 2017, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Catov, J.M.; Simhan, H.N.; Holick, M.F.; Powers, R.W.; Roberts, J.M. Maternal vitamin D deficiency increases the risk of preeclampsia. J. Clin. Endocrinol. Metab. 2007, 92, 3517–3522. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Alobaid, A.; Malhis, T.N.; Khattab, A.F. Effect of vitamin D3 supplementation in pregnancy on risk of pre-eclampsia—Randomized controlled trial. Clin. Nutr. 2019, 38, 557–563. [Google Scholar] [CrossRef]
- Xu, M.L.; Yu, X.J.; Zhao, J.Q.; Du, Y.; Xia, W.J.; Su, Q.; Du, M.M.; Yang, Q.; Qi, J.; Li, Y.; et al. Calcitriol ameliorated autonomic dysfunction and hypertension by down-regulating inflammation and oxidative stress in the paraventricular nucleus of SHR. Toxicol. Appl. Pharmacol. 2020, 394, 114950. [Google Scholar] [CrossRef] [PubMed]
- Sauder, K.A.; Stamatoiu, A.V.; Leshchinskaya, E.; Ringham, B.M.; Glueck, D.H.; Dabelea, D. Cord Blood Vitamin D Levels and Early Childhood Blood Pressure: The Healthy Start Study. J. Am. Heart Assoc. 2019, 8, e011485. [Google Scholar] [CrossRef]
- Yang, J.; Chen, G.; Wang, D.; Chen, M.; Xing, C.; Wang, B. Low serum 25-hydroxyvitamin D level and risk of urinary tract infection in infants. Medicine 2016, 95, e4137. [Google Scholar] [CrossRef]
- Haghdoost, S.; Pazandeh, F.; Darvish, S.; Khabazkhoob, M.; Huss, R.; Lak, T.B. Association of serum vitamin D levels and urinary tract infection in pregnant women: A case control study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 243, 51–56. [Google Scholar] [CrossRef]
- Georgieva, V.; Kamolvit, W.; Herthelius, M.; Luthje, P.; Brauner, A.; Chromek, M. Association between vitamin D, antimicrobial peptides and urinary tract infection in infants and young children. Acta Paediatr. 2019, 108, 551–556. [Google Scholar] [CrossRef]
- Hensel, K.J.; Randis, T.M.; Gelber, S.E.; Ratner, A.J. Pregnancy-specific association of vitamin D deficiency and bacterial vaginosis. Am. J. Obstet. Gynecol. 2011, 204, e41–e49. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Sollid, S.T.; Svartberg, J.; Joakimsen, R.M.; Grimnes, G.; Hutchinson, M.Y. Prevention of urinary tract infections with vitamin D supplementation 20,000 IU per week for five years. Results from an RCT including 511 subjects. Infect. Dis. 2016, 48, 823–828. [Google Scholar] [CrossRef]
- Babikir, I.H.; Abugroun, E.A.; Bilal, N.E.; Alghasham, A.A.; Abdalla, E.E.; Adam, I. The impact of cathelicidin, the human antimicrobial peptide LL-37 in urinary tract infections. BMC Infect. Dis. 2018, 18, 17. [Google Scholar] [CrossRef]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Kaplan, A.T.; Low, J.; Nguyen, L.; Liu, G.Y.; Equils, O.; Hewison, M. Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol. Reprod. 2009, 80, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Chromek, M.; Slamova, Z.; Bergman, P.; Kovacs, L.; Podracka, L.; Ehren, I.; Hokfelt, T.; Gudmundsson, G.H.; Gallo, R.L.; Agerberth, B.; et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 2006, 12, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Akoh, C.C.; Pressman, E.K.; Whisner, C.M.; Thomas, C.; Cao, C.; Kent, T.; Cooper, E.; O’Brien, K.O. Vitamin D mediates the relationship between placental cathelicidin and group B streptococcus colonization during pregnancy. J. Reprod. Immunol. 2017, 121, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Olmos-Ortiz, A.; Noyola-Martinez, N.; Barrera, D.; Zaga-Clavellina, V.; Avila, E.; Halhali, A.; Biruete, B.; Larrea, F.; Diaz, L. IL-10 inhibits while calcitriol reestablishes placental antimicrobial peptides gene expression. J. Steroid Biochem. Mol. Biol. 2015, 148, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Drazancic, A.; Balasa, A.; Zadjelovic, J.; Kralj-Pejakovic, L. The effect of treatment of bacteriuria on pregnancy outcome. Jugosl. Ginekol. Perinatol. 1989, 29, 15–18. [Google Scholar]
- Gibson, C.S.; Goldwater, P.N.; MacLennan, A.H.; Haan, E.A.; Priest, K.; Dekker, G.A.; South Australian Cerebral Palsy Research Group. Fetal exposure to herpesviruses may be associated with pregnancy-induced hypertensive disorders and preterm birth in a Caucasian population. Int. J. Obstet. Gynaecol. 2008, 115, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, J.I.; Raghuraman, N.; Carter, E.B.; Kelly, J.C. Coronavirus disease 2019 infection and hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 2021, 224, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.; Sivalingam, N. Urinary tract infections in pregnancy. Acad. Fam. Physicians Malays. 2007, 2, 54–57. [Google Scholar]
- Yan, L.; Jin, Y.; Hang, H.; Yan, B. The association between urinary tract infection during pregnancy and preeclampsia: A meta-analysis. Medicine 2018, 97, e12192. [Google Scholar] [CrossRef]
- Olmos-Ortiz, A.; Garcia-Quiroz, J.; Lopez-Marure, R.; Gonzalez-Curiel, I.; Rivas-Santiago, B.; Olivares, A.; Avila, E.; Barrera, D.; Halhali, A.; Caldino, F.; et al. Evidence of sexual dimorphism in placental vitamin D metabolism: Testosterone inhibits calcitriol-dependent cathelicidin expression. J. Steroid Biochem. Mol. Biol. 2016, 163, 173–182. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Granger, J.P.; Alexander, B.T.; Bennett, W.A.; Khalil, R.A. Pathophysiology of pregnancy-induced hypertension. Am. J. Hypertens. 2001, 14, 178S–185S. [Google Scholar] [CrossRef]
- Young, B.C.; Levine, R.J.; Karumanchi, S.A. Pathogenesis of preeclampsia. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 173–192. [Google Scholar] [CrossRef]
- Alexander, B.T.; Cockrell, K.L.; Massey, M.B.; Bennett, W.A.; Granger, J.P. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am. J. Hypertens. 2002, 15, 170–175. [Google Scholar] [CrossRef]
- Jayaram, A.; Deer, E.; Amaral, L.M.; Campbell, N.; Vaka, V.R.; Cunningham, M.; Ibrahim, T.; Cornelius, D.C.; LaMarca, B.B. The role of tumor necrosis factor in triggering activation of natural killer cell, multi-organ mitochondrial dysfunction and hypertension during pregnancy. Pregnancy Hypertens. 2021, 24, 65–72. [Google Scholar] [CrossRef]
- Olmos-Ortiz, A.; Flores-Espinosa, P.; Mancilla-Herrera, I.; Vega-Sanchez, R.; Diaz, L.; Zaga-Clavellina, V. Innate Immune Cells and Toll-like Receptor-Dependent Responses at the Maternal-Fetal Interface. Int. J. Mol. Sci. 2019, 20, 3654. [Google Scholar] [CrossRef]
- Olmos-Ortiz, A.; Garcia-Quiroz, J.; Avila, E.; Caldino-Soto, F.; Halhali, A.; Larrea, F.; Diaz, L. Lipopolysaccharide and cAMP modify placental calcitriol biosynthesis reducing antimicrobial peptides gene expression. Am. J. Reprod. Immunol. 2018, 79, e12841. [Google Scholar] [CrossRef] [PubMed]
- Noyola-Martinez, N.; Diaz, L.; Zaga-Clavellina, V.; Avila, E.; Halhali, A.; Larrea, F.; Barrera, D. Regulation of CYP27B1 and CYP24A1 gene expression by recombinant pro-inflammatory cytokines in cultured human trophoblasts. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 106–109. [Google Scholar] [CrossRef]
- Diaz, L.; Noyola-Martinez, N.; Barrera, D.; Hernandez, G.; Avila, E.; Halhali, A.; Larrea, F. Calcitriol inhibits TNF-alpha-induced inflammatory cytokines in human trophoblasts. J. Reprod. Immunol. 2009, 81, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Qiao, G.; Uskokovic, M.; Xiang, W.; Zheng, W.; Kong, J. Vitamin D: A negative endocrine regulator of the renin-angiotensin system and blood pressure. J. Steroid Biochem. Mol. Biol. 2004, 89, 387–392. [Google Scholar] [CrossRef]
- Van Winden, K.R.; Bearden, A.; Kono, N.; Frederick, T.; Operskalski, E.; Stek, A.; Pandian, R.; Barton, L.; Kovacs, A. Low Bioactive Vitamin D Is Associated with Pregnancy-Induced Hypertension in a Cohort of Pregnant HIV-Infected Women Sampled Over a 23-Year Period. Am. J. Perinatol. 2020, 37, 1446–1454. [Google Scholar] [CrossRef]
- Ni, W.; Watts, S.W.; Ng, M.; Chen, S.; Glenn, D.J.; Gardner, D.G. Elimination of vitamin D receptor in vascular endothelial cells alters vascular function. Hypertension 2014, 64, 1290–1298. [Google Scholar] [CrossRef]
- Jia, X.; Gu, Y.; Groome, L.J.; Al-Kofahi, M.; Alexander, J.S.; Li, W.; Wang, Y. 1,25(OH)2D3 Induces Placental Vascular Smooth Muscle Cell Relaxation by Phosphorylation of Myosin Phosphatase Target Subunit 1Ser507: Potential Beneficial Effects of Vitamin D on Placental Vasculature in Humans. Biol. Reprod. 2016, 94, 116. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.B.; Perrier, N.D. Primary hyperparathyroidism and hypertension. Gland. Surg. 2020, 9, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Braun, J.; Bopp, M.; Faeh, D.; Swiss National, C. Inverse association between circulating vitamin D and mortality--dependent on sex and cause of death? Nutr. Metab. Cardiovasc. Dis. 2013, 23, 960–966. [Google Scholar] [CrossRef]
- Sanghera, D.K.; Sapkota, B.R.; Aston, C.E.; Blackett, P.R. Vitamin D Status, Gender Differences, and Cardiometabolic Health Disparities. Ann. Nutr. Metab. 2017, 70, 79–87. [Google Scholar] [CrossRef]
- Ramos, N.L.; Sekikubo, M.; Kironde, F.; Mirembe, F.; Saaf, M.; Brauner, A. The impact of vitamin D on the innate immune response to uropathogenic Escherichia coli during pregnancy. Clin. Microbiol. Infect. 2015, 21, 482.e1–482.e7. [Google Scholar] [CrossRef] [PubMed]
- Hertting, O.; Holm, A.; Luthje, P.; Brauner, H.; Dyrdak, R.; Jonasson, A.F.; Wiklund, P.; Chromek, M.; Brauner, A. Vitamin D induction of the human antimicrobial Peptide cathelicidin in the urinary bladder. PLoS ONE 2010, 5, e15580. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M.; Fraser, A.; Fraser, W.D.; Hypponen, E.; Davey Smith, G.; Deanfield, J.; Hingorani, A.; Sattar, N.; Lawlor, D.A. Associations of maternal 25-hydroxyvitamin D in pregnancy with offspring cardiovascular risk factors in childhood and adolescence: Findings from the Avon Longitudinal Study of Parents and Children. Heart 2013, 99, 1849–1856. [Google Scholar] [CrossRef]
- Meems, L.M.; Mahmud, H.; Buikema, H.; Tost, J.; Michel, S.; Takens, J.; Verkaik-Schakel, R.N.; Vreeswijk-Baudoin, I.; Mateo-Leach, I.V.; van der Harst, P.; et al. Parental vitamin D deficiency during pregnancy is associated with increased blood pressure in offspring via Panx1 hypermethylation. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1459–H1469. [Google Scholar] [CrossRef] [PubMed]
- Ariyawatkul, K.; Lersbuasin, P. Prevalence of vitamin D deficiency in cord blood of newborns and the association with maternal vitamin D status. Eur. J. Pediatr. 2018, 177, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- Ron, M.; Menczel, J.; Schwartz, L.; Palti, Z.; Kidroni, G. Vitamin D3 metabolites in amniotic fluid in relation with maternal and fetal sera in term pregnancies. J. Perinat. Med. 1987, 15, 282–290. [Google Scholar] [CrossRef]
- Li, H.; Ma, J.; Huang, R.; Wen, Y.; Liu, G.; Xuan, M.; Yang, L.; Yang, J.; Song, L. Prevalence of vitamin D deficiency in the pregnant women: An observational study in Shanghai, China. Arch. Public Health 2020, 78, 31. [Google Scholar] [CrossRef]
- Abbasian, M.; Chaman, R.; Amiri, M.; Ajami, M.E.; Jafari-Koshki, T.; Rohani, H.; Taghavi-Shahri, S.M.; Sadeghi, E.; Raei, M. Vitamin D Deficiency in Pregnant Women and Their Neonates. Glob. J. Health Sci. 2016, 8, 54008. [Google Scholar] [CrossRef] [PubMed]
- Olmos-Ortiz, A.; Avila, E.; Durand-Carbajal, M.; Diaz, L. Regulation of calcitriol biosynthesis and activity: Focus on gestational vitamin D deficiency and adverse pregnancy outcomes. Nutrients 2015, 7, 443–480. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Roizen, J.D.; Long, C.; Casella, A.; O’Lear, L.; Caplan, I.; Lai, M.; Sasson, I.; Singh, R.; Makowski, A.J.; Simmons, R.; et al. Obesity Decreases Hepatic 25-Hydroxylase Activity Causing Low Serum 25-Hydroxyvitamin D. J. Bone Miner. Res. 2019, 34, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Bora, S.A.; Kennett, M.J.; Smith, P.B.; Patterson, A.D.; Cantorna, M.T. The Gut Microbiota Regulates Endocrine Vitamin D Metabolism through Fibroblast Growth Factor 23. Front. Immunol. 2018, 9, 408. [Google Scholar] [CrossRef] [PubMed]



| Clinical Data | NP-Female | NP-Male | UTI-Female | UTI-Male | NP Total vs. UTI Total |
|---|---|---|---|---|---|
| n = 24 | n = 20 | n = 22 | n = 26 | (p) | |
| Maternal parameters: | |||||
| Pregestational BMI (kg/m2) | 24.7 ± 0.9 | 24.4 ± 0.9 | 24.8 ± 1.0 | 25.1 ± 0.8 | ns |
| Gestational age (weeks) | 38.6 ± 0.6 | 38.6 ± 0.7 | 35.9 ± 0.7 b | 36.3 ± 0.6 b | <0.001 |
| SBP (mm Hg) | 115 ± 2 | 111 ± 3 | 115 ± 3 | 114 ± 2 | ns |
| DBP (mm Hg) | 72 ± 2 | 66 ± 2 | 70 ± 2 | 70 ± 2 | ns |
| Urinalysis (pH) | 6.0 ± 0.1 | 6.0 ± 0.2 | 6.5 ± 0.1 b | 6.5 ± 0.1 b | 0.005 |
| Urinalysis (Bacteria U/µL) | 1.7 ± 12.6 | 1.0 ± 13.8 | 68.5 ± 14.6 a,b | 23.7 ± 12.6 b | 0.001 |
| Newborn data: | |||||
| Weight (g) | 3009 ± 139 | 3166 ± 149 | 2481 ± 153 b | 2697 ± 133 b | <0.001 |
| Length (cm) | 49.5 ± 0.8 | 50.7 ± 0.9 | 46.6 ± 0.9 b | 47.3 ± 0.8 b | 0.001 |
| Cephalic perimeter (cm) | 34.1 ± 0.4 | 34.2 ± 0.5 | 31.7 ± 0.5 b | 32.9 ± 0.4 | <0.001 |
| Apgar score (1 min) | 8.0 ± 0.2 | 7.9 ± 0.2 | 7.4 ± 0.2 | 7.4 ± 0.2 | 0.016 |
| Apgar score (5 min) | 9.0 ± 0.0 | 9.0 ± 0.0 | 8.8 ± 0.0 b | 8.9 ± 0.0 | 0.007 |
| Subgroups | Groups | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlated | NP-Female | NP-Male | UTI-Female | UTI-Male | NP-Total | UTI-Total | |||||||
| Factors | r | p | r | p | r | p | r | p | r | p | r | p | |
| Cal | SBP | −0.511 | 0.03 | 0.014 | 0.952 | −0.565 | 0.03 | −0.584 | 0.006 | −0.157 | 0.381 | −0.57 | <0.001 |
| Cal | DBP | −0.256 | 0.316 | −0.094 | 0.721 | −0.551 | 0.03 | −0.534 | 0.01 | −0.125 | 0.485 | −0.512 | 0.002 |
| Cal | UB | −0.136 | 0.681 | −0.203 | 0.658 | −0.636 | 0.01 | 0.346 | 0.881 | −0.020 | 0.935 | −0.241 | 0.168 |
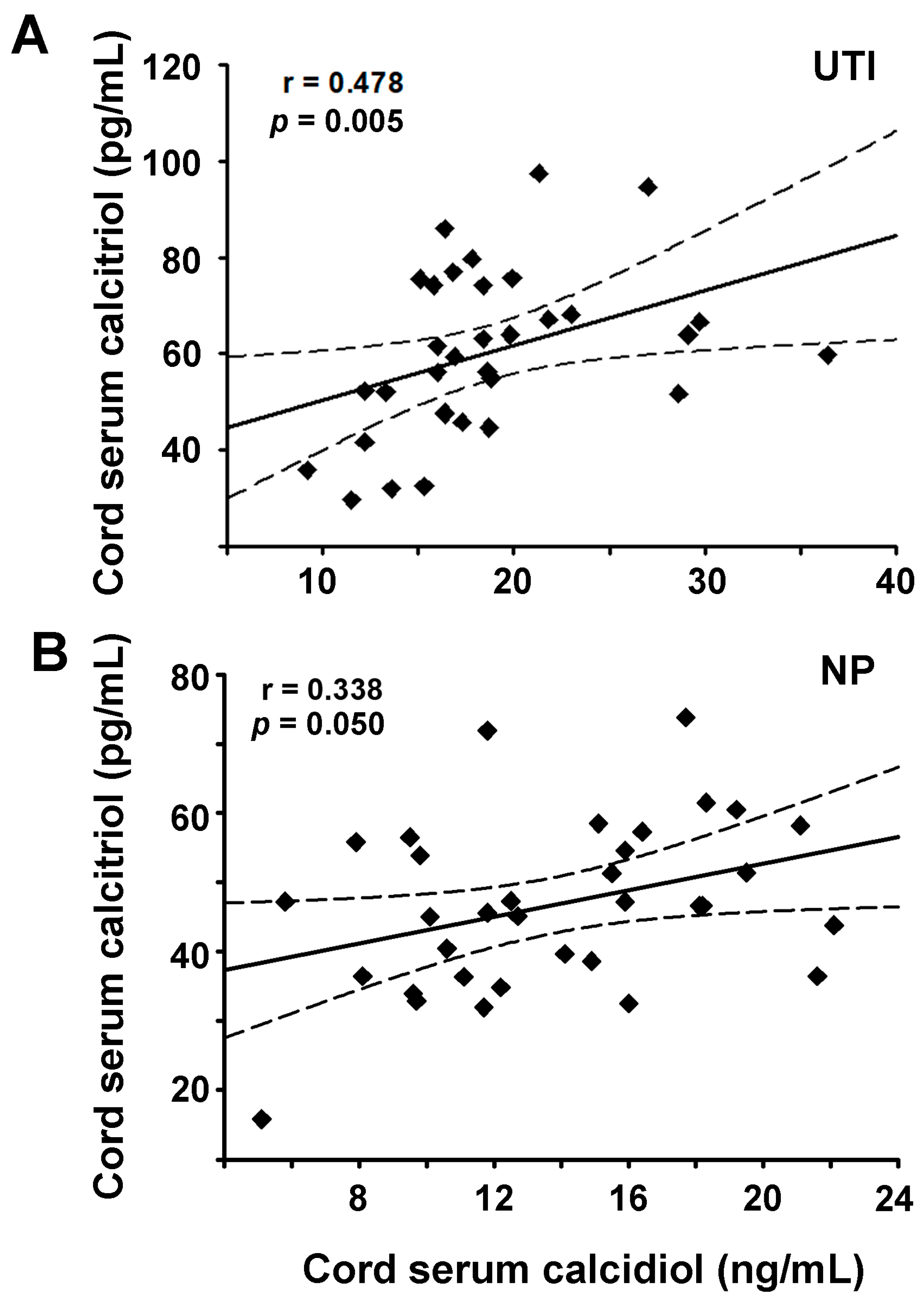
| Cal | calcidiol | 0.418 | 0.082 | 0.232 | 0.378 | 0.011 | 0.964 | 0.703 | <0.001 | 0.338 | 0.05 | 0.478 | 0.005 |
| Cal | LL37 | 0.078 | 0.763 | 0.559 | 0.02 | 0.151 | 0.603 | −0.163 | 0.498 | 0.289 | 0.1 | 0.076 | 0.666 |
| LL37 | NB-L | 0.205 | 0.378 | 0.028 | 0.905 | 0.41 | 0.089 | 0.544 | 0.007 | 0.144 | 0.38 | 0.423 | 0.006 |
| LL37 | CP | 0.181 | 0.44 | 0.055 | 0.82 | 0.523 | 0.02 | 0.255 | 0.237 | 0.101 | 0.53 | 0.201 | 0.207 |
| LL37 | NB-W | 0.494 | 0.026 | −0.017 | 0.94 | 0.45 | 0.058 | 0.54 | 0.008 | 0.33 | 0.04 | 0.38 | 0.01 |
| LL37 | GAD | 0.003 | 0.987 | 0.044 | 0.854 | 0.552 | 0.01 | 0.535 | 0.008 | 0.051 | 0.756 | 0.428 | 0.005 |
| LL37 | Apgar1 | 0.038 | 0.866 | −0.018 | 0.94 | 0.352 | 0.148 | 0.41 | 0.057 | 0.015 | 0.926 | 0.331 | 0.03 |
| NB-W | NB-L | 0.581 | 0.003 | 0.749 | <0.001 | 0.866 | <0.001 | 0.939 | <0.001 | 0.63 | <0.001 | 0.916 | <0.001 |
| NB-W | CP | 0.411 | 0.05 | 0.232 | 0.417 | 0.716 | <0.001 | 0.662 | <0.001 | 0.349 | 0.023 | 0.796 | <0.001 |
| NB-W | GAD | 0.312 | 0.145 | 0.425 | 0.060 | 0.882 | <0.001 | 0.82 | <0.001 | 0.35 | 0.02 | 0.846 | <0.001 |
| SBP | DBP | 0.743 | <0.001 | 0.757 | <0.001 | 0.784 | <0.001 | 0.774 | <0.001 | 0.727 | <0.001 | 0.774 | <0.001 |
| SBP | BMI | 0.532 | 0.009 | 0.196 | 0.403 | 0.522 | 0.02 | 0.105 | 0.615 | 0.384 | 0.01 | 0.302 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmos-Ortiz, A.; Olivares-Huerta, A.; García-Quiroz, J.; Avila, E.; Halhali, A.; Quesada-Reyna, B.; Larrea, F.; Zaga-Clavellina, V.; Díaz, L. Cord Serum Calcitriol Inversely Correlates with Maternal Blood Pressure in Urinary Tract Infection-Affected Pregnancies: Sex-Dependent Immune Implications. Nutrients 2021, 13, 3114. https://doi.org/10.3390/nu13093114
Olmos-Ortiz A, Olivares-Huerta A, García-Quiroz J, Avila E, Halhali A, Quesada-Reyna B, Larrea F, Zaga-Clavellina V, Díaz L. Cord Serum Calcitriol Inversely Correlates with Maternal Blood Pressure in Urinary Tract Infection-Affected Pregnancies: Sex-Dependent Immune Implications. Nutrients. 2021; 13(9):3114. https://doi.org/10.3390/nu13093114
Chicago/Turabian StyleOlmos-Ortiz, Andrea, Alberto Olivares-Huerta, Janice García-Quiroz, Euclides Avila, Ali Halhali, Braulio Quesada-Reyna, Fernando Larrea, Verónica Zaga-Clavellina, and Lorenza Díaz. 2021. "Cord Serum Calcitriol Inversely Correlates with Maternal Blood Pressure in Urinary Tract Infection-Affected Pregnancies: Sex-Dependent Immune Implications" Nutrients 13, no. 9: 3114. https://doi.org/10.3390/nu13093114
APA StyleOlmos-Ortiz, A., Olivares-Huerta, A., García-Quiroz, J., Avila, E., Halhali, A., Quesada-Reyna, B., Larrea, F., Zaga-Clavellina, V., & Díaz, L. (2021). Cord Serum Calcitriol Inversely Correlates with Maternal Blood Pressure in Urinary Tract Infection-Affected Pregnancies: Sex-Dependent Immune Implications. Nutrients, 13(9), 3114. https://doi.org/10.3390/nu13093114









