Clinical Value of Tissue Transglutaminase Antibodies in Celiac Patients over a Long Term Follow-Up
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Antibodies, Endoscopy and Histological Analysis
2.3. Urine Samplings
2.4. Statistical Analysis
3. Results
3.1. Clinical and Demographic Findings
3.2. Endoscopic and Histologic Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.; Cellier, C.; Mulder, C.J.; Lundin, K. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836.e2. [Google Scholar] [CrossRef] [Green Version]
- Catassi, C.; Gatti, S.; Fasano, A. The new epidemiology of celiac disease. J. Pediatr. Gastroenterol. Nutr. 2014, 59, S7–S9. [Google Scholar] [CrossRef] [Green Version]
- Husby, S.; Murray, J.A.; Katzka, D.A. AGA Clinical Practice Update on Diagnosis and Monitoring of Celiac Disease—Changing Utility of Serology and Histologic Measures: Expert Review. Gastroenterology 2019, 156, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasano, A.; Catassi, C. Celiac Disease. N. Engl. J. Med. 2012, 367, 2419–2426. [Google Scholar] [CrossRef]
- Elli, L.; Ferretti, F.; Orlando, S.; Vecchi, M.; Monguzzi, E.; Roncoroni, L.; Schuppan, D. Management of celiac disease in daily clinical practice. Eur. J. Intern. Med. 2019, 61, 15–24. [Google Scholar] [CrossRef]
- Zanini, B.; Lanzarotto, F.; Mora, A.; Bertolazzi, S.; Turini, D.; Cesana, B.; Donato, F.; Ricci, C.; Lonati, F.; Vassallo, F.; et al. Five year time course of celiac disease serology during gluten free diet: Results of a community based ‘CD-Watch’ program. Dig. Liver Dis. 2010, 42, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; King, K.S.; Larson, J.J.; Snyder, M.R.; Wu, T.T.; Gandhi, M.J.; Murray, J.A. Undetectable negative tissue transglutaminase IgA antibodies predict mucosal healing in treated coeliac disease patients. Aliment. Pharmacol. Ther. 2017, 46, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Dipper, C.R.; Maitra, S.; Thomas, R.; Lamb, C.A.; McLean-Tooke, A.P.C.; Ward, R.; Smith, D.; Spickett, G.; Mansfield, J.C. Anti-tissue transglutaminase antibodies in the follow-up of adult coeliac disease. Aliment. Pharmacol. Ther. 2009, 30, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabò, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J. Pediatric Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef]
- Leffler, D.A.; Dennis, M.; Edwards, G.J.B.; Jamma, S.; Magge, S.; Cook, E.F.; Schuppan, D.; Kelly, C.P. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clin. Gastroenterol. Hepatol. 2009, 7, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Rondonotti, E.; Spada, C.; Cadoni, S.; Cannizzaro, R.; Calabrese, C.; De Franchis, R.; Elli, L.; Girelli, C.M.; Hassan, C.; Marmo, R.; et al. Quality performance measures for small capsule endoscopy: Are the ESGE quality standards met? Endosc. Int. Open 2021, 9, E122–E129. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, F.; Branchi, F.; Orlando, S.; Roncoroni, L.; Barigelletti, G.; Fabiano, S.; Vecchi, M.; Penagini, R.; Doneda, L.; Elli, L. Effectiveness of capsule endoscopy and double-balloon enteroscopy in suspected complicated celiac disease. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zammit, S.C.; Elli, L.; Scaramella, L.; Sanders, D.S.; Tontini, G.E.; Sidhu, R. Small bowel capsule endoscopy in refractory celiac disease: A luxury or a necessity? Ann. Gastroenterol. 2021, 34, 188–195. [Google Scholar]
- Branchi, F.; Ferretti, F.; Orlando, S.; Tontini, G.E.; Penagini, R.; Vecchi, M.; Elli, L. Small-bowel capsule endoscopy in patients with celiac disease, axial versus lateral/panoramic view: Results from a prospective randomized trial. Dig. Endosc. 2020, 32, 778–784. [Google Scholar]
- Brotz, C.; Nandi, N.; Conn, M.; Daskalakis, C.; DiMarino, M.; Infantolino, A.; Katz, L.C.; Schroeder, T.; Kastenberg, D. A validation study of 3 grading systems to evaluate small-bowel cleansing for wireless capsule endoscopy: A quantitative index, a qualitative evaluation, and an overall adequacy assessment. Gastrointest. Endosc. 2009, 69, 262–270. [Google Scholar] [CrossRef]
- Tomba, C.; Elli, L.; Bardella, M.T.; Soncini, M.; Contiero, P.; Roncoroni, L.; Locatelli, M.; Conte, D. Enteroscopy for the early detection of small bowel tumours in at-risk celiac patients. Dig. Liver Dis. 2014, 46, 400–404. [Google Scholar] [CrossRef]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef]
- Moreno, M.D.L.; Cebolla, A.; Munõz-Suano, A.; Carrillo-Carrion, C.; Comino, I.; Pizarro, A.; Leon, F.; Rodriguez-Herrera, A.; Sousa, C. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut 2017, 66, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Elli, L.; Bonura, A.; Garavaglia, D.; Rulli, E.; Floriani, I.; Tagliabue, G.; Contiero, P.; Bardella, M.T. Immunological comorbity in coeliac disease: Associations, risk factors and clinical implications. J. Clin. Immunol. 2012, 32, 984–990. [Google Scholar] [CrossRef]
- Corrao, G.; Corazza, G.R.; Bagnardi, V.; Brusco, G.; Ciacci, C.; Cottone, M.; Sategna Guidetti, C.; Usai, P.; Cesari, P.; Pelli, M.A.; et al. Mortality in patients with coeliac disease and their relatives: A cohort study. Lancet 2001, 358, 356–361. [Google Scholar] [CrossRef]
- Ludvigsson, J.F. Mortality and Malignancy in Celiac Disease. Gastrointest. Endosc. Clin. N. Am. 2012, 22, 705–722. [Google Scholar] [CrossRef]
- Bardella, M.T.; Elli, L.; Velio, P.; Fredella, C.; Prampolini, L.; Cesana, B. Silent celiac disease is frequent in the siblings of newly diagnosed celiac patients. Digestion 2007, 75, 182–187. [Google Scholar] [CrossRef]
- Monachesi, C.; Verma, A.K.; Catassi, G.N.; Gatti, S.; Lionetti, E.; Catassi, C. Slow Decrease of Antitissue Transglutaminase Antibody Positivity in Children with Celiac Disease after Starting the Gluten-free Diet. J. Pediatric Gastroenterol. Nutr. 2020, 71, e49. [Google Scholar] [CrossRef]
- Sharkey, L.M.; Corbett, G.; Currie, E.; Lee, J.; Sweeney, N.; Woodward, J.M. Optimising delivery of care in coeliac disease-Comparison of the benefits of repeat biopsy and serological follow-up. Aliment. Pharmacol. Ther. 2013, 38, 1278–1291. [Google Scholar] [CrossRef]
- Kaukinen, K.; Peräaho, M.; Lindfors, K.; Partanen, J.; Woolley, N.; Pikkarainen, P.; Karvonen, A.L.; Laasanen, T.; Sievänen, H.; Mäki, M.; et al. Persistent small bowel mucosal villous atrophy without symptoms in coeliac disease. Aliment. Pharmacol. Ther. 2007, 25, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Brandimarte, G.; Giorgetti, G.M. Lack of Usefulness of Anti-Transglutaminase Antibodies in Assessing Histologic Recovery After Gluten-Free Diet in Celiac Disease. J. Clin. Gastroenterol. 2003, 37, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Lanzini, A.; Lanzarotto, F.; Villanacci, V.; Mora, A.; Bertolazzi, S.; Turini, D.; Carella, G.; Malagoli, A.; Ferrante, G.; Cesana, B.M.; et al. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment. Pharmacol. Ther. 2009, 29, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Rahim, M.W.; See, J.A.; Lahr, B.D.; Wu, T.T.; Murray, J.A. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am. J. Gastroenterol. 2010, 105, 1412–1420. [Google Scholar] [CrossRef] [Green Version]
- Bardella, M.T.; Velio, P.; Cesana, B.M.; Prampolini, L.; Casella, G.; Di Bella, C.; Lanzini, A.; Gambarotti, M.; Bassotti, G.; Villanacci, V. Coeliac disease: A histological follow-up study. Histopathology 2007, 50, 465–471. [Google Scholar] [CrossRef]
- Elli, L.; Zini, E.; Tomba, C.; Bardella, M.T.; Bosari, S.; Conte, D.; Runza, L.; Roncoroni, L.; Ferrero, S. Histological evaluation of duodenal biopsies from coeliac patients: The need for different grading criteria during follow-up. BMC Gastroenterol. 2015, 15, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvester, J.A.; Kurada, S.; Szwajcer, A.; Kelly, C.P.; Leffler, D.A.; Duerksen, D.R. Tests for Serum Transglutaminase and Endomysial Antibodies Do Not Detect Most Patients with Celiac Disease and Persistent Villous Atrophy on Gluten-free Diets: A Meta-analysis. Gastroenterology 2017, 153, 689–701.e1. [Google Scholar] [CrossRef]
- Lau, M.S.; Mooney, P.D.; White, W.L.; Rees, M.A.; Wong, S.H.; Kurien, M.; Trott, N.; Leffler, D.A.; Hadjivassiliou, M.; Sanders, d.S. The Role of an IgA/IgG-Deamidated Gliadin Peptide Point-of-Care Test in Predicting Persistent Villous Atrophy in Patients with Celiac Disease on a Gluten-Free Diet. Am. J. Gastroenterol. 2017, 112, 1859–1867. [Google Scholar] [CrossRef]
- Selby, W.S.; Prakoso, E. The inability to visualize the ampulla of Vater is an inherent limitation of capsule endoscopy. Eur. J. Gastroenterol. Hepatol. 2011, 23, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Casazza, G.; Locatelli, M.; Branchi, F.; Ferretti, F.; Conte, D.; Fraquelli, M. Use of enteroscopy for the detection of malignant and premalignant lesions of the small bowel in complicated celiac disease: A meta-analysis. Gastrointest. Endosc. 2017, 86, 264–273.e1. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Contiero, P.; Tagliabue, G.; Tomba, C.; Bardella, M.T. Risk of intestinal lymphoma in undiagnosed coeliac disease: Results from a registered population with different coeliac disease prevalence. Dig. Liver Dis. 2012, 44, 743–747. [Google Scholar] [CrossRef]
- Elli, L.; Baskuñán, K.; Di Lernia, L.; Bardella, M.T.; Doneda, L.; Soldati, L.; Orlando, S.; Ferretti, F.; Lombardo, V.; Barigelletti, G.; et al. Safety of occasional ingestion of gluten in patients with celiac disease: A real-life study. BMC Med. 2020, 18, 42. [Google Scholar] [CrossRef] [Green Version]
- Vahedi, K.; Mascart, F.; Mary, J.Y.; Laberenne, J.E.; Bouhnik, Y.; Morin, M.C.; Ocmant, A.; Velly, C.; Colombel, J.F.; Matuchansky, C. Reliability of antitransglutaminase antibodies as predictors of gluten-free diet compliance in adult celiac disease. Am. J. Gastroenterol. 2003, 98, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Itzlinger, A.; Branchi, F.; Elli, L.; Schumann, M. Gluten-free diet in celiac disease—forever and for all? Nutrients 2018, 10, 1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]


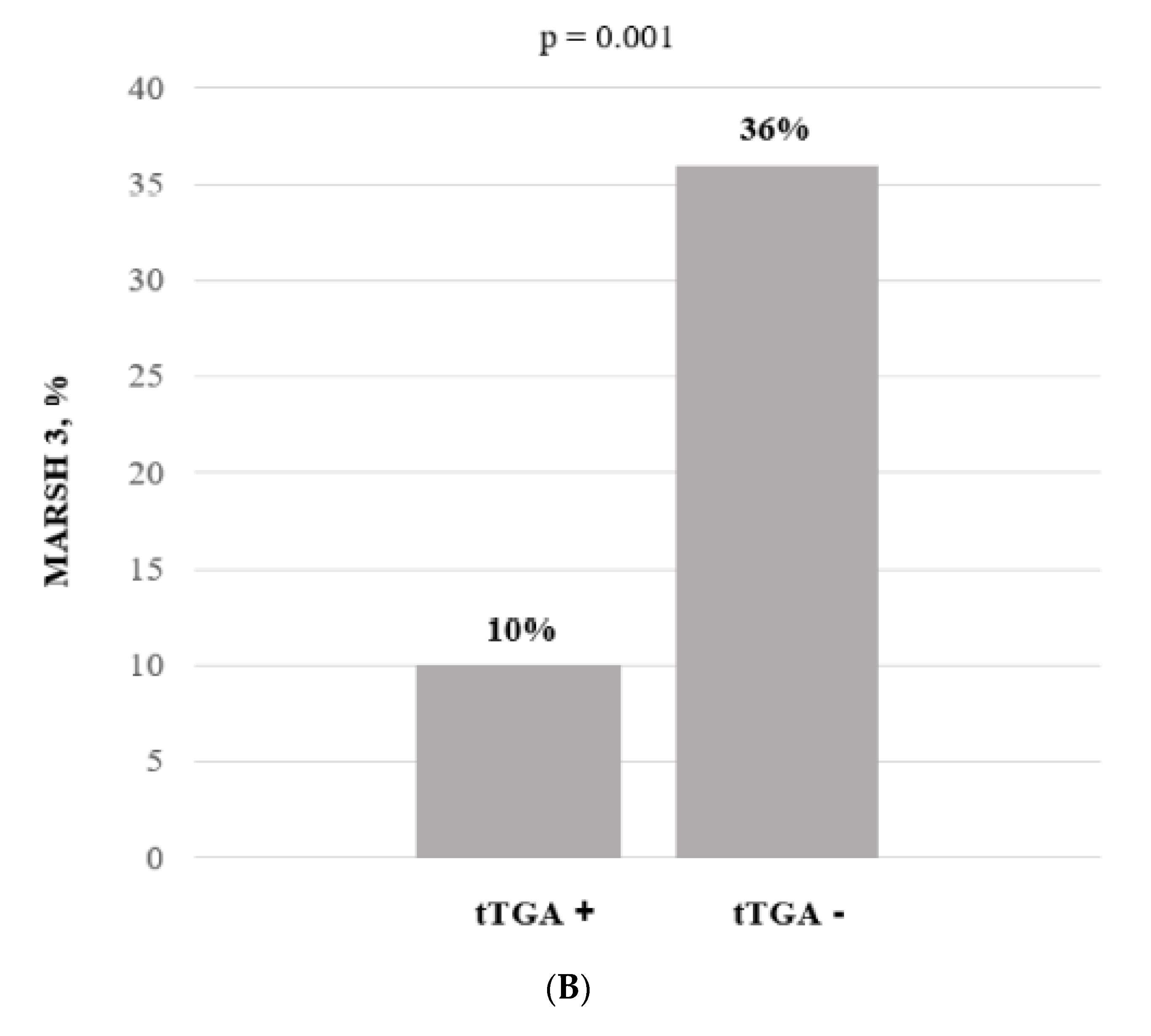
| Baseline Variables | CD Patients with IgA-TG2 Positive (n = 65) | CD Patients with Negative IgA-TG2 (n = 212) | p-Value Time Independent |
|---|---|---|---|
| Age at diagnosis, years * | 31 (1–76) | 29 (1–67) | 0.659 |
| Age at enrollment, years * | 37 (14–86) | 39 (16–78) | 0.015 |
| Male, n (%) | 8 (12%) | 38 (18%) | 0.256 |
| Autoimmune disease, n (%) | 13 (20%) | 52 (25%) | 0.409 |
| Onset, n (%) | |||
| Infancy ° | 5 (8%) | 12 (6%) | 0.567 |
| Pediatric °° | 8 (12%) | 32 (15%) | 0.546 |
| Adult ^ | 52 (80%) | 161 (76%) | 0.504 |
| Elderly § | 0 | 7 (3%) | 0.159 |
| Phenotype, n (%) | |||
| Classic | 15 (23%) | 111 (52%) | <0.001 |
| Mono-paucisymptomatic | 35 (54%) | 84 (40%) | 0.047 |
| Dermatitis herpetiformis | 4 (6%) | 4 (2%) | 0.095 |
| Familiar screening | 5 (8%) | 8 (4%) | 0.194 |
| Screening for other diseases | 6 (9%) | 5 (2%) | 0.009 |
| Baseline Variables | Class 1 (n = 36) | Class 2 (n = 16) | Class 3 (n = 13) | p-Value |
|---|---|---|---|---|
| Age at diagnosis, years * | 34 (14–76) | 31 (1–54) | 30 (5–56) | 0.425 |
| Age at enrolling, years * | 37 (17–86) | 37 (20–63) | 37 (14–69) | 0.975 |
| Male, n (%) | 5 (14%) | 1 (7%) | 2 (15%) | 0.690 |
| Autoimmune disease, n (%) | 6 (17%) | 4 (33%) | 3 (23%) | 0.749 |
| Onset, n (%) | 0.104 | |||
| Pediatric | 0 | 3 (19%) | 2 (15%) | 0.033 |
| Teenage | 6 (17%) | 1 (6%) | 1 (8%) | 0.488 |
| Adult | 30 (83%) | 12 (75%) | 10 (77%) | 0.749 |
| Elderly | 0 | 0 | 0 | - |
| Onset fenotype, n (%) | 0.640 | |||
| Classic | 6 (17%) | 5 (3%) | 4 (31%) | 0.393 |
| Mono-paucisymptomatic | 23 (65%) | 6 (38%) | 6 (46%) | 0.175 |
| Dermatitis herpetiformis | 3 (8%) | 1 (6%) | 0 | 0.563 |
| Familiar screening | 2 (5%) | 2 (12%) | 1 (8%) | 0.686 |
| Screening for other diseases | 2 (5%) | 2 (12%) | 2 (15%) | 0.504 |
| TTGA titer, (x UNL) | 3 (1–24) | 2 (1–7) | 3 (1–74) | 0.314 |
| Follow-up endoscopy available | 15 (42%) | 12 (75%) | 12 (92%) | 0.002 |
| Marsh 3 at follow-up endoscopy, n (%) | 2 (13%) | 0 | 2 (17%) | 0.357 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farina, E.; Roncoroni, L.; Lombardo, V.; Scricciolo, A.; Vecchi, M.; Doneda, L.; Elli, L. Clinical Value of Tissue Transglutaminase Antibodies in Celiac Patients over a Long Term Follow-Up. Nutrients 2021, 13, 3057. https://doi.org/10.3390/nu13093057
Farina E, Roncoroni L, Lombardo V, Scricciolo A, Vecchi M, Doneda L, Elli L. Clinical Value of Tissue Transglutaminase Antibodies in Celiac Patients over a Long Term Follow-Up. Nutrients. 2021; 13(9):3057. https://doi.org/10.3390/nu13093057
Chicago/Turabian StyleFarina, Elisa, Leda Roncoroni, Vincenza Lombardo, Alice Scricciolo, Maurizio Vecchi, Luisa Doneda, and Luca Elli. 2021. "Clinical Value of Tissue Transglutaminase Antibodies in Celiac Patients over a Long Term Follow-Up" Nutrients 13, no. 9: 3057. https://doi.org/10.3390/nu13093057
APA StyleFarina, E., Roncoroni, L., Lombardo, V., Scricciolo, A., Vecchi, M., Doneda, L., & Elli, L. (2021). Clinical Value of Tissue Transglutaminase Antibodies in Celiac Patients over a Long Term Follow-Up. Nutrients, 13(9), 3057. https://doi.org/10.3390/nu13093057






