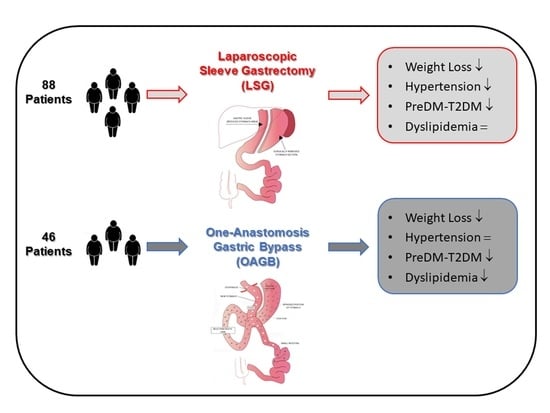
Improvement of Lipid Profile after One-Anastomosis Gastric Bypass Compared to Sleeve Gastrectomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Settings
2.2. Anthropometric Measurements
2.3. Biochemical Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sjöström, L. Review of the key results from the Swedish Obese Subjects (SOS) trial—A prospective controlled intervention study of bariatric surgery. J. Intern. Med. 2013, 273, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Formisano, G.; Buchwald, H.; Scopinaro, N. Bariatric surgery worldwide 2013. Obes. Surg. 2015, 25, 1822–1832. [Google Scholar] [CrossRef]
- Carbajo, M.A.; Luque-de-León, E.; Jiménez, J.M.; Ortiz-de-Solórzano, J.; Pérez-Miranda, M.; Castro-Alija, M.J. Laparoscopic one-anastomosis gastric bypass: Technique, results, and long-term follow-up in 1200 patients. Obes. Surg. 2017, 27, 1153–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, M.; Espalieu, P.; Pelascini, E.; Caiazzo, R.; Sterkers, A.; Khamphommala, L.; Poghosyan, T.; Chevallier, J.M.; Malherbe, V.; Chouillard, E.; et al. Efficacy and safety of one anastomosis gastric bypass versus Roux-en-Y gastric bypass for obesity (YOMEGA): A multicentre, randomised, open-label, non-inferiority trial. Lancet 2019, 393, 1299–1309. [Google Scholar] [CrossRef]
- Lee, W.J.; Almalki, O.M.; Ser, K.H.; Chen, J.C.; Lee, Y.C. Randomized controlled trial of one anastomosis gastric bypass versus Roux-En-Y gastric bypass for obesity: Comparison of the YOMEGA and Taiwan studies. Obes. Surg. 2019, 29, 3047–3053. [Google Scholar] [CrossRef]
- Ahuja, A.; Tantia, O.; Goyal, G.; Chaudhuri, T.; Khanna, S.; Poddar, A.; Gupta, S.; Majumdar, K. MGB-OAGB: Effect of biliopancreatic limb length on nutritional deficiency, weight loss, and comorbidity resolution. Obes. Surg. 2018, 28, 3439–3445. [Google Scholar] [CrossRef]
- Chang, S.H.; Stoll, C.R.T.; Song, J.; Varela, J.E.; Eagon, C.J.; Colditz, G.A. The effectiveness and risks of bariatric surgery an updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014, 149, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Seetharamaiah, S.; Tantia, O.; Goyal, G.; Chaudhuri, T.; Khanna, S.; Singh, J.P.; Ahuja, A. LSG vs OAGB—1 year follow-up data—A randomized control trial. Obes. Surg. 2017, 27, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Chong, K.; Ser, K.H.; Lee, Y.C.; Chen, S.C.; Chen, J.C.; Tsai, M.H.; Chuang, L.M. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: A randomized controlled trial. Arch. Surg. 2011, 146, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Singla, V.; Aggarwal, S.; Singh, B.; Tharun, G.; Katiyar, V.; Bhambri, A. Outcomes in super obese patients undergoing one anastomosis gastric bypass or laparoscopic sleeve gastrectomy. Obes. Surg. 2019, 29, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Tantia, O.; Goyal, G.; Chaudhuri, T.; Khanna, S.; Poddar, A.; Majumdar, K.; Gupta, S. LSG vs. MGB-OAGB: 5-year follow-up data and comparative outcome of the two procedures over long term—Results of a Randomised control trial. Obes. Surg. 2021, 31, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.; Clarke, M.G.; Evennett, N.J.; John Robinson, S.; Lee Humphreys, M.; Hammodat, H.; Jones, B.; Kim, D.D.; Cutfield, R.; Johnson, M.H.; et al. Laparoscopic sleeve gastrectomy versus banded Roux-en-Y gastric bypass for diabetes and obesity: A prospective Randomised double-blind trial. Obes. Surg. 2018, 28, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.P.; Bhattacharya, S.; Kumar, S.S.; Sabnis, S.C.; Parthasarathi, R.; Swamy, P.D.K.; Palanivelu, C. Comparison of effects of sleeve gastrectomy and gastric bypass on lipid profile parameters in Indian obese: A case matched analysis. Obes. Surg. 2017, 27, 2606–2612. [Google Scholar] [CrossRef]
- Kular, K.S.; Manchanda, N.; Rutledge, R. Analysis of the five-year outcomes of sleeve gastrectomy and mini gastric bypass: A report from the Indian sub-continent. Obes. Surg. 2014, 24, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European guidelines for obesity management in adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Bernante, P.; Foletto, M.; Busetto, L.; Pomerri, F.; Pesenti, F.F.; Pelizzo, M.R.; Nitti, D. Feasibility of laparoscopic sleeve gastrectomy as a revision procedure for prior laparoscopic gastric banding. Obes. Surg. 2006, 16, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2007, 25, 1105–1187. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Siekmann, L.; Bonora, R.; Burtis, C.A.; Ceriotti, F.; Clerc-Renaud, P.; Férard, G.; Ferrero, C.A.; Forest, J.-G.; Franck, P.F.H.; Gella, F.-J.; et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 7. Certification of four reference materials for the determ. Clin. Chem. Lab. Med. 2002, 40, 739–745. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S.; William, T. Friedewald.pdf. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The fatty liver index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, J.M.; Carbajo, M.A.; López, M.; Cao, M.J.; Rúiz-Tovar, J.; García, S.; Castro, M.J. Changes in lipid profile, body weight variables and cardiovascular risk in obese patients undergoing one-anastomosis gastric bypass. Int. J. Environ. Res. Public Health 2020, 17, 5858. [Google Scholar] [CrossRef]
- Musella, M.; Apers, J.; Rheinwalt, K.; Ribeiro, R.; Manno, E.; Greco, F.; Čierny, M.; Milone, M.; Di Stefano, C.; Guler, S.; et al. Efficacy of Bariatric Surgery in Type 2 Diabetes Mellitus Remission: The Role of Mini Gastric Bypass/One Anastomosis Gastric Bypass and Sleeve Gastrectomy at 1 Year of Follow-up. A European survey. Obes. Surg. 2016, 26, 933–940. [Google Scholar] [CrossRef]
- Shivakumar, S.; Tantia, O.; Goyal, G.; Chaudhuri, T.; Khanna, S.; Ahuja, A.; Poddar, A.; Majumdar, K. LSG vs MGB-OAGB—3 Year Follow-up Data: A Randomised Control Trial. Obes. Surg. 2018, 28, 2820–2828. [Google Scholar] [CrossRef]
- Ding, L.; Fan, Y.; Li, H.; Zhang, Y.; Qi, D.; Tang, S.; Cui, J.; He, Q.; Zhuo, C.; Liu, M. Comparative effectiveness of bariatric surgeries in patients with obesity and type 2 diabetes mellitus: A network meta-analysis of randomized controlled trials. Obes. Rev. 2020, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carswell, K.A.; Belgaumkar, A.P.; Amiel, S.A.; Patel, A.G. A systematic review and meta-analysis of the effect of gastric bypass surgery on plasma lipid levels. Obes. Surg. 2016, 26, 843–855. [Google Scholar] [CrossRef]
- Jammu, G.S.; Sharma, R. A 7-year clinical audit of 1107 cases comparing sleeve gastrectomy, Roux-En-Y gastric bypass, and Mini-Gastric bypass, to determine an effective and safe bariatric and metabolic procedure. Obes. Surg. 2016, 26, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Pihlajamäki, J.; Grönlund, S.; Simonen, M.; Käkelä, P.; Moilanen, L.; Pääkkönen, M.; Pirinen, E.; Kolehmainen, M.; Kärjä, V.; Kainulainen, S.; et al. Cholesterol absorption decreases after Roux-en-Y gastric bypass but not after gastric banding. Metabolism 2010, 59, 866–872. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Varela, J.E. Bariatric surgery for obesity and metabolic disorders: State of the art. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Carbajo, M.A.; Fong-Hirales, A.; Luque-de-León, E.; Molina-Lopez, J.F.; Ortiz-de-Solórzano, J. Weight loss and improvement of lipid profiles in morbidly obese patients after laparoscopic one-anastomosis gastric bypass: 2-year follow-up. Surg. Endosc. 2017, 31, 416–421. [Google Scholar] [CrossRef] [PubMed]

| LSG (n = 88) | OAGB (n = 46) | p | |
|---|---|---|---|
| Sex (M/F) | 20/68 | 4/42 | 0.076 |
| Age (years) | 47.9 ± 10.5 | 46.5 ± 8.8 | 0.473 |
| Weight (kg) | 127 (108.6–144.8) | 123.5 (112.3–137) | 0.598 |
| BMI (kg/m2) | 46 (41.6–51.7) | 44.9 (41.2–48.1) | 0.159 |
| Waist (cm) | 127 (120.8–144) | 125.5 (116–132) | 0.094 |
| TC (mg/dL) | 186 (166–205) | 187 (167–207) | 0.752 |
| HDL (mg/dL) | 49 (39–56) | 47 (37–59) | 0.699 |
| NHDLC (mg/dL) | 137 (115–156) | 145 (115–161) | 0.689 |
| LDL (mg/dL) | 115 (97–135) | 118 (91–130) | 0.591 |
| TG (mg/dL) | 95 (70–134) | 129 (92–165) | 0.007 |
| HOMA-IR | 3.6 (2–6) | 4.7 (2.9–9.5) | 0.092 |
| FPG (mmol/L) | 5.4 (5–6.2) | 5.5 (4.9–6.3) | 0.528 |
| Insulin (mU/L) | 13.5 (7.3–23.3) | 16.5 (10.3–33.6) | 0.163 |
| Hb1Ac (%) | 6.9 (6.2–7.6) | 6.1 (5.8–8.2) | 0.696 |
| ALT (U/L) | 23 (17–33) | 23 (19–35) | 0.757 |
| AST (U/L) | 22 (17–30) | 18 (16–24) | 0.059 |
| Metabolic profile (N, preDM, T2DM) | 45 (51%)/22 (25%)/21 (24%) | 23 (50%)/14 (30%)/9 (20%) | 0.479 |
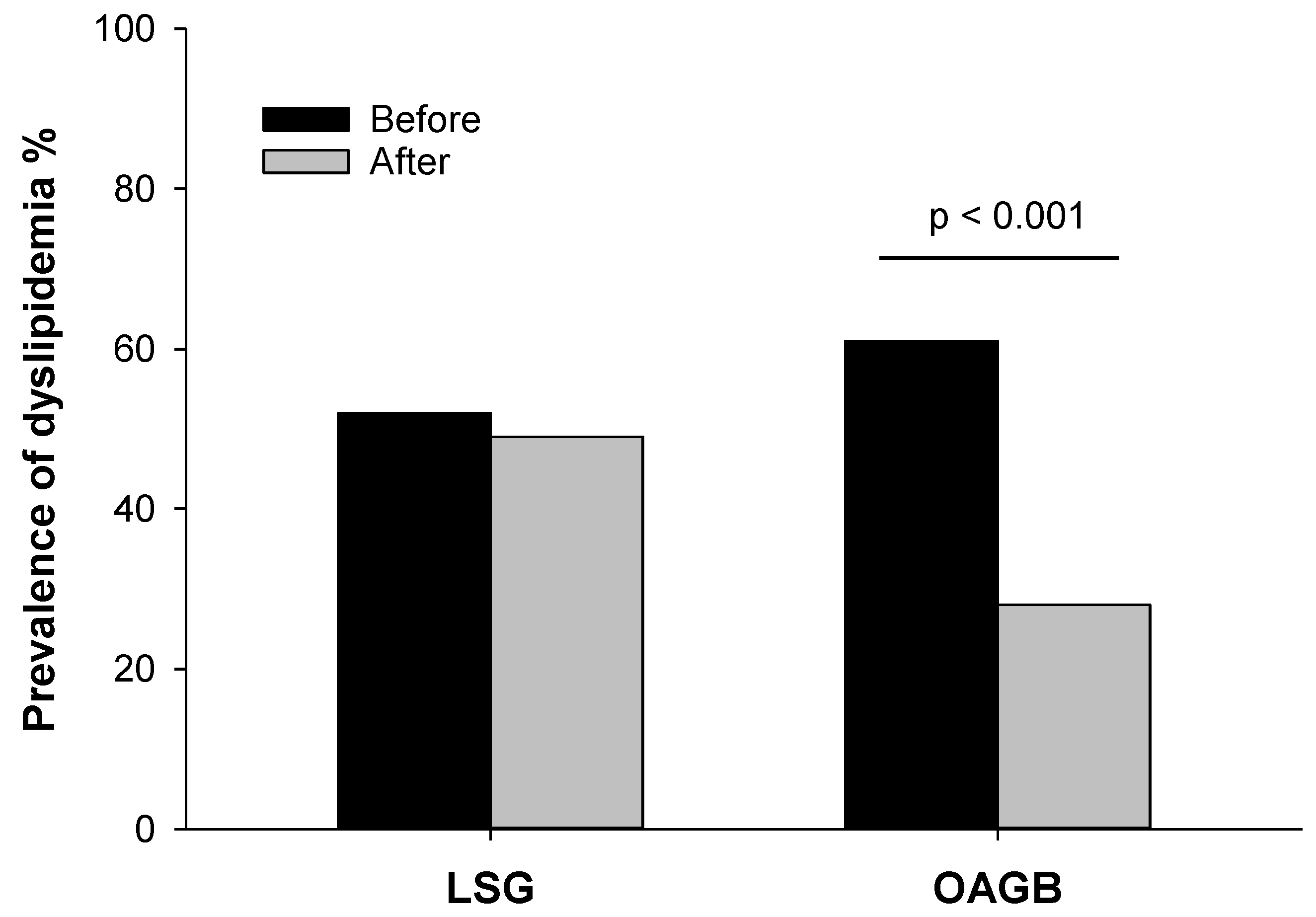
| Dyslipidemia | 46 (52%) | 28 (61%) | 0.443 |
| Hypertension | 42 (48%) | 18 (39%) | 0.737 |
| Anti-dyslipidemic drugs * | 18 (20%) | 4 (9%) | 0.134 |
| Before | After | Delta | |
|---|---|---|---|
| Weight (kg) | 127 (108.6–144.8) | 92.2 (74.1–105.8) *** | 37.6 (24.2–51.1), 29.6% |
| BMI (kg/m2) | 47.4 ± 8.2 | 33.7 ± 7 *** | 13.7 ± 5.4, 28.9% |
| Waist (cm) | 131.1 ± 16.3 | 105.1 ± 16.7 *** | 26 ± 12.3, 19.8% |
| TC (mg/dL) | 189 ± 40 | 182 ± 37 | 7 ± 32, 3.7% |
| HDL (mg/dL) | 49 (39–56) | 57 (49–69) *** | −10 ((−18)–(−5)), 20.4% |
| NHDLC (mg/dL) | 140 ± 38 | 123 ± 35 *** | 16 ± 30, 11.4% |
| LDL (mg/dL) | 115 (97–135) | 103 (85–123) *** | 9 ((−1)–(24)), 7.8% |
| TG (mg/dL) | 95 (70–134) | 75 (57–98) *** | 23 (3–49), 24.2% |
| FPG (mmol/L) | 5.4 (5–6.2) | 4.6 (4.4–4.9) *** | 0.8 (0.5–1.3), 14.8% |
| Hb1Ac (%) | 6.9 (6.2–7.6) | 5.9 (5.2–6.3) *** | 1 (0.5–2), 19% |
| ALT (U/L) | 23 (17–33) | 16 (12–20) *** | 6 (2–15), 26.1% |
| AST (U/L) | 22 (17–30) | 16 (14–20) *** | 5 (1–12), 22.7% |
| Metabolic profile (N, preDM, T2DM) | 45 (51%)/22 (25%)/21 (24%) | 71 (81%)/13 (15%)/4 (4%) *** | - |
| Hypertension | 42 (48%) | 25 (28%) *** | - |
| Anti-dyslipidemic drugs * | 18 (20%) | 7 (8) *** | - |
| Before | After | Delta | |
|---|---|---|---|
| Weight (kg) | 126.5 ± 18.4 | 83.3 ± 14.2 *** | 42 (33–50), 33.2% |
| BMI (kg/m2) | 45.2 ± 6.1 | 29.8 ± 4.6 *** | 15.5 ± 4, 34.3% |
| TC (mg/dL) | 190 ± 33 | 166 ± 27 *** | 24 ± 36, 12.6% |
| HDL (mg/dL) | 48 ± 12 | 55 ± 13 *** | −8 ± 13, 16.6% |
| NHDLC (mg/dL) | 141 ± 33 | 111 ± 25 *** | 29 ± 34, 20.6% |
| LDL (mg/dL) | 113 ± 32 | 95 ± 24 *** | 19 ± 32, 16.8% |
| TG (mg/dL) | 129 (92–164) | 84 (62–107) *** | 42 (8–59), 32.6% |
| FPG (mmol/L) | 5.5 (4.9–6.3) | 4.7 (4.4–5) *** | 0.7 (0.2–1.8), 12.7% |
| Hb1Ac (%) | 6.1 (5.8–8.2) | 5.6 (5–6) * | 0.5 (0.5–1.9), 8% |
| ALT (U/L) | 23 (19–35) | 27 (19–33) | −4 ((−10)–(8)), 17.4% |
| AST (U/L) | 22 ± 12 | 25 ± 10 | −4 ((−12)–(2)), 18.2% |
| Metabolic profile (N, preDM, T2DM) | 23 (50%)/14 (30%)/9 (20%) | 37 (80%)/7 (15%)/2 (5%) ** | - |
| Hypertension | 18 (39%) | 18 (39%) | - |
| Anti-dyslipidemic drugs * | 4 (9%) | 1 (2%) |
| LSG | OAGB | p | |
|---|---|---|---|
| Δ Weight (kg) | 37.6 (24.2–51.1) | 42 (33–50) | 0.024 |
| Δ BMI (kg/m2) | 13.7 ± 5.4 | 15.5 ± 4 | 0.032 |
| Δ TC (mg/dL) | 7 ± 32 | 24 ± 36 | 0.009 |
| Δ TC adj (mg/dL) | −13 ((−20)–(−7)) | 24 ((−9)–54) | <0.001 |
| Δ HDL (mg/dL) | −10 ((−18)–(−5)) | −5 ((−20)–2) | 0.078 |
| Δ HDL adj (mg/dL) | −10 ((−17.8)–(−5.3)) | −5 ((−20)–(2.3)) | 0.078 |
| Δ NHDLC (mg/dL) | 16 ± 30 | 29 ± 34 | 0.032 |
| Δ NHDLC adj (mg/dL) | −7 ((−15–0) | 27 (4–53) | <0.001 |
| Δ LDL (mg/dL) | 9 ((−1)–(24)) | 15 ((−4)–40) | 0.218 |
| Δ LDL adj (mg/dL) | −7 ((−11)–1) | 15 ((−4)–40) | <0.001 |
| Δ TG (mg/dL) | 23 (3–49) | 42 (8–59) | 0.034 |
| Δ TG adj (mg/dL) | 22 (3–49) | 44 (21–63) | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bettini, S.; Segato, G.; Prevedello, L.; Fabris, R.; Prà, C.D.; Zabeo, E.; Compagnin, C.; De Luca, F.; Finco, C.; Foletto, M.; et al. Improvement of Lipid Profile after One-Anastomosis Gastric Bypass Compared to Sleeve Gastrectomy. Nutrients 2021, 13, 2770. https://doi.org/10.3390/nu13082770
Bettini S, Segato G, Prevedello L, Fabris R, Prà CD, Zabeo E, Compagnin C, De Luca F, Finco C, Foletto M, et al. Improvement of Lipid Profile after One-Anastomosis Gastric Bypass Compared to Sleeve Gastrectomy. Nutrients. 2021; 13(8):2770. https://doi.org/10.3390/nu13082770
Chicago/Turabian StyleBettini, Silvia, Gianni Segato, Luca Prevedello, Roberto Fabris, Chiara Dal Prà, Eva Zabeo, Chiara Compagnin, Fabio De Luca, Cristiano Finco, Mirto Foletto, and et al. 2021. "Improvement of Lipid Profile after One-Anastomosis Gastric Bypass Compared to Sleeve Gastrectomy" Nutrients 13, no. 8: 2770. https://doi.org/10.3390/nu13082770
APA StyleBettini, S., Segato, G., Prevedello, L., Fabris, R., Prà, C. D., Zabeo, E., Compagnin, C., De Luca, F., Finco, C., Foletto, M., Vettor, R., Busetto, L., & on behalf of the Veneto Obesity Network. (2021). Improvement of Lipid Profile after One-Anastomosis Gastric Bypass Compared to Sleeve Gastrectomy. Nutrients, 13(8), 2770. https://doi.org/10.3390/nu13082770