Relationship between Circulating Galectin-3, Systemic Inflammation, and Protein-Energy Wasting in Chronic Hemodialysis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Design and Patients
2.2. Laboratory Measurements
2.3. Measurement of Lean Body Mass
2.4. Assessment of Nutritional Status
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Hemodialysis Patients Stratified by the Plasma Galectin-3 Level
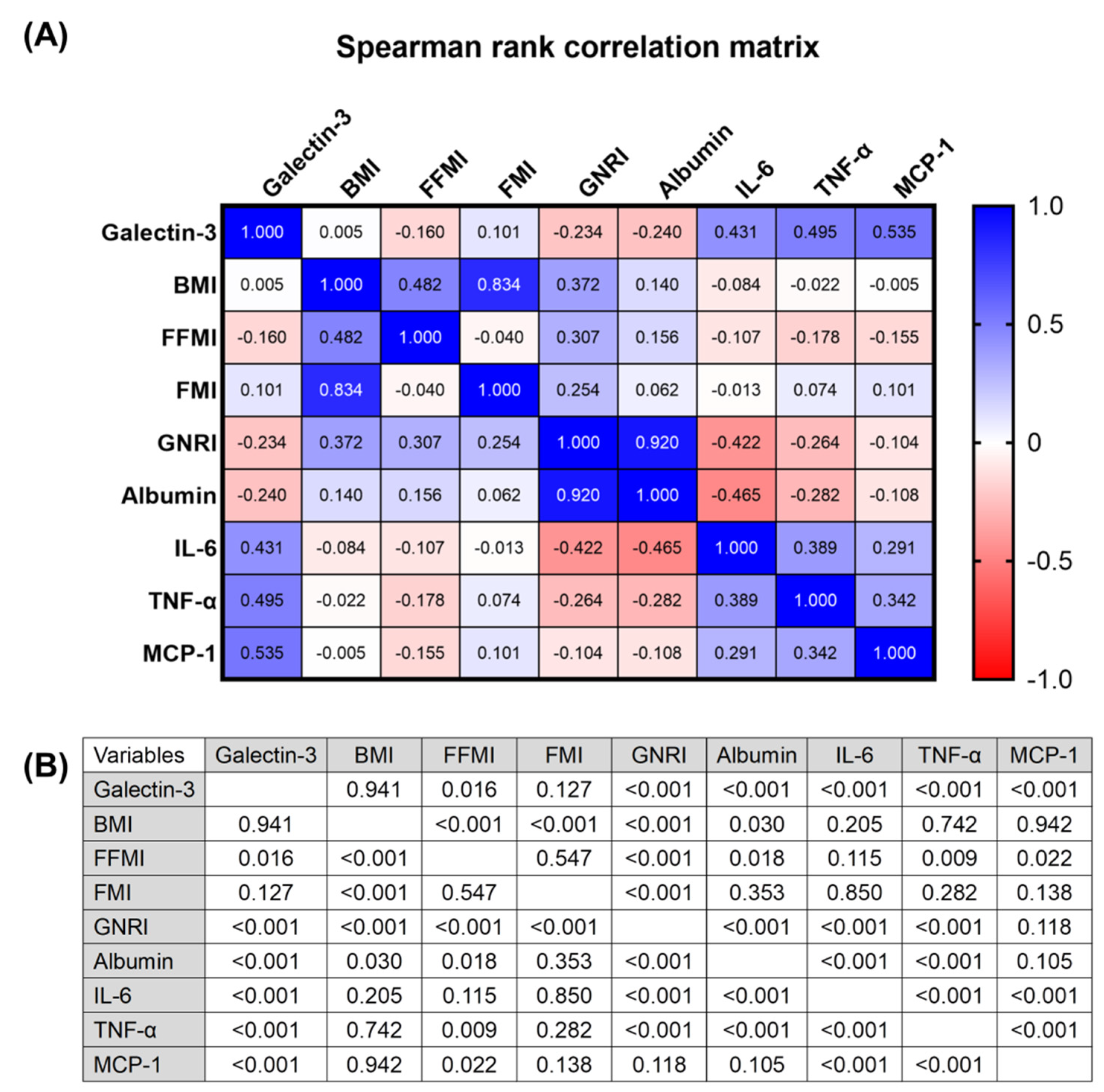
3.2. Correlation Analysis between Plasma Galectin-3 Levels, Body Composition, Circulating Inflammatory Markers, and Gnri in Hemodialysis Patients
3.3. Factors Associated with Increased Plasma Galectin-3 Levels in Hemodialysis Patients
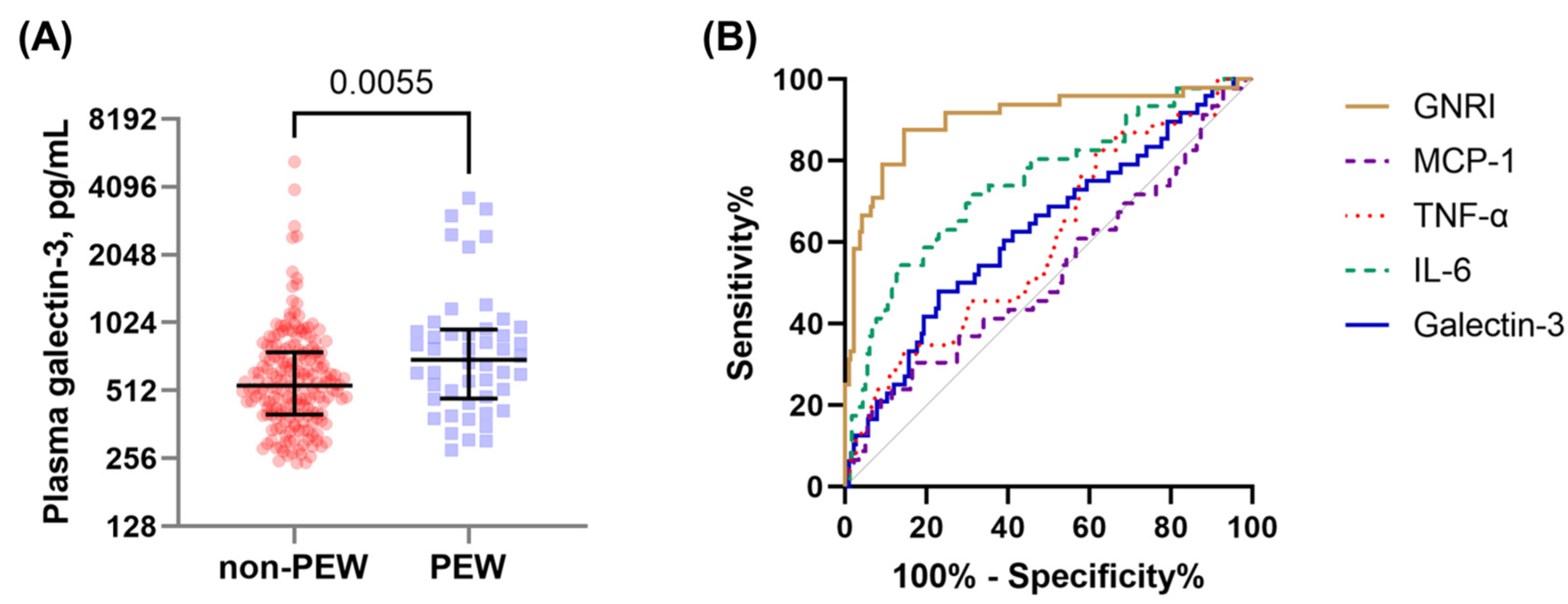
3.4. Factors Associated with the Presence of PEW in Hemodialysis Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Cockwell, P.; Fisher, L.A. The global burden of chronic kidney disease. Lancet 2020, 395, 662–664. [Google Scholar] [CrossRef] [Green Version]
- Cobo, G.; Lindholm, B.; Stenvinkel, P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol. Dial. Transplant. 2018, 33, iii35–iii40. [Google Scholar] [CrossRef] [Green Version]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Tsai, M.T.; Hu, F.H.; Lien, T.J.; Chen, P.J.; Huang, T.P.; Tarng, D.C. Interaction between geriatric nutritional risk index and decoy receptor 3 predicts mortality in chronic hemodialysis patients. Am. J. Nephrol. 2014, 40, 191–199. [Google Scholar] [CrossRef]
- Onal, E.M.; Afsar, B.; Covic, A.; Vaziri, N.D.; Kanbay, M. Gut microbiota and inflammation in chronic kidney disease and their roles in the development of cardiovascular disease. Hypertens Res. 2019, 42, 123–140. [Google Scholar] [CrossRef] [Green Version]
- Kooman, J.P.; Dekker, M.J.; Usvyat, L.A.; Kotanko, P.; van der Sande, F.M.; Schalkwijk, C.G.; Shiels, P.G.; Stenvinkel, P. Inflammation and premature aging in advanced chronic kidney disease. Am. J. Physiol. Renal Physiol. 2017, 313, F938–F950. [Google Scholar] [CrossRef] [Green Version]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [Green Version]
- Koppe, L.; Fouque, D.; Kalantar-Zadeh, K. Kidney cachexia or protein-energy wasting in chronic kidney disease: Facts and numbers. J. Cachexia Sarcopenia Muscle 2019, 10, 479–484. [Google Scholar] [CrossRef] [Green Version]
- Obi, Y.; Qader, H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr. Opin. Clin. Nutr. Metab Care 2015, 18, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Lodebo, B.T.; Shah, A.; Kopple, J.D. Is it Important to Prevent and Treat Protein-Energy Wasting in Chronic Kidney Disease and Chronic Dialysis Patients? J. Ren. Nutr. 2018, 28, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.T.; Liu, H.C.; Huang, T.P. The impact of malnutritional status on survival in elderly hemodialysis patients. J. Chin. Med. Assoc. 2016, 79, 309–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef]
- Thiemann, S.; Baum, L.G. Galectins and Immune Responses-Just How Do They Do Those Things They Do? Annu. Rev. Immunol. 2016, 34, 243–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.C.; Kuo, P.L. The Role of Galectin-3 in the Kidneys. Int. J. Mol. Sci. 2016, 17, 565. [Google Scholar] [CrossRef] [Green Version]
- Koch, A.; Poirier, F.; Jacob, R.; Delacour, D. Galectin-3, a novel centrosome-associated protein, required for epithelial morphogenesis. Mol. Biol. Cell 2010, 21, 219–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desmedt, V.; Desmedt, S.; Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Galectin-3 in Renal Pathology: More than Just an Innocent Bystander. Am. J. Nephrol. 2016, 43, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, M.; Coutrot, M.; Michel, T.; Boutin, L.; Genest, M.; Poirier, F.; Launay, J.M.; Kane, B.; Kinugasa, S.; Prakoura, N.; et al. Acute Kidney Injury Induces Remote Cardiac Damage and Dysfunction Through the Galectin-3 Pathway. JACC Basic. Transl. Sci. 2019, 4, 717–732. [Google Scholar] [CrossRef]
- O’Seaghdha, C.M.; Hwang, S.J.; Ho, J.E.; Vasan, R.S.; Levy, D.; Fox, C.S. Elevated galectin-3 precedes the development of CKD. J. Am. Soc. Nephrol. 2013, 24, 1470–1477. [Google Scholar] [CrossRef] [Green Version]
- Drechsler, C.; Delgado, G.; Wanner, C.; Blouin, K.; Pilz, S.; Tomaschitz, A.; Kleber, M.E.; Dressel, A.; Willmes, C.; Krane, V.; et al. Galectin-3, Renal Function, and Clinical Outcomes: Results from the LURIC and 4D Studies. J. Am. Soc. Nephrol. 2015, 26, 2213–2221. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Cao, S.; Yang, H.; Li, J. Prognostic impact of galectin-3 in chronic kidney disease patients: A systematic review and meta-analysis. Int. Urol. Nephrol. 2019, 51, 1005–1011. [Google Scholar] [CrossRef]
- Shimura, T.; Shibata, M.; Gonda, K.; Kofunato, Y.; Okada, R.; Ishigame, T.; Kimura, T.; Kenjo, A.; Kono, K.; Marubashi, S. Significance of Circulating Galectin-3 in Patients with Pancreatobiliary Cancer. Anticancer Res. 2017, 37, 4979–4986. [Google Scholar] [CrossRef] [Green Version]
- Shimura, T.; Shibata, M.; Gonda, K.; Nakajima, T.; Chida, S.; Noda, M.; Suzuki, S.; Nakamura, I.; Ohki, S.; Takenoshita, S. Association between circulating galectin-3 levels and the immunological, inflammatory and nutritional parameters in patients with colorectal cancer. Biomed. Rep. 2016, 5, 203–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, M.T.; Tseng, W.C.; Ou, S.M.; Lee, K.H.; Yang, C.Y.; Tarng, D.C. Comparison of Simplified Creatinine Index and Systemic Inflammatory Markers for Nutritional Evaluation of Hemodialysis Patients. Nutrients 2021, 13, 1870. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.M.; Kuo, H.C.; Chang, J.S.; Chen, S.P.; Liu, Y.M.; Chen, H.W.; Tsai, F.J.; Lee, Y.C.; Chen, C.H.; Wu, J.Y.; et al. CXCL10/IP-10 is a biomarker and mediator for Kawasaki disease. Circ. Res. 2015, 116, 876–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daugirdas, J.T. Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J. Am. Soc. Nephrol. 1993, 4, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Daugirdas, J.T. Simplified equations for monitoring Kt/V, PCRn, eKt/V, and ePCRn. Adv. Ren. Replace Ther. 1995, 2, 295–304. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Hsu, D.K. The role of galectin-3 in promotion of the inflammatory response. Drug News Perspect. 2007, 20, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.C.; Choy, C.S.; Lin, W.N.; Chang, S.W.; Liou, J.C.; Tung, T.H.; Hsieh, C.Y.; Chang, J.F. Galectin-3 Interacts with Vascular Cell Adhesion Molecule-1 to Increase Cardiovascular Mortality in Hemodialysis Patients. J. Clin. Med. 2018, 7, 300. [Google Scholar] [CrossRef] [Green Version]
- Tuegel, C.; Katz, R.; Alam, M.; Bhat, Z.; Bellovich, K.; de Boer, I.; Brosius, F.; Gadegbeku, C.; Gipson, D.; Hawkins, J.; et al. GDF-15, Galectin 3, Soluble ST2, and Risk of Mortality and Cardiovascular Events in CKD. Am. J. Kidney Dis. 2018, 72, 519–528. [Google Scholar] [CrossRef]
- Kopple, J.D. Pathophysiology of protein-energy wasting in chronic renal failure. J. Nutr. 1999, 129, 247s–251s. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Block, G.; McAllister, C.J.; Humphreys, M.H.; Kopple, J.D. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am. J. Clin. Nutr. 2004, 80, 299–307. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef]
- Pellegrinelli, V.; Rouault, C.; Rodriguez-Cuenca, S.; Albert, V.; Edom-Vovard, F.; Vidal-Puig, A.; Clément, K.; Butler-Browne, G.S.; Lacasa, D. Human Adipocytes Induce Inflammation and Atrophy in Muscle Cells During Obesity. Diabetes 2015, 64, 3121–3134. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Liu, S.; Lu, M.; Bandyopadhyay, G.; Oh, D.; Imamura, T.; Johnson, A.M.F.; Sears, D.; Shen, Z.; Cui, B.; et al. Hematopoietic-Derived Galectin-3 Causes Cellular and Systemic Insulin Resistance. Cell 2016, 167, 973–984.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rancourt, A.; Dufresne, S.S.; St-Pierre, G.; Lévesque, J.C.; Nakamura, H.; Kikuchi, Y.; Satoh, M.S.; Frenette, J.; Sato, S. Galectin-3 and N-acetylglucosamine promote myogenesis and improve skeletal muscle function in the mdx model of Duchenne muscular dystrophy. FASEB J. 2018, 32, fj201701151RRR. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.N.; Su, Y.; Vizi, D.; Fang, L.; Ellims, A.H.; Zhao, W.B.; Kiriazis, H.; Gao, X.M.; Sadoshima, J.; Taylor, A.J.; et al. Mechanisms responsible for increased circulating levels of galectin-3 in cardiomyopathy and heart failure. Sci. Rep. 2018, 8, 8213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.W.; Hsu, L.W.; Chang, H.Y.; Huang, T.C.; Yu, J.R.; Liao, H.Y.; Lee, C.H.; Liu, P.Y. Elevated Platelet Galectin-3 and Rho-Associated Protein Kinase Activity Are Associated with Hemodialysis Arteriovenous Shunt Dysfunction among Subjects with Diabetes Mellitus. Biomed. Res. Int. 2019, 2019, 8952414. [Google Scholar] [CrossRef] [Green Version]
- Meijers, W.C.; van der Velde, A.R.; Ruifrok, W.P.; Schroten, N.F.; Dokter, M.M.; Damman, K.; Assa, S.; Franssen, C.F.; Gansevoort, R.T.; van Gilst, W.H.; et al. Renal handling of galectin-3 in the general population, chronic heart failure, and hemodialysis. J. Am. Heart Assoc. 2014, 3, e000962. [Google Scholar] [CrossRef] [Green Version]
| Parameters a | All Patients (n = 240) | Galectin-3 ≤ 567 pg/mL (n = 120) | Galectin-3 > 567 pg/mL (n = 120) | p Value |
|---|---|---|---|---|
| Galectin-3, pg/mL | 567.3 (406.5−808.5) | 406.9 (332.0–480.2) | 806.3 (657.5–987.0) | <0.001 |
| Demographic and clinical characteristics | ||||
| Age, years | 64.5 (55.3–74.0) | 61.0 (52.5–69.0) | 68.5 (58.3–76.0) | 0.001 |
| Women, n (%) | 86 (35.8) | 41 (34.2) | 45 (37.5) | 0.590 |
| Dialysis vintage, months | 49.0 (29.0–93.8) | 48.5 (28.0–84.8) | 49.0 (29.0–108.3) | 0.740 |
| spKt/Vurea | 1.53 ± 0.26 | 1.53 ± 0.25 | 1.53 ± 0.26 | 0.983 |
| nPCR, g/kg/day | 1.13 (1.00–1.30) | 1.11 (1.00–1.26) | 1.15 (0.98–1.35) | 0.258 |
| Hypertension, n (%) | 212 (88.3) | 108 (90.0) | 104 (86.7) | 0.421 |
| Diabetes mellitus, n (%) | 134 (55.8) | 62 (51.7) | 72 (60.0) | 0.194 |
| Cardiovascular disease, n (%) | 113 (47.1) | 52 (43.3) | 61 (50.8) | 0.244 |
| Mean arterial pressure, mmHg | 101 ± 18 | 105 ± 17 | 97 ± 18 | 0.001 |
| Dialyzer | 0.776 | |||
| High-flux, n (%) | 170 (70.8) | 84 (70.0) | 86 (71.7) | |
| Low-flux, n (%) | 70 (29.2) | 36 (30.0) | 34 (28.3) | |
| Vascular access | 0.001 | |||
| AV fistula, n (%) | 188 (78.3) | 102 (85.0) | 86 (71.7) | |
| AV graft, n (%) | 28 (11.7) | 15 (12.5) | 13 (10.8) | |
| Tunneled catheter, n (%) | 24 (10.0) | 3 (2.5) | 21 (17.5) | |
| Anthropometric measurements and nutritional scores | ||||
| Body mass index, kg/m2 | 24.3 (21.9–27.3) | 24.0 (22.2–27.0) | 24.9 (21.3–27.6) | 0.642 |
| Fat-free mass index, kg/m2 | 16.4 (14.7–18.1) | 16.8 (15.1–18.4) | 15.9 (14.5–17.7) | 0.026 |
| Fat mass index, kg/m2 | 8.1 (5.2–10.6) | 7.5 (5.0–10.2) | 8.8 (5.3–10.7) | 0.069 |
| ECW/TBW, % | 40.1 (39.2–41.2) | 40.0 (39.1–40.9) | 40.4 (39.3–41.5) | 0.107 |
| GNRI | 101.3 (97.2–105.7) | 102.7 (98.3–106.7) | 100.3 (95.7–102.7) | 0.003 |
| GNRI ≤ 98, n (%) | 62 (25.8) | 25 (20.8) | 37 (30.8) | 0.077 |
| Presence of PEW b, n (%) | 48 (20.0) | 17 (14.2) | 31 (25.8) | 0.024 |
| Laboratory test results | ||||
| Hemoglobin, g/dL | 9.8 (9.0–10.7) | 9.9 (9.0–10.7) | 9.8 (9.1–10.7) | 0.993 |
| Leuocytes, 103/μL | 5.7 (4.7–7.0) | 5.9 (5.0–7.0) | 5.4 (4.6–7.1) | 0.232 |
| Albumin, g/dL | 4.1 (3.9–4.3) | 4.1 (3.9–4.4) | 4.0 (3.8–4.1) | 0.002 |
| Calcium, mg/dL | 9.1 (8.5–9.6) | 9.2 (8.7–9.7) | 9.0 (8.5–9.6) | 0.252 |
| Phosphorous, mg/dL | 5.1 (4.3–6.0) | 5.1 (4.3–5.9) | 5.1 (4.3–6.1) | 0.999 |
| Total cholesterol, mg/dL | 142 (124–167) | 145 (125–173) | 138 (123–165) | 0.099 |
| Glucose, mg/dL | 123 (99–165) | 114 (94–170) | 131 (102–165) | 0.070 |
| Plasma biomarkers | ||||
| IL-6, pg/mL | 11.9 (6.9–22.8) | 8.8 (3.8–15.5) | 16.0 (9.6–32.3) | <0.001 |
| TNF-α, pg/mL | 2.9 (1.2–5.1) | 1.3 (1.0–3.9) | 4.0 (2.4–6.1) | <0.001 |
| MCP-1, pg/mL | 173.6 (129.2–231.8) | 139.1 (102.6–180.3) | 208.9 (167.6–271.8) | <0.001 |
| High Plasma Level of Galectin-3 | ||||
|---|---|---|---|---|
| Univariate | Multivariate a | |||
| Variables | Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value |
| Age (per year) | 1.034 (1.015–1.054) | 0.001 | 1.009 (0.984–1.035) | 0.483 |
| Gender (male:female) | 0.865 (0.510–1.467) | 0.590 | 1.269 (0.612–2.634) | 0.522 |
| Dialysis vintage (per year) | 1.015 (0.961–1.073) | 0.589 | – | – |
| spKt/Vurea (per 0.1 unit) | 1.001 (0.906–1.106) | 0.983 | – | – |
| Hypertension (yes:no) | 0.722 (0.326–1.600) | 0.423 | – | – |
| Diabetes mellitus (yes:no) | 1.403 (0.841–2.340) | 0.194 | – | – |
| Cardiovascular disease (yes:no) | 1.352 (0.813–2.248) | 0.245 | – | – |
| MAP (per 10 mmHg) | 0.773 (0.663–0.901) | 0.001 | 0.761 (0.614–0.943) | 0.013 |
| High-flux (yes:no) | 1.084 (0.621–1.892) | 0.776 | – | – |
| Tunnel catheter (yes:no) | 8.273 (2.396–28.558) | 0.001 | 7.313 (1.396–38.318) | 0.019 |
| ECW/TBW, % | 1.076 (0.918–1.261) | 0.366 | – | – |
| Presence of PEW (yes:no) | 2.110 (1.095–4.067) | 0.026 | 1.231 (0.476–3.180) | 0.668 |
| Hemoglobin (per g/dL) | 1.093 (0.896–1.333) | 0.381 | – | – |
| Leuocytes (per 103/μL) | 0.922 (0.815–1.043) | 0.196 | – | – |
| Calcium (per mg/dL) | 0.856 (0.632–1.159) | 0.313 | – | – |
| Phosphorous (per mg/dL) | 1.020 (0.861–1.207) | 0.820 | – | – |
| Total cholesterol (per mg/dL) | 0.994 (0.987–1.001) | 0.079 | 1.000 (0.990–1.010) | 0.989 |
| Glucose (per mg/dL) | 1.003 (0.999–1.006) | 0.166 | – | – |
| IL-6 (per pg/mL) | 1.034 (1.014–1.055) | 0.001 | 1.020 (0.999–1.041) | 0.064 |
| TNF-α (per pg/mL) | 1.459 (1.267–1.681) | <0.001 | 1.374 (1.167–1.617) | <0.001 |
| MCP-1 (per pg/mL) | 1.012 (1.008–1.017) | <0.001 | 1.009 (1.004–1.014) | <0.001 |
| AUC (95% CI) | p-Value | SEN a (%) | SPEC a (%) | PPV a (%) | NPV a (%) | PLR a | NLR a | |
|---|---|---|---|---|---|---|---|---|
| GNRI | 0.90 (0.86–0.94) b | <0.0001 | 87.5 | 83.3 | 56.8 | 96.4 | 5.25 | 0.15 |
| IL-6 | 0.75 (0.69–0.80) c | <0.0001 | 54.4 | 86.3 | 50.0 | 88.2 | 3.96 | 0.53 |
| Gal-3 | 0.63 (0.56–0.69) d | 0.0057 | 47.9 | 77.1 | 34.3 | 85.5 | 2.09 | 0.68 |
| TNF-α | 0.60 (0.54–0.67) | 0.0270 | 82.6 | 38.5 | 25.3 | 89.7 | 1.34 | 0.45 |
| MCP-1 | 0.53 (0.46–0.59) | 0.5997 | 30.4 | 83.5 | 31.8 | 82.6 | 1.85 | 0.83 |
| Variables a | Model 1 b | Model 2 c | Model 3 d | Model 4 e | ||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value | |
| Galectin-3 (per pg/mL) | 1.001 (1.000–1.001) | 0.013 | 1.000 (1.000–1.001) | 0.058 | 1.000 (0.999–1.001) | 0.888 | 1.000 (0.999–1.001) | 0.630 |
| Age (per year) | 1.032 (1.007–1.059) | 0.013 | 1.014 (0.986–1.041) | 0.329 | 0.984 (0.948–1.021) | 0.388 | ||
| Gender (male:female) | 0.709 (0.365–1.378) | 0.311 | 0.657 (0.289–1.494) | 0.316 | 1.003 (0.225–4.462) | 0.997 | ||
| Tunnel catheter (yes:no) | 1.876 (0.433–8.119) | 0.400 | 1.038 (0.084–12.770) | 0.977 | ||||
| Hemoglobin (per g/dL) | 0.718 (0.510–1.012) | 0.058 | 0.906 (0.582–1.410) | 0.662 | ||||
| Calcium (per mg/dL) | 0.671 (0.419–1.074) | 0.097 | 1.268 (0.689–2.334) | 0.446 | ||||
| Phosphorous (per mg/dL) | 0.685 (0.512–0.916) | 0.011 | 0.952 (0.615–1.473) | 0.825 | ||||
| Total cholesterol (per mg/dL) | 0.977 (0.964–0.991) | 0.001 | 0.978 (0.959–0.997) | 0.025 | ||||
| MCP-1 (per pg/mL) | 1.000 (0.998–1.003) | 0.680 | 1.001 (0.997–1.004) | 0.724 | ||||
| nPCR (per g/kg/day) | 0.666 (0.507–0.875) | 0.003 | ||||||
| GNRI (per 1 unit) | 0.691 (0.595–0.804) | < 0.001 | ||||||
| FFMI (per 1 unit) | 0.912 (0.667–1.246) | 0.563 | ||||||
| ECW/TBW, % | 1.018 (0.774–1.340) | 0.896 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-T.; Ou, S.-M.; Chen, H.-Y.; Tseng, W.-C.; Lee, K.-H.; Yang, C.-Y.; Yang, R.-B.; Tarng, D.-C. Relationship between Circulating Galectin-3, Systemic Inflammation, and Protein-Energy Wasting in Chronic Hemodialysis Patients. Nutrients 2021, 13, 2803. https://doi.org/10.3390/nu13082803
Tsai M-T, Ou S-M, Chen H-Y, Tseng W-C, Lee K-H, Yang C-Y, Yang R-B, Tarng D-C. Relationship between Circulating Galectin-3, Systemic Inflammation, and Protein-Energy Wasting in Chronic Hemodialysis Patients. Nutrients. 2021; 13(8):2803. https://doi.org/10.3390/nu13082803
Chicago/Turabian StyleTsai, Ming-Tsun, Shuo-Ming Ou, Huan-Yuan Chen, Wei-Cheng Tseng, Kuo-Hua Lee, Chih-Yu Yang, Ruey-Bing Yang, and Der-Cherng Tarng. 2021. "Relationship between Circulating Galectin-3, Systemic Inflammation, and Protein-Energy Wasting in Chronic Hemodialysis Patients" Nutrients 13, no. 8: 2803. https://doi.org/10.3390/nu13082803
APA StyleTsai, M.-T., Ou, S.-M., Chen, H.-Y., Tseng, W.-C., Lee, K.-H., Yang, C.-Y., Yang, R.-B., & Tarng, D.-C. (2021). Relationship between Circulating Galectin-3, Systemic Inflammation, and Protein-Energy Wasting in Chronic Hemodialysis Patients. Nutrients, 13(8), 2803. https://doi.org/10.3390/nu13082803








