Creatine Supplementation Upregulates mTORC1 Signaling and Markers of Synaptic Plasticity in the Dentate Gyrus While Ameliorating LPS-Induced Cognitive Impairment in Female Rats
Abstract
:1. Introduction
2. Materials and Methods
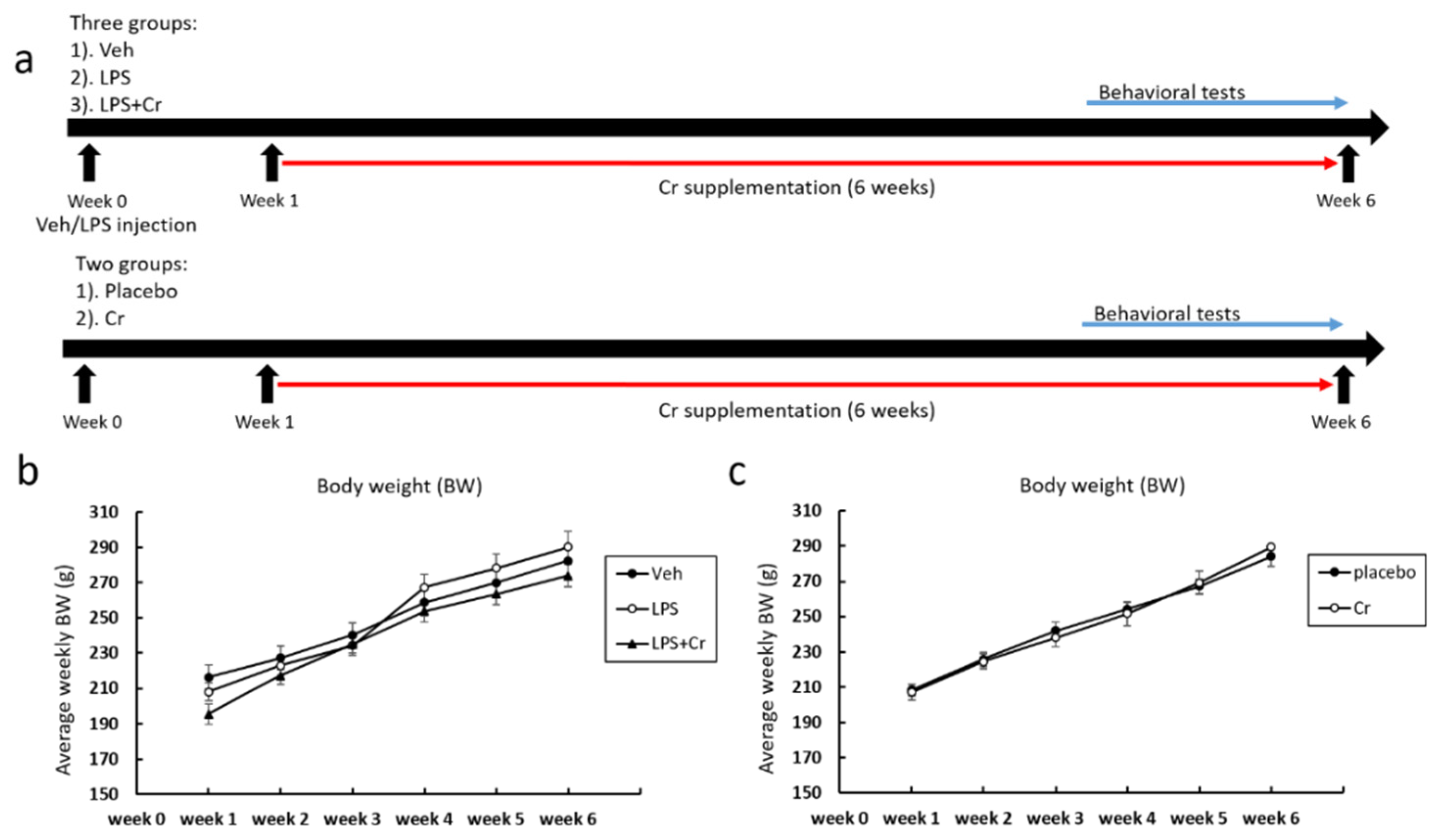
2.1. Animals and Experimental Design
2.2. Creatine Supplementation
2.3. Surgery and Induction of MCI
2.4. Behavioral Tests
2.4.1. Barnes Maze Test
2.4.2. Novel Object Recognition Test
2.5. Euthanasia and Tissue Harvesting
2.6. RNA Isolation, cDNA Synthesis, and Real-Time Polymerase Chain Reaction (RT-PCR)
2.7. Immunoblotting
2.8. Statistical Analysis
3. Results
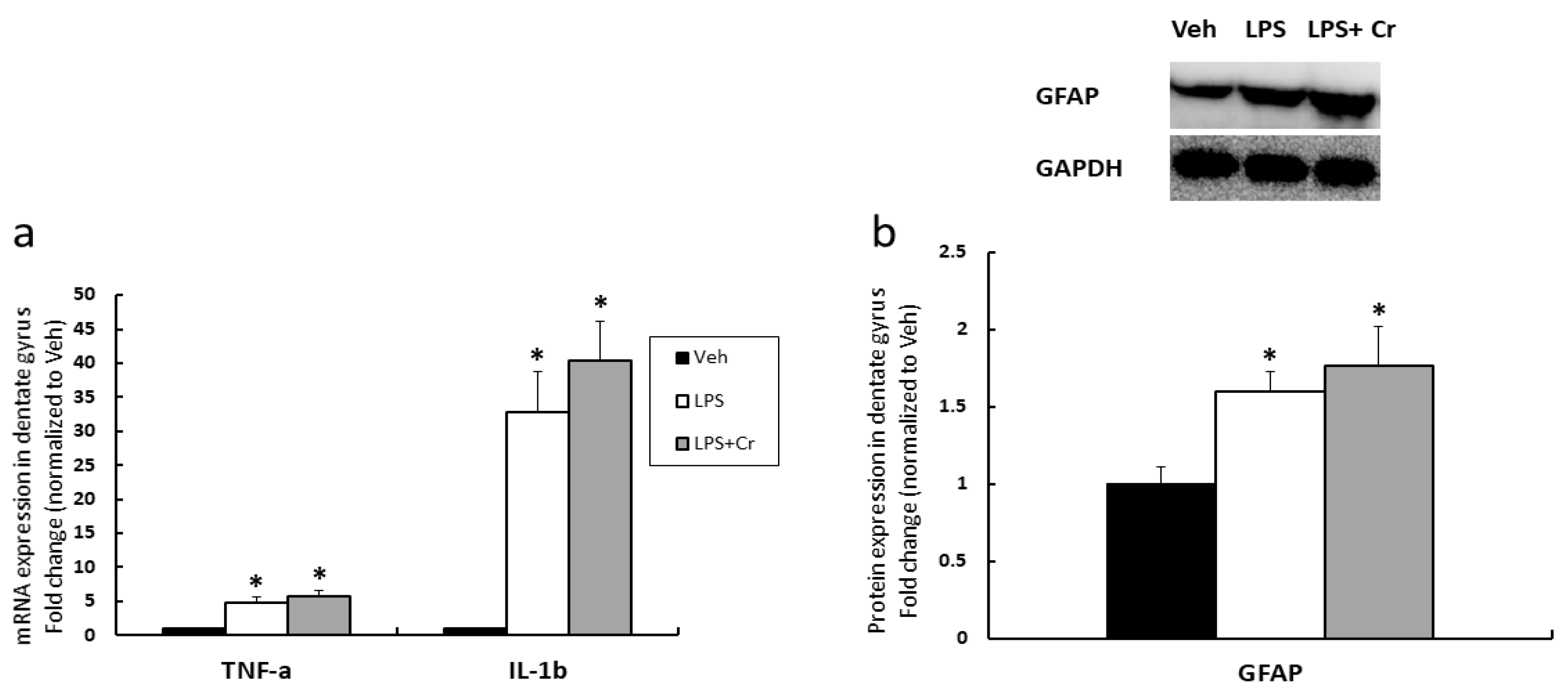
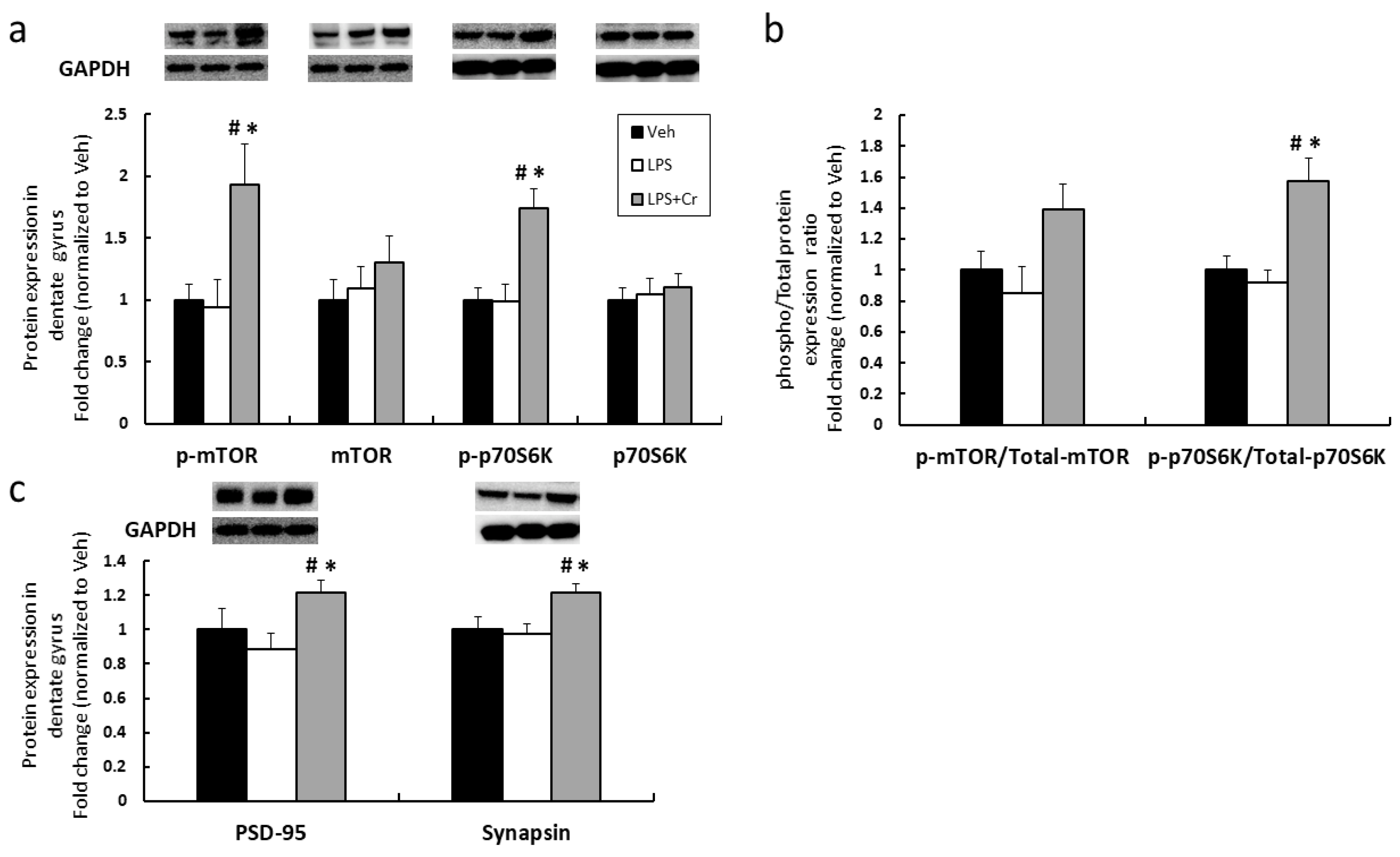
3.1. Experiment 1
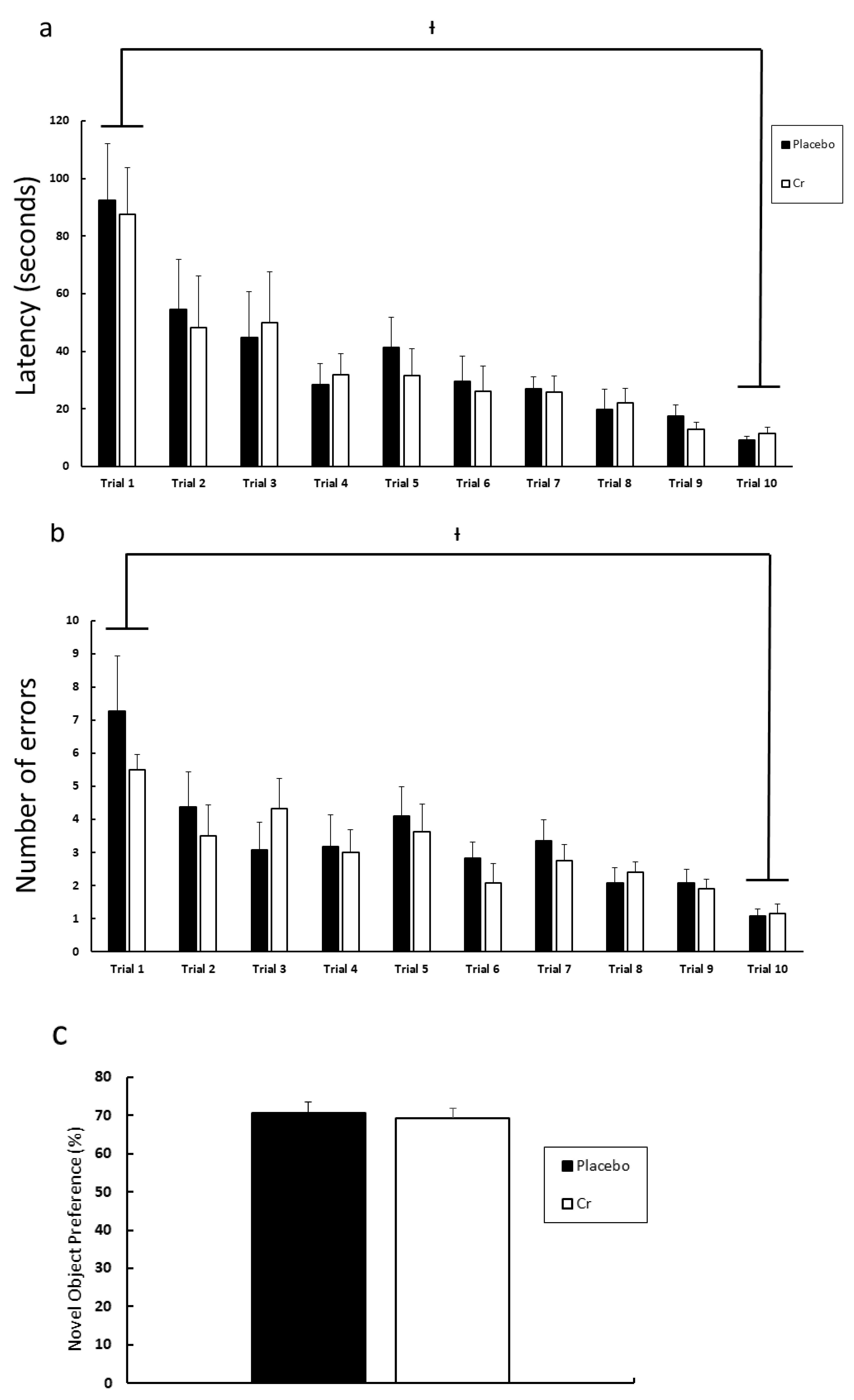
3.2. Experiment 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef] [PubMed]
- Collie, A.; Maruff, P. The neuropsychology of preclinical Alzheimer’s disease and mild cognitive impairment. Neurosci. Biobehav. Rev. 2000, 24, 365–374. [Google Scholar] [CrossRef]
- Xu, H.; Yang, R.; Dintica, C.S.; Qi, X.; Song, R.; Bennett, D.A.; Xu, W. Association of lifespan cognitive reserve indicator with the risk of mild cognitive impairment and its progression to dementia. Alzheimers Dement. 2020, 16, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Mujika, I.; Padilla, S. Creatine Supplementation as an Ergogenic Aid for Sports Performance in Highly Trained Athletes: A Critical Review. Int. J. Sports Med. 1997, 18, 491–496. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Bougioukas, K.I.; Kapogiannis, D. Effects of creatine supplementation on cognitive function of healthy individuals: A systematic review of randomized controlled trials. Exp. Gerontol. 2018, 108, 166–173. [Google Scholar] [CrossRef]
- Owen, L.; Sunram-Lea, S.I. Metabolic Agents that Enhance ATP can Improve Cognitive Functioning: A Review of the Evidence for Glucose, Oxygen, Pyruvate, Creatine, and l-Carnitine. Nutrients 2011, 3, 735–755. [Google Scholar] [CrossRef] [Green Version]
- Rae, C.; Digney, A.L.; McEwan, S.R.; Bates, T.C. Oral creatine monohydrate supplementation improves brain performance: A double-blind, placebo-controlled, cross-over trial. Proc. R. Soc. B Boil. Sci. 2003, 270, 2147–2150. [Google Scholar] [CrossRef] [Green Version]
- McMorris, T.; Mielcarz, G.; Harris, R.C.; Swain, J.P.; Howard, A.N. Creatine Supplementation and Cognitive Performance in Elderly Individuals. Aging Neuropsychol. Cogn. 2007, 14, 517–528. [Google Scholar] [CrossRef]
- McMorris, T.; Harris, R.C.; Swain, J.; Corbett, J.; Collard, K.; Dyson, R.J.; Dye, L.; Hodgson, C.; Draper, N. Effect of creatine supplementation and sleep deprivation, with mild exercise, on cognitive and psychomotor performance, mood state, and plasma concentrations of catecholamines and cortisol. Psychopharmacology 2006, 185, 93–103. [Google Scholar] [CrossRef]
- McMorris, T.; Harris, R.; Howard, A.; Langridge, G.; Hall, B.; Corbett, J.; Dicks, M.; Hodgson, C. Creatine supplementation, sleep deprivation, cortisol, melatonin and behavior. Physiol. Behav. 2007, 90, 21–28. [Google Scholar] [CrossRef]
- Turner, C.; Byblow, W.; Gant, N. Creatine Supplementation Enhances Corticomotor Excitability and Cognitive Performance during Oxygen Deprivation. J. Neurosci. 2015, 35, 1773–1780. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling at a glance. J. Cell Sci. 2009, 122, 3589–3594. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.J.; Reis, G.; Kang, H.; Gingras, A.-C.; Sonenberg, N.; Schuman, E.M. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc. Natl. Acad. Sci. USA 2002, 99, 467–472. [Google Scholar] [CrossRef] [Green Version]
- Bekinschtein, P.; Katche, C.; Slipczuk, L.N.; Igaz, L.M.; Cammarota, M.; Izquierdo, I.; Medina, J.H. mTOR signaling in the hippocampus is necessary for memory formation. Neurobiol. Learn. Mem. 2007, 87, 303–307. [Google Scholar] [CrossRef]
- Pazini, F.L.; Cunha, M.; Rosa, J.; Colla, A.R.S.; Lieberknecht, V.; Oliveira-Giacomelli, Á.; Rodrigues, A.L.S. Creatine, similar to Ketamine, Counteracts Depressive-Like Behavior Induced by Corticosterone via PI3K/Akt/mTOR Pathway. Mol. Neurobiol. 2016, 53, 6818–6834. [Google Scholar] [CrossRef] [PubMed]
- Pazini, F.L.; Rosa, J.M.; Camargo, A.; Fraga, D.B.; Moretti, M.; Siteneski, A.; Rodrigues, A.L.S. mTORC1-dependent signaling pathway underlies the rapid effect of creatine and ketamine in the novelty-suppressed feeding test. Chem. Interact. 2020, 332, 109281. [Google Scholar] [CrossRef] [PubMed]
- Kelty, T.J.; Schachtman, T.R.; Mao, X.; Grigsby, K.B.; Childs, T.E.; Olver, T.D.; Michener, P.N.; Richardson, R.A.; Roberts, C.K.; Booth, F.W. Resistance-exercise training ameliorates LPS-induced cognitive impairment concurrent with molecular signaling changes in the rat dentate gyrus. J. Appl. Physiol. 2019, 127, 254–263. [Google Scholar] [CrossRef]
- Jessberger, S.; Clark, R.E.; Broadbent, N.J.; Clemenson, J.G.D.; Consiglio, A.; Lie, D.C.; Squire, L.R.; Gage, F.H. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 2009, 16, 147–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hainmueller, T.; Bartos, M. Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nat. Rev. Neurosci. 2020, 21, 153–168. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Grigsby, K.B.; Ruegsegger, G.N.; Childs, T.E.; Booth, F.W. Overexpression of Protein Kinase Inhibitor Alpha Reverses Rat Low Voluntary Running Behavior. Mol. Neurobiol. 2018, 56, 1782–1797. [Google Scholar] [CrossRef]
- Gawel, K.; Gibula, E.; Marszalek-Grabska, M.; Filarowska, J.; Kotlinska, J.H. Assessment of spatial learning and memory in the Barnes maze task in rodents—methodological consideration. Naunyn-Schmiedebergs Arch. Pharmacol. 2019, 392, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Ruegsegger, G.N.; Toedebusch, R.G.; Childs, T.E.; Grigsby, K.B.; Booth, F.W. Loss of Cdk5 function in the nucleus accumbens decreases wheel running and may mediate age-related declines in voluntary physical activity. J. Physiol. 2016, 595, 363–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Lee, Y.K.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflamm. 2008, 5, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef] [Green Version]
- Perez-Dominguez, M.; Ávila-Muñoz, E.; Domínguez-Rivas, E.; Zepeda, A. The detrimental effects of lipopolysaccharide-induced neuroinflammation on adult hippocampal neurogenesis depend on the duration of the pro-inflammatory response. Neural Regen. Res. 2019, 14, 817–825. [Google Scholar] [CrossRef]
- Dean, P.J.A.; Arikan, G.; Opitz, B.; Sterr, A. Potential for use of creatine supplementation following mild traumatic brain injury. Concussion 2017, 2, CNC34. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wu, M.; Peng, C.; Zhao, G.; Guanjie, Z. GFAP expression in injured astrocytes in rats. Exp. Ther. Med. 2017, 14, 1905–1908. [Google Scholar] [CrossRef] [Green Version]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-R.; Liu, J.-C.; Bao, J.-S.; Bai, Q.-Q.; Wang, G. Interaction of Microglia and Astrocytes in the Neurovascular Unit. Front. Immunol. 2020, 11, 1024. [Google Scholar] [CrossRef]
- Bender, A.; Beckers, J.; Schneider, I.; Hölter-Koch, S.; Haack, T.; Ruthsatz, T.; Vogt-Weisenhorn, D.; Becker, L.; Genius, J.; Rujescu, D.; et al. Creatine improves health and survival of mice. Neurobiol. Aging 2008, 29, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Allahyar, R.; Akbar, A.; Iqbal, F. Effect of creatine monohydrate supplementation on learning, memory and neuromuscular coordination in female albino mice. Acta Neuropsychiatr. 2016, 29, 27–34. [Google Scholar] [CrossRef]
- Graber, T.E.; McCamphill, P.; Sossin, W.S. A recollection of mTOR signaling in learning and memory. Learn. Mem. 2013, 20, 518–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, R.; Gafford, G.M.; Helmstetter, F. Translational Control via the Mammalian Target of Rapamycin Pathway Is Critical for the Formation and Stability of Long-Term Fear Memory in Amygdala Neurons. J. Neurosci. 2006, 26, 12977–12983. [Google Scholar] [CrossRef]
- Deli, A.; Schipany, K.; Rosner, M.; Höger, H.; Pollak, A.; Li, L.; Hengstschläger, M.; Lubec, G. Blocking mTORC1 activity by rapamycin leads to impairment of spatial memory retrieval but not acquisition in C57BL/6J mice. Behav. Brain Res. 2012, 229, 320–324. [Google Scholar] [CrossRef]
- Jobim, P.F.; Pedroso, T.R.; Christoff, R.R.; Werenicz, A.; Maurmann, N.; Reolon, G.K.; Roesler, R. Inhibition of mTOR by rapamycin in the amygdala or hippocampus impairs formation and reconsolidation of inhibitory avoidance memory. Neurobiol. Learn. Mem. 2012, 97, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Hoeffer, C.A.; Capetillo-Zarate, E.; Yu, F.; Wong, H.; Lin, M.T.; Tampellini, D.; Klann, E.; Blitzer, R.D.; Gouras, G.K. Dysregulation of the mTOR Pathway Mediates Impairment of Synaptic Plasticity in a Mouse Model of Alzheimer’s Disease. PLoS ONE 2010, 5, e12845. [Google Scholar] [CrossRef] [PubMed]
- Cesca, F.; Baldelli, P.; Valtorta, F.; Benfenati, F. The synapsins: Key actors of synapse function and plasticity. Prog. Neurobiol. 2010, 91, 313–348. [Google Scholar] [CrossRef]
- Keith, D.J. Excitation control: Balancing PSD-95 function at the synapse. Front. Mol. Neurosci. 2008, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Bond, P. Regulation of mTORC1 by growth factors, energy status, amino acids and mechanical stimuli at a glance. J. Int. Soc. Sports Nutr. 2016, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wrigley, S.; Arafa, D.; Tropea, D. Insulin-Like Growth Factor 1: At the Crossroads of Brain Development and Aging. Front. Cell. Neurosci. 2017, 11, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodges, S.L.; Reynolds, C.D.; Smith, G.D.; Jefferson, T.S.; Nolan, S.; Lugo, J.N. Molecular interplay between hyperactive mammalian target of rapamycin signaling and Alzheimer’s disease neuropathology in the NS-Pten knockout mouse model. NeuroReport 2018, 29, 1109–1113. [Google Scholar] [CrossRef]
- Snow, W.M.; Cadonic, C.; Cortes-Perez, C.; Chowdhury, S.K.R.; Djordjevic, J.; Thomson, E.; Bernstein, M.J.; Suh, M.; Fernyhough, P.; Albensi, B.C. Chronic dietary creatine enhances hippocampal-dependent spatial memory, bioenergetics, and levels of plasticity-related proteins associated with NF-κB. Learn. Mem. 2018, 25, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Rawson, E.S.; Lieberman, H.R.; Walsh, T.M.; Zuber, S.M.; Harhart, J.M.; Matthews, T.C. Creatine supplementation does not improve cognitive function in young adults. Physiol. Behav. 2008, 95, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Woolley, C.; Gould, E.; Frankfurt, M.; McEwen, B. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 1990, 10, 4035–4039. [Google Scholar] [CrossRef] [Green Version]


| Gene | Forward (5′→3′) | Reverse (5′→3′) | Accession No. |
|---|---|---|---|
| 18S | GCTCGCTCCTCTCCTACTTG | GATAAATGCACGCGTTCCCC | NR_046237.2 |
| TNF-α | AACACACGAGACGCTGAAGT | TCCAGTGAGTTCCGAAAGCC | NM_012675.3 |
| IL-1β | TGACTTCACCATGGAACCCG | GACCTGACTTGGCAGAGGAC | NM_031512.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, X.; Kelty, T.J.; Kerr, N.R.; Childs, T.E.; Roberts, M.D.; Booth, F.W. Creatine Supplementation Upregulates mTORC1 Signaling and Markers of Synaptic Plasticity in the Dentate Gyrus While Ameliorating LPS-Induced Cognitive Impairment in Female Rats. Nutrients 2021, 13, 2758. https://doi.org/10.3390/nu13082758
Mao X, Kelty TJ, Kerr NR, Childs TE, Roberts MD, Booth FW. Creatine Supplementation Upregulates mTORC1 Signaling and Markers of Synaptic Plasticity in the Dentate Gyrus While Ameliorating LPS-Induced Cognitive Impairment in Female Rats. Nutrients. 2021; 13(8):2758. https://doi.org/10.3390/nu13082758
Chicago/Turabian StyleMao, Xuansong, Taylor J. Kelty, Nathan R. Kerr, Thomas E. Childs, Michael D. Roberts, and Frank W. Booth. 2021. "Creatine Supplementation Upregulates mTORC1 Signaling and Markers of Synaptic Plasticity in the Dentate Gyrus While Ameliorating LPS-Induced Cognitive Impairment in Female Rats" Nutrients 13, no. 8: 2758. https://doi.org/10.3390/nu13082758
APA StyleMao, X., Kelty, T. J., Kerr, N. R., Childs, T. E., Roberts, M. D., & Booth, F. W. (2021). Creatine Supplementation Upregulates mTORC1 Signaling and Markers of Synaptic Plasticity in the Dentate Gyrus While Ameliorating LPS-Induced Cognitive Impairment in Female Rats. Nutrients, 13(8), 2758. https://doi.org/10.3390/nu13082758







