Hypoglycaemic and Antioxidant Properties of Acrocomia aculeata (Jacq.) Lodd Ex Mart. Extract Are Associated with Better Vascular Function of Type 2 Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Botanical Material and Isolation of Extract
2.3. Cell Culture and Viability Assays
2.4. Animal Maintenance and Treatment
2.4.1. In Vivo Procedures and Sample Collection
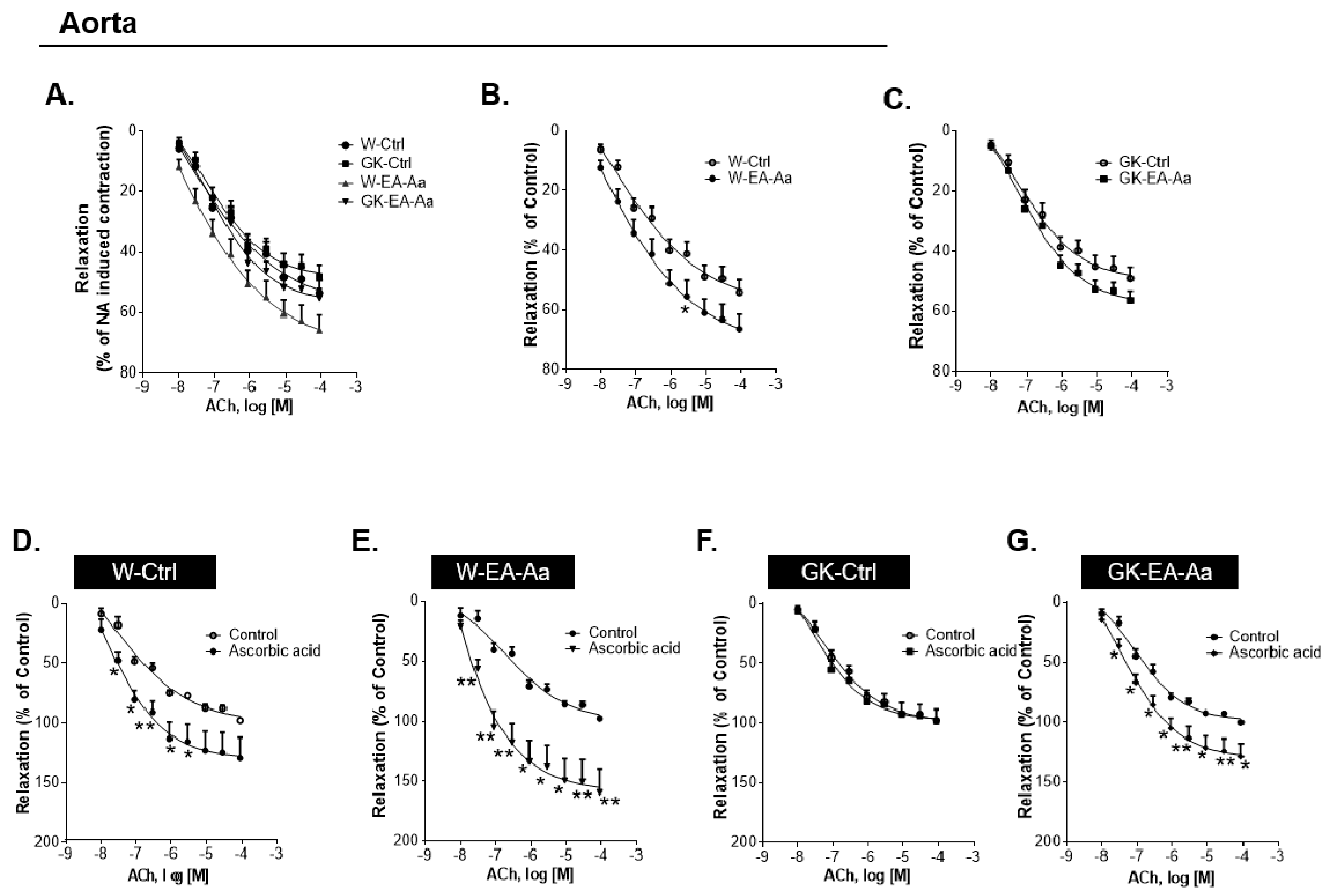
2.4.2. Studies of Isometric Tension of Aorta
2.5. Biochemical Analyses
2.6. Fluorescence Immunocytochemistry and Immunohistochemistry
2.7. Western Blot
2.8. Statistical Analysis
3. Results
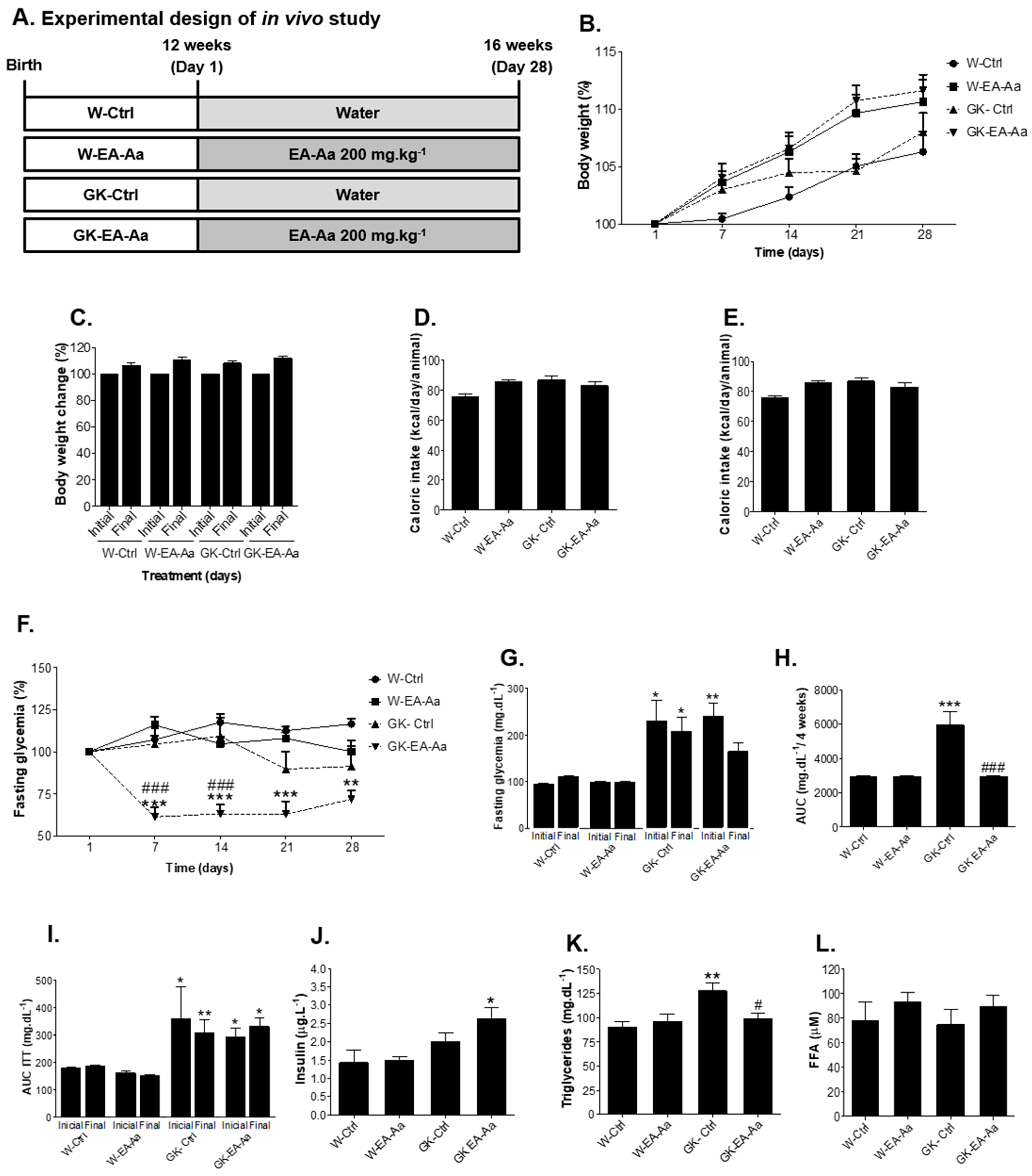
3.1. EA-Aa Improves the Metabolic Profile of Diabetic Rats
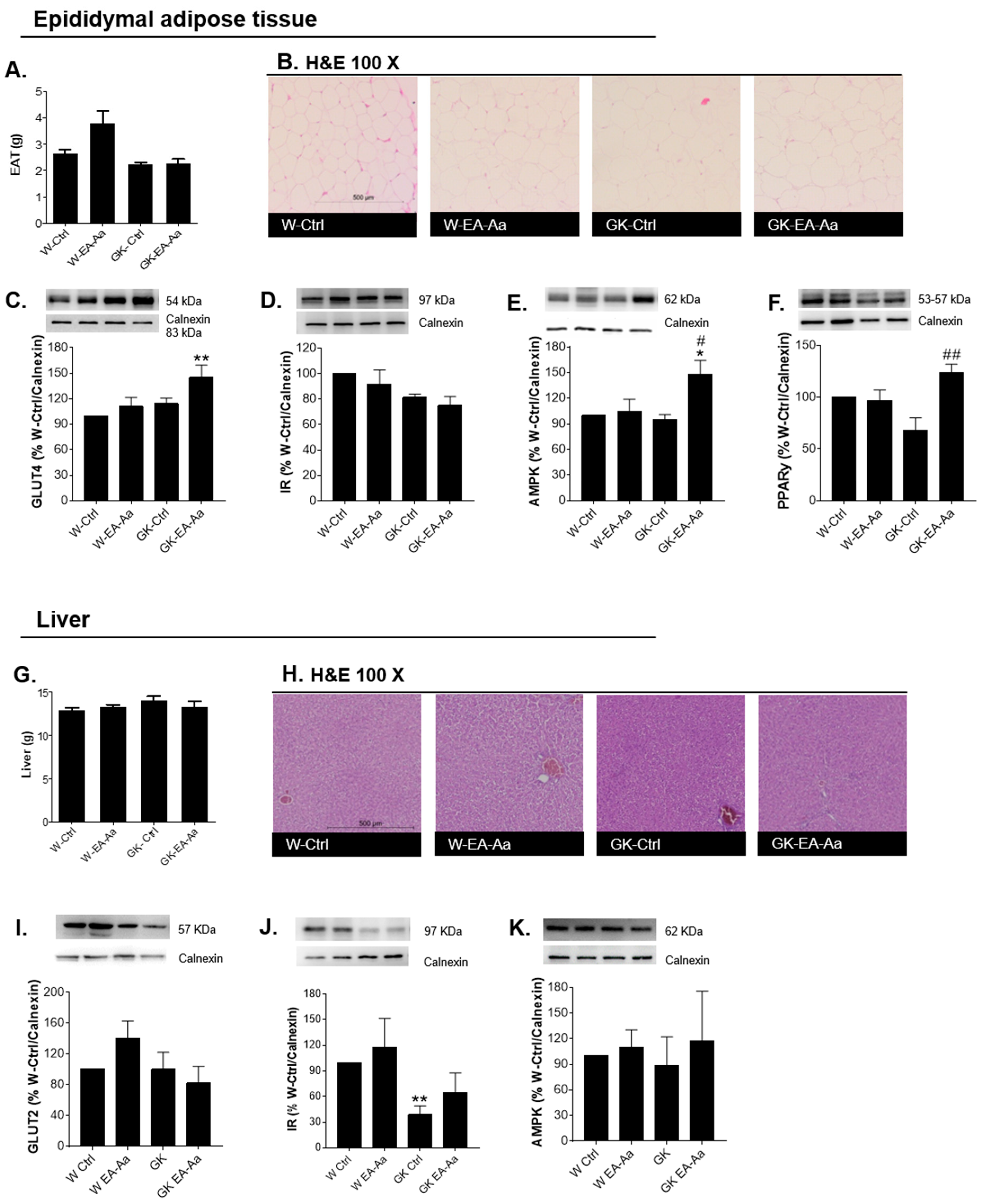
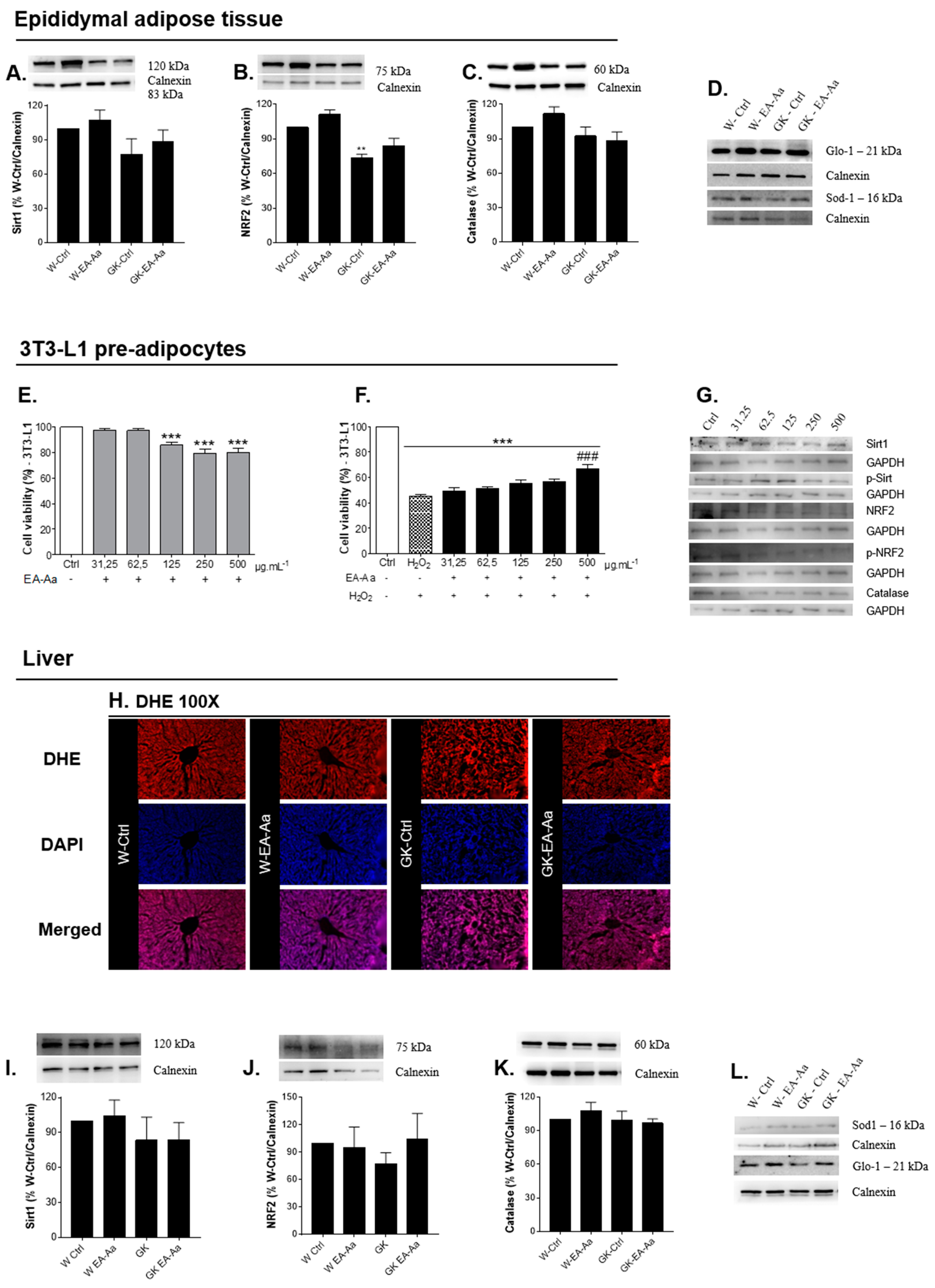
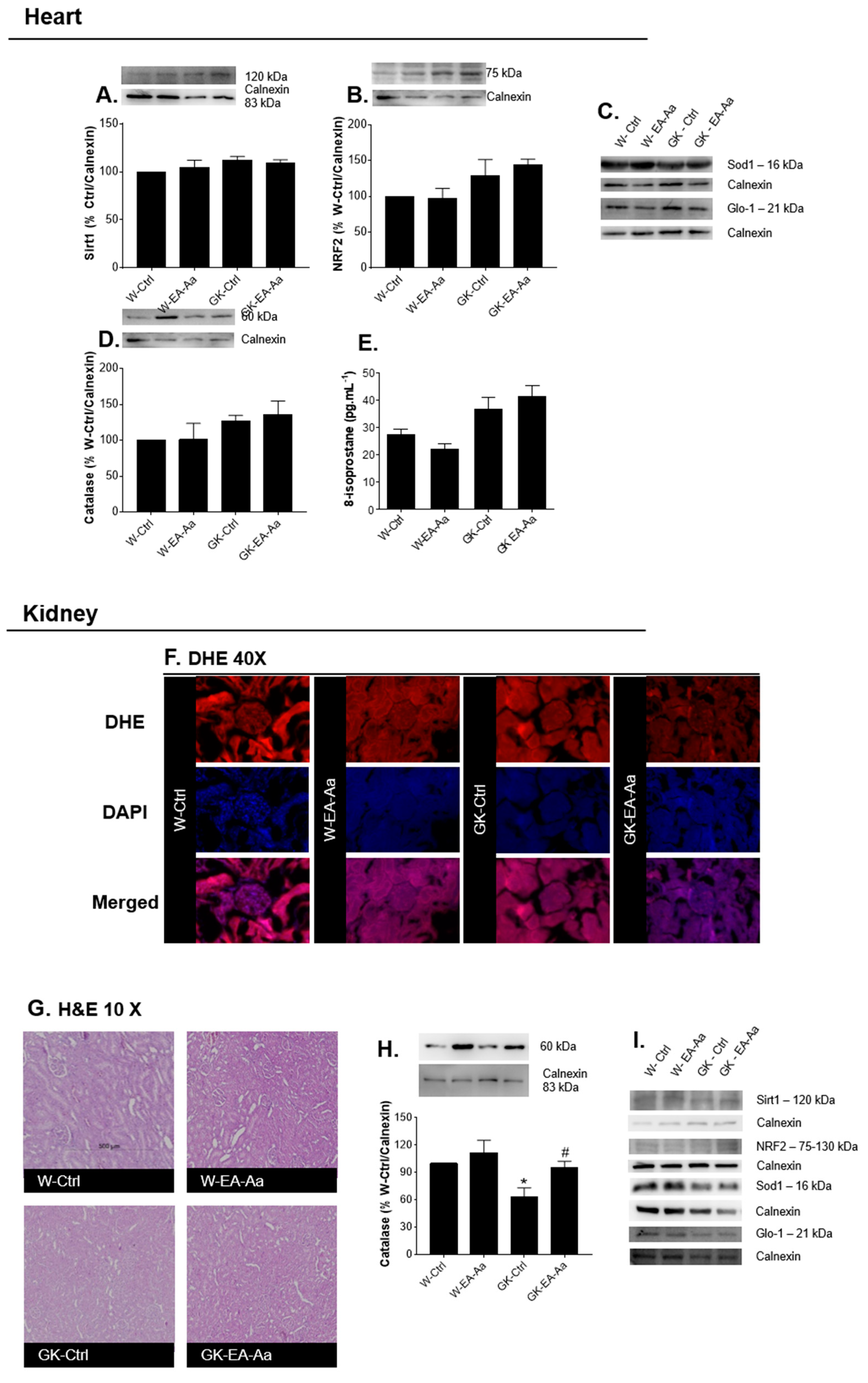
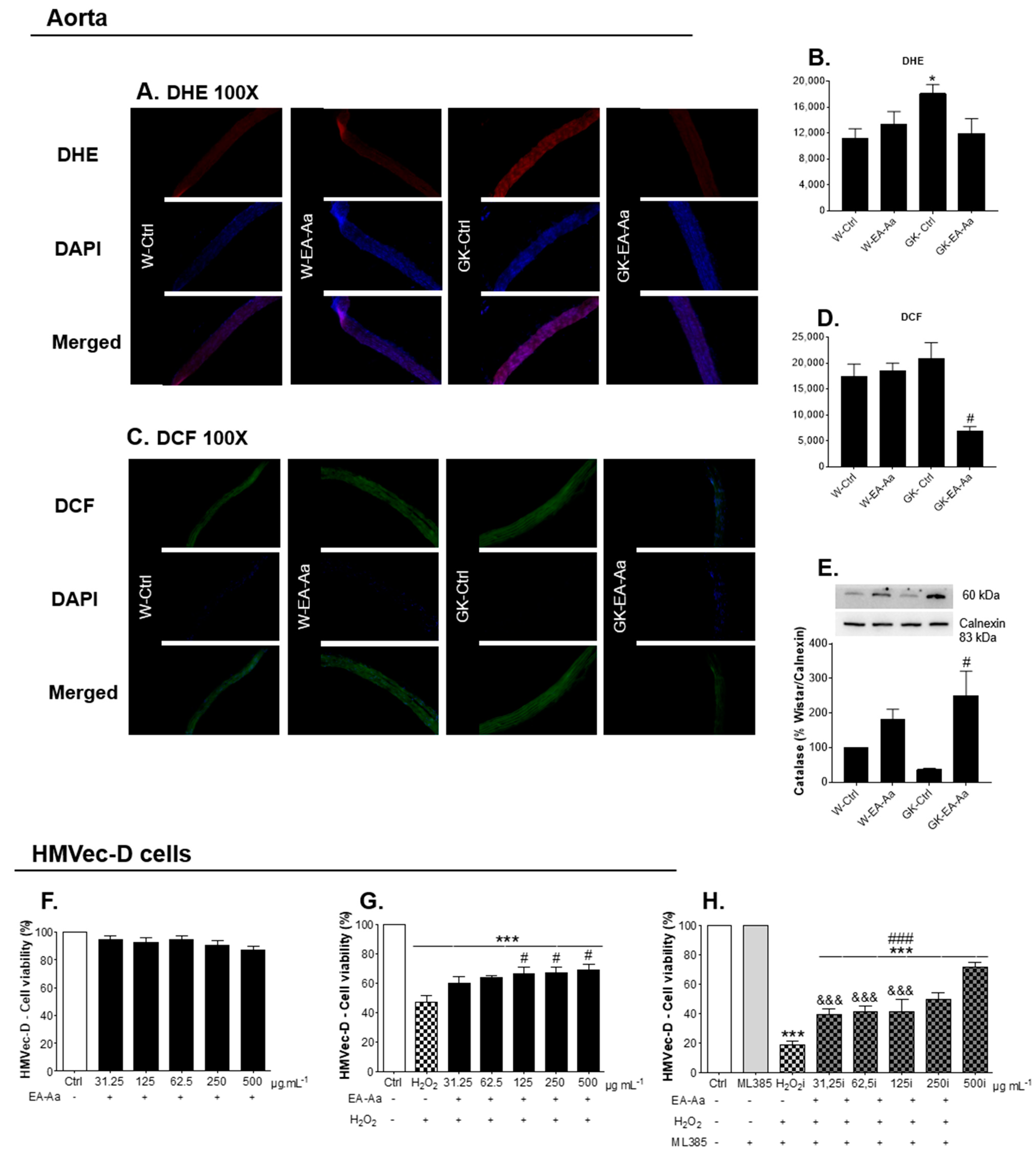
3.2. EA-Aa Has Tissue-Specific Protective Antioxidant Effects
3.3. EA-Aa Improves Diabetic Endothelial Dysfunction Reducing Vascular Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef] [PubMed]
- IDF Diabetes Atlas Worldwide Toll of Diabetes. Available online: https://www.diabetesatlas.org/en/sections/worldwide-toll-of-diabetes.html (accessed on 28 April 2020).
- Papatheodorou, K.; Banach, M.; Bekiari, E.; Rizzo, M.; Edmonds, M. Complications of Diabetes 2017. Available online: https://www.hindawi.com/journals/jdr/2018/3086167/ (accessed on 28 April 2020).
- Huang, D.-D.; Shi, G.; Jiang, Y.; Yao, C.; Zhu, C. A review on the potential of resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed. Pharmacother. 2020, 125, 109767. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Kapoor, B.; Gulati, M.; Kumar, R.; Ramanunny, A.K.; Awasthi, A.; Dua, K. Treatment strategies against diabetes: Success so far and challenges ahead. Eur. J. Pharmacol. 2019, 862, 172625. [Google Scholar] [CrossRef]
- Figueiredo de Santana Aquino, D.; Monteiro, T.A.; Lima Cardoso, C.A.; Heredia Vieira, S.C.; do Carmo Vieira, M.; de Picoli Souza, K.; Amaya-Farfan, J.; Borges Castro Carvalho, G.C.; Moura, C.S.; Morato, P.N. Investigation of the antioxidant and hypoglycemiant properties of Alibertia edulis (L.C. Rich.) A.C. Rich. leaves. J. Ethnopharmacol. 2020, 253, 112648. [Google Scholar] [CrossRef]
- MMA Ministério Do Meio Ambiente—O Bioma Cerrado. Available online: https://www.mma.gov.br/biomas/cerrado (accessed on 9 September 2019).
- Ribeiro, D.A.; de Oliveira, L.G.S.; de Macêdo, D.G.; de Menezes, I.R.A.; da Costa, J.G.M.; da Silva, M.A.P.; Lacerda, S.R.; de Almeida Souza, M.M. Promising medicinal plants for bioprospection in a Cerrado area of Chapada do Araripe, Northeastern Brazil. J. Ethnopharmacol. 2014, 155, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Neto, J.A.; Pimenta Tarôco, B.R.; Batista dos Santos, H.; Thomé, R.G.; Wolfram, E.; de A Ribeiro, R.I.M. Using the plants of Brazilian Cerrado for wound healing: From traditional use to scientific approach. J. Ethnopharmacol. 2020, 112547. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira Agostini-Costa, T. Bioactive compounds and health benefits of some palm species traditionally used in Africa and the Americas—A review. J. Ethnopharmacol. 2018, 224, 202–229. [Google Scholar] [CrossRef]
- Silva, P. Caracterização Química e Avaliação Do Potencialantidiabético e Citotóxico de Oleo Extraído de Acrocomia aculeata (Macaúba). Master’s Thesis, Universidade Federal da Grande Dourados, Dourados, Brazil, 2012. [Google Scholar]
- Monteiro-Alfredo, T.; Matafome, P.; Iacia, B.P.; Antunes, K.Á.; dos Santos, J.M.; da Silva Melo da Cunha, J.; Oliveira, S.; Oliveira, A.S.; Campos, J.F.; Magalhães, M.; et al. Acrocomia aculeata (Jacq.) Lodd. Ex Mart. Leaves Increase SIRT1 Levels and Improve Stress Resistance. Available online: https://www.hindawi.com/journals/omcl/2020/5238650/ (accessed on 20 March 2020).
- 3T3-L1 ATCC® CL-173TM Mus Musculus Embryo. Available online: https://www.lgcstandards-atcc.org/products/all/CL-173.aspx?geo_country=ro#culturemethod (accessed on 8 February 2021).
- Soares, J.; Espadinha, M.; Raimundo, L.; Ramos, H.; Gomes, A.S.; Gomes, S.; Loureiro, J.B.; Inga, A.; Reis, F.; Gomes, C.; et al. DIMP53-1: A novel small-molecule dual inhibitor of p53–MDM2/X interactions with multifunctional p53-dependent anticancer properties. Mol. Oncol. 2017, 11, 612–627. [Google Scholar] [CrossRef]
- EGM-2MV Microvascular Endothelial Cell Growth Medium-2|Lonza. Available online: https://bioscience.lonza.com/lonza_bs/CH/en/Primary-and-Stem-Cells/p/000000000000185321/EGM--2-MV-Microvascular-Endothelial-Cell-Growth-Medium-2-BulletKit (accessed on 8 February 2021).
- Rodrigues, T.; Borges, P.; Mar, L.; Marques, D.; Albano, M.; Eickhoff, H.; Carrêlo, C.; Almeida, B.; Pires, S.; Abrantes, M.; et al. GLP-1 improves adipose tissue glyoxalase activity and capillarization improving insulin sensitivity in type 2 diabetes. Pharmacol. Res. 2020, 161, 105198. [Google Scholar] [CrossRef]
- Matafome, P.; Santos-Silva, D.; Crisóstomo, J.; Rodrigues, T.; Rodrigues, L.; Sena, C.M.; Pereira, P.; Seiça, R. Methylglyoxal causes structural and functional alterations in adipose tissue independently of obesity. Arch. Physiol. Biochem. 2012, 118, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Matafome, P.; Louro, T.; Nunes, E.; Seiça, R.M. Effects of atorvastatin and insulin in vascular dysfunction associated with type 2 diabetes. Physiol. Res. 2014, 63, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Shabab, S.; Gholamnezhad, Z.; Mahmoudabady, M. Protective effects of medicinal plant against diabetes induced cardiac disorder: A review. J. Ethnopharmacol. 2021, 265, 113328. [Google Scholar] [CrossRef] [PubMed]
- King, G.L.; Brownlee, M. The cellular and molecular mechanisms of diabetic complications. Endocrinol. Metab. Clin. North. Am. 1996, 25, 255–270. [Google Scholar] [CrossRef]
- Santos, R.X.; Cardoso, S.; Silva, S.; Correia, S.; Carvalho, C.; Crisóstomo, J.; Rodrigues, L.; Amaral, C.; Louro, T.; Matafome, P.; et al. Food deprivation promotes oxidative imbalance in rat brain. J. Food Sci. 2009, 74, H8–H14. [Google Scholar] [CrossRef]
- Da Silva, P.V.B.; Ramiro, M.M.; Iriguchi, E.K.K.; Corrêa, W.A.; Lowe, J.; Cardoso, C.A.L.; Arena, A.C.; Kassuya, C.A.L.; Muzzi, R.M. Antidiabetic, cytotoxic and antioxidant activities of oil extracted from Acrocomia aculeata pulp. Nat. Prod. Res. 2019, 33, 2413–2416. [Google Scholar] [CrossRef] [Green Version]
- Nonato, C.D.F.A.; Leite, D.O.D.; Pereira, R.C.; Boligon, A.A.; Ribeiro-Filho, J.; Rodrigues, F.F.G.; da Costa, J.G.M. Chemical analysis and evaluation of antioxidant and antimicrobial activities of fruit fractions of Mauritia flexuosa L. f. (Arecaceae). PeerJ 2018, 6, e5991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, Â.A.; Buccini, D.F.; Jaques, J.A.S.; Portugal, L.C.; Guimarães, R.C.A.; Favaro, S.P.; Caldas, R.A.; Carvalho, C.M.E. Effect of Acrocomia aculeata kernel oil on adiposity in type 2 diabetic rats. Plant. Foods Hum. Nutr. 2018, 73, 61–67. [Google Scholar] [CrossRef]
- Nunes, Â.A.; Buccini, D.F.; dos Santos Jaques, J.A.; Portugal, L.C.; Guimarães, R.C.A.; Favaro, S.P.; de Araújo Caldas, R.; Carvalho, C.M.E. Effect of dietary Acrocomia aculeata kernel oil rich in medium chain fatty acids on type 2 diabetic rats. J. Funct. Foods 2020, 75, 104295. [Google Scholar] [CrossRef]
- Abdelaziz, D.H.A.; Ali, S.A.; Mostafa, M.M.A. Phoenix dactylifera seeds ameliorate early diabetic complications in streptozotocin-induced diabetic rats. Pharm. Biol. 2015, 53, 792–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, F.G.; de Araújo, F.F.; de Paulo Farias, D.; Zanotto, A.W.; Neri-Numa, I.A.; Pastore, G.M. Brazilian fruits of Arecaceae family: An overview of some representatives with promising food, therapeutic and industrial applications. Food Res. Int. 2020, 138, 109690. [Google Scholar] [CrossRef]
- Preetha, P.P.; Girija Devi, V.; Rajamohan, T. Comparative effects of mature coconut water (Cocos Nucifera) and glibenclamide on some biochemical parameters in alloxan induced diabetic rats. Rev. Bras. De Farmacogn. 2013, 23, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Renjith, R.S.; Chikku, A.M.; Rajamohan, T. Cytoprotective, antihyperglycemic and phytochemical properties of Cocos nucifera (L.) inflorescence. Asian Pac. J. Trop. Med. 2013, 6, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Egawa, T.; Tsuda, S.; Oshima, R.; Goto, A.; Ma, X.; Goto, K.; Hayashi, T. Regulatory mechanism of skeletal muscle glucose transport by phenolic acids. Phenolic Compd.—Biol. Act. 2017. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Gnanaraj, C.; Arulselvan, P.; El-Seedi, H.; Teng, H. A review on advanced microencapsulation technology to enhance bioavailability of phenolic compounds: Based on its activity in the treatment of type 2 diabetes. Trends Food Sci. Technol. 2019, 85, 149–162. [Google Scholar] [CrossRef]
- Eid, H.M.; Vallerand, D.; Muhammad, A.; Durst, T.; Haddad, P.S.; Martineau, L.C. Structural constraints and the importance of lipophilicity for the mitochondrial uncoupling activity of naturally occurring caffeic acid esters with potential for the treatment of insulin resistance. Biochem. Pharmacol. 2010, 79, 444–454. [Google Scholar] [CrossRef]
- Tsuda, S.; Egawa, T.; Ma, X.; Oshima, R.; Kurogi, E.; Hayashi, T. Coffee polyphenol caffeic acid but not chlorogenic acid increases 5′AMP-activated protein kinase and insulin-independent glucose transport in rat skeletal muscle. J. Nutr. Biochem. 2012, 23, 1403–1409. [Google Scholar] [CrossRef]
- Huang, D.-W.; Chang, W.-C.; Wu, J.S.-B.; Shih, R.-W.; Shen, S.-C. Gallic acid ameliorates hyperglycemia and improves hepatic carbohydrate metabolism in rats fed a high-fructose diet. Nutr. Res. 2016, 36, 150–160. [Google Scholar] [CrossRef]
- Prasad, C.N.V.; Anjana, T.; Banerji, A.; Gopalakrishnapillai, A. Gallic acid induces GLUT4 translocation and glucose uptake activity in 3T3-L1 cells. FEBS Lett. 2010, 584, 531–536. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.-J.; Li, Y.; Cao, Q.-H.; Wu, H.-X.; Tang, X.-Y.; Gao, X.-H.; Yu, J.-Q.; Chen, Z.; Yang, Y. In Vitro and In Vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef]
- Akuodor, G.; Udia, P.; Bassey, A.; Chilaka, K.; Okezie, O. Antihyperglycemic and antihyperlipidemic properties of aqueous root extract of Icacina senegalensis in alloxan induced diabetic rats. J. Acute Dis. 2014, 3, 99–103. [Google Scholar] [CrossRef]
- AL-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginsberg, H.N.; Zhang, Y.-L.; Hernandez-Ono, A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch. Med. Res. 2005, 36, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Chen, G.; Lee, Y.; Unger, R.H. Tissue triglycerides, insulin resistance, and insulin production: Implications for hyperinsulinemia of obesity. Am. J. Physiol. -Endocrinol. Metab. 1997, 273, E708–E713. [Google Scholar] [CrossRef] [PubMed]
- Kraegen, E.W.; Cooney, G.J.; Ye, J.; Thompson, A.L. Triglycerides, fatty acids and insulin resistance—Hyperinsulinemia. Exp. Clin. Endocrinol. Diabetes 2001, 109, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Feng, J.; Jiang, D.; Zhou, X.; Jiang, Q.; Cai, M.; Wang, X.; Shan, T.; Wang, Y. AMPK regulates lipid accumulation in skeletal muscle cells through FTO-dependent demethylation of N 6-methyladenosine. Sci. Rep. 2017, 7, 41606. [Google Scholar] [CrossRef] [Green Version]
- Zong, H.; Ren, J.M.; Young, L.H.; Pypaert, M.; Mu, J.; Birnbaum, M.J.; Shulman, G.I. AMP Kinase Is Required for Mitochondrial Biogenesis in Skeletal Muscle in Response to Chronic Energy Deprivation. Proc. Natl. Acad. Sci. USA 2002, 99, 15983–15987. [Google Scholar] [CrossRef] [Green Version]
- Fusi, F.; Saponara, S.; Pessina, F.; Gorelli, B.; Sgaragli, G. Effects of quercetin and rutin on vascular preparations: A comparison between mechanical and electrophysiological phenomena. Eur. J. Nutr. 2003, 42, 10–17. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Venema, D.P.; Peters, T.H.F.; Koenen, M.E.; Arts, I.C.W.; Vincken, J.-P.; Gruppen, H.; Keijer, J.; Hollman, P.C.H. Some phenolic compounds increase the nitric oxide level in endothelial cells In Vitro. J. Agric. Food Chem. 2009, 57, 7693–7699. [Google Scholar] [CrossRef]
- Taubert, D.; Berkels, R.; Klaus, W.; Roesen, R. Nitric oxide formation and corresponding relaxation of porcine coronary arteries induced by plant phenols: Essential structural features. J. Cardiovasc. Pharmacol. 2002, 40, 701–713. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Il Kim, H.; Hag Park, S.; Jung Lee, M.; Yeoul Jun, J.; Lee Kim, H.; Hoon Chung, J.; Ho Yeum, C. Endothelium-dependent vasodilation by ferulic acid in aorta from chronic renal hypertensive rats. Kidney Res. Clin. Pract. 2012, 31, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.-Y.; Xu, J.-Q.; Zhao, W.-R.; Chen, X.-L.; Jin, Y.; Tang, N.; Tang, J.-Y. Ferulic acid relaxed rat aortic, small mesenteric and coronary arteries by blocking voltage-gated calcium channel and calcium desensitization via dephosphorylation of ERK1/2 and MYPT1. Eur. J. Pharmacol. 2017, 815, 26–32. [Google Scholar] [CrossRef]
- De Oliveira, L.M.; de Oliveira, T.S.; da Costa, R.M.; de Souza Gil, E.; Costa, E.A.; de Cassia Aleixo Tostes Passaglia, R.; Filgueira, F.P.; Ghedini, P.C. The vasorelaxant effect of gallic acid involves endothelium-dependent and -independent mechanisms. Vasc. Pharmacol. 2016, 81, 69–74. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Traka, M.H.; Mithen, R.F. Plant science and human nutrition: Challenges in assessing health-promoting properties of phytochemicals. Plant Cell 2011, 23, 2483–2497. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of gallic acid on gut health: Focus on the gut microbiome, immune response, and mechanisms of action. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Lafay, S.; Morand, C.; Manach, C.; Besson, C.; Scalbert, A. Absorption and metabolism of caffeic acid and chlorogenic acid in the small intestine of rats. Br. J. Nutr. 2006, 96, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Sova, M.; Saso, L. Natural sources, pharmacokinetics, biological activities and health benefits of hydroxycinnamic acids and their metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef] [PubMed]
- Topakas, E.; Kalogeris, E.; Kekos, D.; Macris, B.J.; Christakopoulos, P. Bioconversion of ferulic acid into vanillic acid by the thermophilic fungus sporotrichum thermophile. LWT Food Sci. Technol. 2003, 36, 561–565. [Google Scholar] [CrossRef]
- Winterbone, M.S.; Tribolo, S.; Needs, P.W.; Kroon, P.A.; Hughes, D.A. Physiologically relevant metabolites of quercetin have no effect on adhesion molecule or chemokine expression in human vascular smooth muscle cells. Atherosclerosis 2009, 202, 431–438. [Google Scholar] [CrossRef]
- Pashikanti, S.; de Alba, D.R.; Boissonneault, G.A.; Cervantes-Laurean, D. Rutin metabolites: Novel inhibitors of nonoxidative advanced glycation end products. Free Radic. Biol. Med. 2010, 48, 656–663. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro-Alfredo, T.; Oliveira, S.; Amaro, A.; Rosendo-Silva, D.; Antunes, K.; Pires, A.S.; Teixo, R.; Abrantes, A.M.; Botelho, M.F.; Castelo-Branco, M.; et al. Hypoglycaemic and Antioxidant Properties of Acrocomia aculeata (Jacq.) Lodd Ex Mart. Extract Are Associated with Better Vascular Function of Type 2 Diabetic Rats. Nutrients 2021, 13, 2856. https://doi.org/10.3390/nu13082856
Monteiro-Alfredo T, Oliveira S, Amaro A, Rosendo-Silva D, Antunes K, Pires AS, Teixo R, Abrantes AM, Botelho MF, Castelo-Branco M, et al. Hypoglycaemic and Antioxidant Properties of Acrocomia aculeata (Jacq.) Lodd Ex Mart. Extract Are Associated with Better Vascular Function of Type 2 Diabetic Rats. Nutrients. 2021; 13(8):2856. https://doi.org/10.3390/nu13082856
Chicago/Turabian StyleMonteiro-Alfredo, Tamaeh, Sara Oliveira, Andreia Amaro, Daniela Rosendo-Silva, Katia Antunes, Ana Salomé Pires, Ricardo Teixo, Ana Margarida Abrantes, Maria Filomena Botelho, Miguel Castelo-Branco, and et al. 2021. "Hypoglycaemic and Antioxidant Properties of Acrocomia aculeata (Jacq.) Lodd Ex Mart. Extract Are Associated with Better Vascular Function of Type 2 Diabetic Rats" Nutrients 13, no. 8: 2856. https://doi.org/10.3390/nu13082856
APA StyleMonteiro-Alfredo, T., Oliveira, S., Amaro, A., Rosendo-Silva, D., Antunes, K., Pires, A. S., Teixo, R., Abrantes, A. M., Botelho, M. F., Castelo-Branco, M., Seiça, R., Silva, S., de Picoli Souza, K., & Matafome, P. (2021). Hypoglycaemic and Antioxidant Properties of Acrocomia aculeata (Jacq.) Lodd Ex Mart. Extract Are Associated with Better Vascular Function of Type 2 Diabetic Rats. Nutrients, 13(8), 2856. https://doi.org/10.3390/nu13082856









