The Metabolism of Glucosinolates by Gut Microbiota
Abstract
1. Introduction
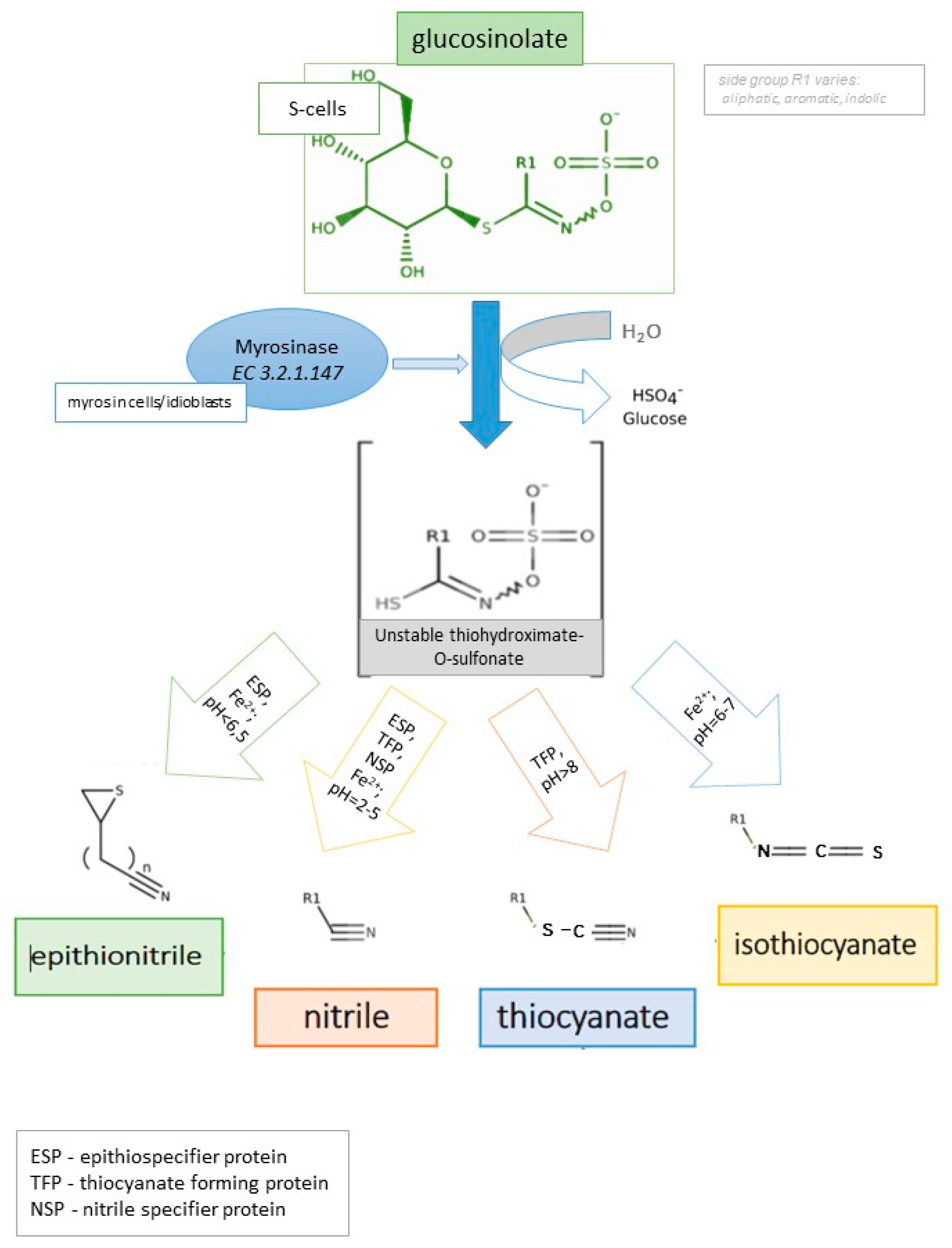
2. Glucosinolates and Their Derivatives
- Aliphatic group from Met, Ala, Leu, Ile, and Val;
- Aromatic group from Phe and Tyr; and
- Indolic group from Trp.
- Epithionitriles
- b.
- Nitriles
- c.
- Isothiocyanates
- d.
- Thiocyanates
3. Plant Composition and Human Gut Microbiome
4. Glucosinolates’ and Their Derivatives’ Influence on Human Gut Microbiota
5. The Role of Microbial Communities in the GLS Metabolism in the Human Gut
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sikorska-Zimny, K.; Beneduce, L. The glucosinolates and their bioactive derivatives in Brassica: A review on classification, biosynthesis and content in plant tissues, fate during and after processing, effect on the human organism and interaction with the gut microbiota. Crit. Rev. Food Sci. Nutr. 2020, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Integrated Taxonomy System. Available online: https://www.itis.gov/ (accessed on 5 November 2020).
- Martínez, S.; Armesto, J.; Gómez-Limia, L.; Carballo, J. Impact of processing and storage on the nutritional and sensory properties and bioactive components of Brassica spp. A review. Food Chem. 2020, 313, 126065. [Google Scholar] [CrossRef]
- Nagata, N.; Xu, L.; Kohno, S.; Ushida, Y.; Aoki, Y.; Umeda, R.; Fuke, N.; Zhuge, F.; Ni, Y.; Nagashimada, M.; et al. Glucoraphanin ameliorates obesity and insulin resistance through adipose tissue browning and reduction of metabolic endotoxemia in mice. Diabetes 2017, 66, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Jaafaru, M.S.; Abd Karim, N.A.; Enas, M.E.; Rollin, P.; Mazzon, E.; Abdull Razis, A.F. Protective effect of glucosinolates hydrolytic products in neurodegenerative diseases (NDDs). Nutrients 2018, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.; Gardner, S.; Jupp, O.; Bullough, A.; Butters, S.; Watts, L.; Donell, S.; Traka, M.; Saha, S.; Mithen, R.; et al. Isothiocyanates are detected in human synovial fluid following broccoli consumption and can affect the tissues of the knee joint. Sci. Rep. 2017, 7, 3398. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, D.L.; Graham, N.S.; Klinder, A.; van Ommen Kloeke, A.E.; Marcotrigiano, A.R.; Wagstaff, C.; Verkerk, R.; Sonnante, G.; Aarts, M.G. Overexpression of the MYB29 transcription factor affects aliphatic glucosinolate synthesis in Brassica oleracea. Plant Mol. Biol. 2019, 101, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Sturm, C.; Wagner, A.E. Brassica-derived plant bioactives as modulators of chemopreventive and inflammatory signaling pathways. Int. J. Mol. Sci. 2017, 8, 1890. [Google Scholar] [CrossRef]
- Tian, S.; Liu, X.; Lei, P.; Zhang, X.; Shan, Y. Microbiota: A mediator to transform glucosinolate precursors in cruciferous vegetables to the active isothiocyanates. J. Sci. Food Agric. 2018, 98, 1255–1260. [Google Scholar] [CrossRef]
- Sikorska-Zimny, K. Chosen glucosinolates and its derivatives: Sources, characteristic and influences on human body. Bromat. Chem. Toksykol. 2016, 49, 96–105. (In Polish) [Google Scholar]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of glucosinolates and their breakdown products: Impact of processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef]
- Liou, C.S.; Sirk, S.J.; Diaz, C.A.; Klein, A.P.; Fischer, C.R.; Higginbottom, S.K.; Sonnenburg, J.L.; Sattely, E.S. A metabolic pathway for glucosinolate activation by the human gut symbiont Bacteroides thetaiotaomicron. bioRxiv 2019, 626366. [Google Scholar]
- Narbad, A.; Rossiter, J.T. Gut glucosinolate metabolism and isothiocyanate production. Mol. Nutr. Food Res. 2018, 62, 1700991. [Google Scholar] [CrossRef] [PubMed]
- Castro-Torres, I.G.; Castro-Torres, V.A.; Hernández-Lozano, M.; Naranjo-Rodríguez, E.B.; Domínguez-Ortiz, M.Á. Chapter 4: Glucosinolates and metabolism. In Glucosinolates: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 107–141. [Google Scholar]
- Tani, N.; Ohtsuru, M.; Hata, T. Isolation of myrosinase producing microorganism. Agric. Biol. Chem. 1974, 38, 1617–1622. [Google Scholar] [CrossRef]
- Albaser, A.; Kazana, E.; Bennett, M.H.; Cebeci, F.; Luang-In, V.; Spanu, P.D.; Rossiter, J.T. Discovery of a bacterial glycoside hydrolase family 3 (GH3) β-glucosidase with myrosinase activity from a Citrobacter strain isolated from soil. J. Agric. Food Chem. 2016, 64, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, B.; Rybakova, D.; Müller, C.; Berg, G. Harnessing the microbiomes of Brassica vegetables for health issues. Sci. Rep. 2017, 7, 17649. [Google Scholar] [CrossRef]
- Cordeiro, R.P.; Doria, J.H.; Zhanel, G.G.; Sparling, R.; Holley, R.A. Role of glycoside hydrolase genes in sinigrin degradation by E. coli O157:H7. Int. J. Food Microbiol. 2015, 205, 105–111. [Google Scholar] [CrossRef] [PubMed]
- El-Shora, H.M.; El-Shobaky, A.M.; El-Atrozy, M.M. Activity of purified bacterial myrosinase and its essential residues. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 567–578. [Google Scholar] [CrossRef]
- Weir, T.L.; Trikha, S.R.J.; Thompson, H.J. Diet and cancer risk reduction: The role of diet-microbiota interactions and microbial metabolites. Semin. Cancer Biol. 2021, 70, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Palop, M.L.; Smiths, J.P.; ten Brink, B. Degradation of sinigrin by Lactobacillus agilis strain R16. Int. J. Food Microbiol. 1995, 26, 219–229. [Google Scholar] [CrossRef]
- Elfoul, L.; Rabot, S.; Khelifa, N.; Quinsac, A.; Duguay, A.; Rimbault, A. Formation of allyl isothiocyanate from sinigrin in the digestive tract of rats monoassociated with a human colonic strain of Bacteroides thetaiotaomicron. FEMS Microbiol. Lett. 2001, 197, 99–103. [Google Scholar] [CrossRef]
- Mullaney, J.A.; Kelly, W.J.; McGhie, T.K.; Ansell, J.; Heyes, J.A. Lactic acid bacteria convert glucosinolates to nitriles efficiently yet differently from Enterobacteriaceae. J. Agric. Food Chem. 2013, 61, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Kim, J.S. Understanding of MYB transcription factors involved in glucosinolate biosynthesis in Brassicaceae. Molecules 2017, 22, 1549. [Google Scholar] [CrossRef] [PubMed]
- Malka, S.K.; Cheng, Y. Possible interactions between the biosynthetic pathways of indole glucosinolate and auxin. Front. Plant Sci. 2017, 14, 2131. [Google Scholar] [CrossRef] [PubMed]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An overview of their antimicrobial activity against human infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef]
- PubChem Web Site, National Center for Biotechnology Information, National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 28 July 2021).
- Shokri, S.; Jegasothy, H.; Augustin, M.A.; Terefe, N.S. Thermosonication for the Production of Sulforaphane Rich Broccoli Ingredients. Biomolecules 2021, 11, 321. [Google Scholar] [CrossRef]
- Daxenbichler, M.E.; VanEtten, C.H.; Wolff, I.A. (S)- and (R)-1-cyano-2-hydroxy-3-butene from myrosinase hydrolysis of epi-progoitrin and progoitrin. Biochemistry 1966, 5, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Wang, J.; Cai, C.; Chang, J.; Zhao, Y.; Wang, Q. Accumulation of Glucosinolates in Broccoli; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2017; pp. 133–162. [Google Scholar] [CrossRef]
- Maina, S.; Misinzo, G.; Bakari, G.; Kim, H.Y. Human, Animal and Plant Health Benefits of Glucosinolates and Strategies for Enhanced Bioactivity: A Systematic Review. Molecules 2020, 25, 3682. [Google Scholar] [CrossRef]
- Collett, M.G.; Stegelmeier, B.L.; Tapper, B.A. Could nitrile derivatives of turnip (Brassica rapa) glucosinolates be hepato-or cholangiotoxic in cattle? J. Agric. Food Chem. 2014, 62, 7370–7375. [Google Scholar] [CrossRef]
- Cabello-Hurtado, F.; Gicquel, M.; Esnault, M.A. Evaluation of the antioxidant potential of cauliflower (Brassica oleracea) from a glucosinolate content perspective. Food Chem. 2012, 132, 1003–1009. [Google Scholar] [CrossRef]
- Al-Gendy, A.A.; El-Gindi, O.D.; Hafez, A.S.; Ateya, A.M. Glucosinolates, volatile constituents and biological activities of Erysimum corinthium Boiss. (Brassicaceae). Food Chem. 2010, 118, 519–524. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Berhow, M.A. Glucosinolate hydrolysis products from various plant sources: pH effects, isolation, and purification. Ind. Crops Prod. 2005, 21, 193–202. [Google Scholar] [CrossRef]
- Joint FAO; WHO Expert Committee on Food Additives. Safety Evaluation of Certain Food Additives and Contaminants; World Health Organization: Rome, Italy, 2002. [Google Scholar]
- Song, L.; Iori, R.; Thornalley, P.J. Purification of major glucosinolates fromBrassicaceae seeds and preparation of isothiocyanate and amine metabolites. J. Sci. Food Agric. 2006, 86, 1271–1280. [Google Scholar] [CrossRef]
- Lim, T.K. Brassica napus var. napobrassica. In Edible Medicinal and Non Medicinal Plants; Springer: Dordrecht, The Netherlands, 2015; pp. 761–767. [Google Scholar]
- Musk, S.R.R.; Smith, T.K.; Johnson, I.T. On the cytotoxicity and genotoxicity of allyl and phenethyl isothiocyanates and their parent glucosinolates sinigrin and gluconasturtiin. Mutat. Res. Lett. 1995, 348, 19–23. [Google Scholar] [CrossRef]
- Slominski, B.A.; Campbell, L.D. Gas chromatographic determination of indoleacetonitriles in rapeseed and brassica vegetables. J. Chromatogr. A 1988, 454, 285–291. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Hossain, S. Interaction of cruciferous phytoanticipins with plant fungal pathogens: Indole glucosinolates are not metabolised but the corresponding desulfo-derivatives and nitriles are. Phytochemistry 2011, 72, 2308–2316. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Mellon, F.A.; Rosa, E.A.; Perkins, L.; Kroon, P.A. Profiling glucosinolates, flavonoids, alkaloids, and other secondary metabolites in tissues of Azima tetracantha L. (Salvadoraceae). J. Agric. Food Chem. 2004, 52, 5856–5862. [Google Scholar] [CrossRef] [PubMed]
- Agerbirk, N.; Olsen, C.E.; Heimes, C.; Christensen, S.; Bak, S.; Hauser, T.P. Multiple hydroxyphenethyl glucosinolate isomers and their tandem mass spectrometric distinction in a geographically structured polymorphism in the crucifer Barbarea vulgaris. Phytochemistry 2015, 115, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Schulz, M.; Pagnotta, E.; Ugolini, L.; Yang, T.; Matthes, A.; Lazzeri, L.; Agerbirk, N. The Role of the Glucosinolate-Myrosinase System in Mediating Greater Resistance of Barbarea verna than B. vulgaris to Mamestra brassicae Larvae. J. Chem. Ecol. 2018, 44, 1190–1205. [Google Scholar] [CrossRef]
- Shakour, Z.T.; Shehab, N.G.; Gomaa, A.S.; Wessjohann, L.A.; Farag, M.A. Metabolic and biotransformation effects on dietary glucosinolates, their bioavailability, catabolism and biological effects in different organisms. Biotechnol. Adv. 2021, 107784. [Google Scholar] [CrossRef]
- Bell, L.; Wagstaff, C. Enhancement of glucosinolate and isothiocyanate profiles in brassicaceae crops: Addressing challenges in breeding for cultivation, storage, and consumer-related traits. J. Agric. Food Chem. 2017, 65, 9379–9403. [Google Scholar] [CrossRef]
- Wittstock, U.; Burow, M. Tipping the scales—Specifier proteins in glucosinolate hydrolysis. IUBMB Life 2007, 59, 744–751. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Kaufmann, M.; Kupke, F.; Hackl, T.; Kroh, L.W.; Rohn, S.; Schreiner, M. Brassica vegetables as sources of epithionitriles: Novel secondary products formed during cooking. Food Chem. 2018, 245, 564–569. [Google Scholar] [CrossRef]
- Witzel, K.; Risha, M.A.; Albers, P.; Börnke, F.; Hanschen, F.S. Identification and characterisation of three epithiospecifier protein isoforms in Brassica oleracea. Front. Plant Sci. 2019, 10, 1552. [Google Scholar] [CrossRef] [PubMed]
- Backenköhler, A.; Eisenschmidt, D.; Schneegans, N.; Strieker, M.; Brandt, W.; Wittstock, U. Iron is a centrally bound cofactor of specifier proteins involved in glucosinolate breakdown. PLoS ONE 2018, 13, e0205755. [Google Scholar] [CrossRef]
- Whiteman, N.K.; Peláez, J.N. Taste-testing tarsi: Gustatory receptors for glucosinolates in cabbage butterflies. PLoS Genet. 2021, 17, e1009616. [Google Scholar] [CrossRef]
- Chodur, G.M.; Olson, M.E.; Wade, K.L.; Stephenson, K.K.; Nouman, W.; Garima, K.; Fahey, J.W. Wild and domesticated Moringa oleifera differ in taste, glucosinolate composition, and antioxidant potential, but not myrosinase activity or protein content. Sci. Rep. 2018, 8, 7995. [Google Scholar] [CrossRef]
- Bradlow, H.L. Indole-3-carbinol as a chemoprotective agent in breast and prostate cancer. In Vivo 2008, 22, 441–445. [Google Scholar]
- Javaheri, B.; Poulet, B.; Aljazzar, A.; de Souza, R.L.; Piles, M.; Hopkinson, M.; Shervill, E.; Pollard, A.; Chan, B.; Chang, Y.-M.; et al. Stable sulforaphane protects against gait anomalies and modifies bone microarchitecture in the spontaneous STR/Ort model of osteoarthritis. Bone 2017, 103, 308–317. [Google Scholar] [CrossRef]
- Tortorella, S.M.; Royce, S.G.; Licciardi, P.V.; Karagiannis, T.C. Dietary Sulforaphane in Cancer Chemoprevention: The Role of Epigenetic Regulation and HDAC Inhibition. Antioxid. Redox Signal. 2015, 22, 1382–1424. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.G.; Riley, H.; Borge, G.I.A.; Lea, P.; Rødbotten, M.; Bengtsson, G.B. Short running title: Soil type and fertiliser affect swede quality. Eur. J. Hortic. Sci. 2017, 82, 294–305. [Google Scholar] [CrossRef]
- Deng, Q.; Zinoviadou, K.G.; Galanakis, C.M.; Orlien, V.; Grimi, N.; Vorobiev, E.; Lebovka, N.; Barba, F.J. The Effects of Conventional and Non-conventional Processing on Glucosinolates and Its Derived Forms, Isothiocyanates: Extraction, Degradation, and Applications. Food Eng. Rev. 2015, 7, 357–381. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Ercolini, D.; Fogliano, V. Food design to feed the human gut microbiota. J. Agric. Food Chem. 2018, 66, 3754–3758. [Google Scholar] [CrossRef]
- Riccio, P.; Rossano, R. Undigested food and gut microbiota may cooperate in the pathogenesis of neuroinflammatory diseases: A matter of barriers and a proposal on the origin of organ specificity. Nutrients 2019, 11, 2714. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Wilmes, P. Human gut microbiome: Function matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar] [CrossRef]
- Korpela, K.; de Vos, W.M. Early life colonisation of the human gut: Microbes matter everywhere. Curr. Opin. Microbiol. 2018, 44, 70–78. [Google Scholar] [CrossRef]
- Mota de Carvalho, N.; Costa, E.M.; Silva, S.; Pimentel, L.; Fernandes, T.H.; Pintado, M.E. Fermented foods and beverages in human diet and their influence on gut microbiota and health. Ferment 2018, 4, 90. [Google Scholar] [CrossRef]
- LoSasso, C.; Eckert, E.; Mastrorilli, E.; Villiger, J.; Mancin, M.; Patuzzi, I.; Di Cesare, A.; Cibin, V.; Barrucci, F.; Pernthaler, J.; et al. Assessing the Influence of Vegan, Vegetarian and Omnivore Oriented Westernized Dietary Styles on Human Gut Microbiota: A Cross Sectional Study. Front. Microbiol. 2018, 9, 317. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012, 10, 323–335. [Google Scholar] [CrossRef]
- Maukonen, J.; Saarela, M. Human gut microbiota: Does diet matter? Proc. Nutr. Soc. 2015, 74, 23–36. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bai, Y.; Tao, S.; Zhang, G.; Wang, J.; Liu, L.; Zhang, S. Fiber-rich foods affected gut bacterial community and short-chain fatty acids production in pig model. J. Funct. Foods 2019, 57, 266–274. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Bachem, A.; Makhlouf, C.; Binger, K.J.; de Souza, D.P.; Tull, D.; Hochheiser, K.; Whitney, P.G.; Fernandez-Ruiz, D.; Dähling, S.; Kastenmüller, W.; et al. Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated CD8+ T Cells. Immunity 2019, 51, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Do Prado, S.B.R.; Castro-Alves, V.C.; Ferreira, G.F.; Fabi, J.P. Ingestion of non-digestible carbohydrates from plant-source foods and decreased risk of colorectal cancer: A review on the biological effects and the mechanisms of action. Front. Nutr. 2019, 6, 72. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59, S130–S152. [Google Scholar] [CrossRef]
- Aggett, P.J.; Agostoni, C.; Axelsson, I.; Edwards, C.A.; Goulet, O.; Hernell, O.; Koletzko, B.; Lafeber, H.N.; Micheli, J.-L.; Michaelsen, K.F.; et al. Nondigestible Carbohydrates in the Diets of Infants and Young Children: A Commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 329–337. [Google Scholar] [CrossRef]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomised, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Seppänen-Laakso, T.; Nohynek, L. Effects of ellagitannin rich berries on blood lipid profiles, gut microbiota and metabolism of phenolic compounds in metabolic syndrome. Mol. Nutr. Food Res. 2013, 57, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hullar, M.A.; Schwarz, Y.; Lampe, J.W. Human gut bacterial communities are altered by addition of cruciferous vegetables to a controlled fruit- and vegetable-free diet. J. Nutr. 2009, 139, 1685–1691. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Hoeflinger, J.L.; Neme, B.P.; Jeffery, E.H.; Miller, M.J. Dietary Broccoli Alters Rat Cecal Microbiota to Improve Glucoraphanin Hydrolysis to Bioactive Isothiocyanates. Nutrients 2017, 9, 262. [Google Scholar] [CrossRef]
- Kaczmarek, J.L.; Liu, X.; Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Seifried, H.E.; Ross, S.A.; Miller, M.J.; Swanson, K.S.; Holscher, H.D. Broccoli consumption affects the human gastrointestinal microbiota. J. Nutr. Biochem. 2019, 63, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Charron, C.S.; Vinyard, B.T.; Ross, S.A.; Seifried, H.E.; Jeffery, E.H.; Novotny, J.A. Absorption and metabolism of isothiocyanates formed from broccoli glucosinolates: Effects of BMI and daily consumption in a randomised clinical trial. Br. J. Nutr. 2018, 120, 1370–1379. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, Y.; Zhu, Y.; Mupunga, J.; Zou, L.; Liu, C.; Mao, J. Broccoli ingestion increases the glucosinolate hydrolysis activity of microbiota in the mouse gut. Int. J. Food Sci. Nutr. 2019, 70, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Kellingray, L.; Le Gall, G.; Doleman, J.F.; Narbad, A.; Mithen, R.F. Effects of in vitro metabolism of a broccoli leachate, glucosinolates and S-methylcysteine sulphoxide on the human faecal microbiome. Eur. J. Nutr. 2020, 60, 2141–2154. [Google Scholar] [CrossRef]
- Li, F.; Hullar, M.A.; Beresford, S.A.; Lampe, J.W. Variation of glucoraphanin metabolism in vivo and ex vivo by human gut bacteria. Br. J. Nutr. 2011, 106, 408–416. [Google Scholar] [CrossRef]
- Luang-In, V.; Narbad, A.; Nueno-Palop, C.; Mithen, R.; Bennett, M.; Rossiter, J.T. The metabolism of methylsulfinylalkyl- and methylthioalkyl-glucosinolates by a selection of human gut bacteria. Mol. Nutr. Food Res. 2014, 58, 875–883. [Google Scholar] [CrossRef]
- Lai, R.H.; Miller, M.J.; Jeffery, E. Glucoraphanin hydrolysis by microbiota in the rat cecum results in sulforaphane absorption. Food. Funct. 2010, 1, 161–166. [Google Scholar] [CrossRef]
- Cheng, D.L.; Hashimoto, K.; Uda, Y. In vitro digestion of sinigrin and glucotropaeolin by single strains of Bifidobacterium and identification of the digestive products. Food Chem. Toxicol. 2004, 42, 351–357. [Google Scholar] [CrossRef]
- Huber, J.; Kranz, G.; Kreibich, G.; Beining, K.; Krüger, M.; Weissbach, F. Microbiological degradation of glucosinolates in defatted rapeseed meal. Die Nahr. 1983, 27, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Oginsky, E.L.; Stein, A.E.; Greer, M.A. Myrosinase Activity in Bacteria as Demonstrated by the Conversion of Progoitrin to Goitrin. Proc. Soc. Exp. Biol. Med. 1965, 119, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Brabban, A.D.; Edwards, C. Isolation of glucosinolate degrading microorganisms and their potential for reducing the glucosinolate content of rapemeal. FEMS Microb. Lett. 1994, 119, 83–88. [Google Scholar] [CrossRef]
- Luang-In, V.; Albaser, A.A.; Nueno-Palop, C.; Bennett, M.H.; Narbad, A.; Rossiter, J.T. Glucosinolate and Desulfo-glucosinolate Metabolism by a Selection of Human Gut Bacteria. Curr. Microbiol. 2016, 73, 442–451. [Google Scholar] [CrossRef]
- Luciano, F.B.; Belland, J.; Holley, R.A. Microbial and chemical origins of the bactericidal activity of thermally treated yellow mustard powder toward Escherichia coli O157:H7 during dry sausage ripening. Int. J. Food Microbiol. 2011, 145, 69–76. [Google Scholar] [CrossRef]
- Nugon-Baudon, L.; Rabot, S.; Wal, J.M.; Szylit, O. Interactions of the intestinal microflora with glucosinolates in rapeseed meal toxicity: First evidence of an intestinal lactobacillus possessing a myrosinase-like activity in vivo. J. Sci. Food Agric. 1990, 52, 547–559. [Google Scholar] [CrossRef]
- Herzallah, S.; Lledó, M.L.; Holley, R.A. Influence of NaCl and NaNO3 on sinigrin hydrolysis by foodborne bacteria. J. Food Prot. 2011, 74, 2162–2168. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Sobhi, B.; Holley, R.A. Influence of temperature, glucose, and iron on sinigrin degradation by Salmonella and Listeria monocytogenes. J. Food Prot. 2014, 77, 2133–2138. [Google Scholar] [CrossRef][Green Version]
- Liou, C.S.; Sirk, S.J.; Diaz, C.A.; Klein, A.P.; Fischer, C.R.; Higginbottom, S.K.; Erez, A.; Donia, M.S.; Sonnenburg, J.L.; Sattely, E.S. A metabolic pathway for activation of dietary glucosinolates by a human gut symbiont. Cell 2020, 180, 717–728. [Google Scholar] [CrossRef]
- Luang-In, V.; Narbad, A.; Cebeci, F.; Bennett, M.; Rossiter, J.T. Identification of proteins possibly involved in glucosinolate metabolism in L. agilis R16 and E. coli VL8. Prot. J. 2015, 34, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Dufour, V.; Stahl, M.; Baysse, C. The antibacterial properties of isothiocyanates. Microbiology 2015, 161, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.P.; Beran, F. Gut microbiota degrades toxic isothiocyanates in a flea beetle pest. Mol. Ecol. 2020, 29, 4692–4705. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.L.; Tan, H.Q.; Chua, K.J.; Kang, A.; Lim, K.H.; Ling, K.L.; Yew, W.S.; Lee, Y.S.; Thiery, J.P.; Chang, M.W. Author Correction: Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat. Biomed. Eng. 2020, 4, 754–755. [Google Scholar] [CrossRef]


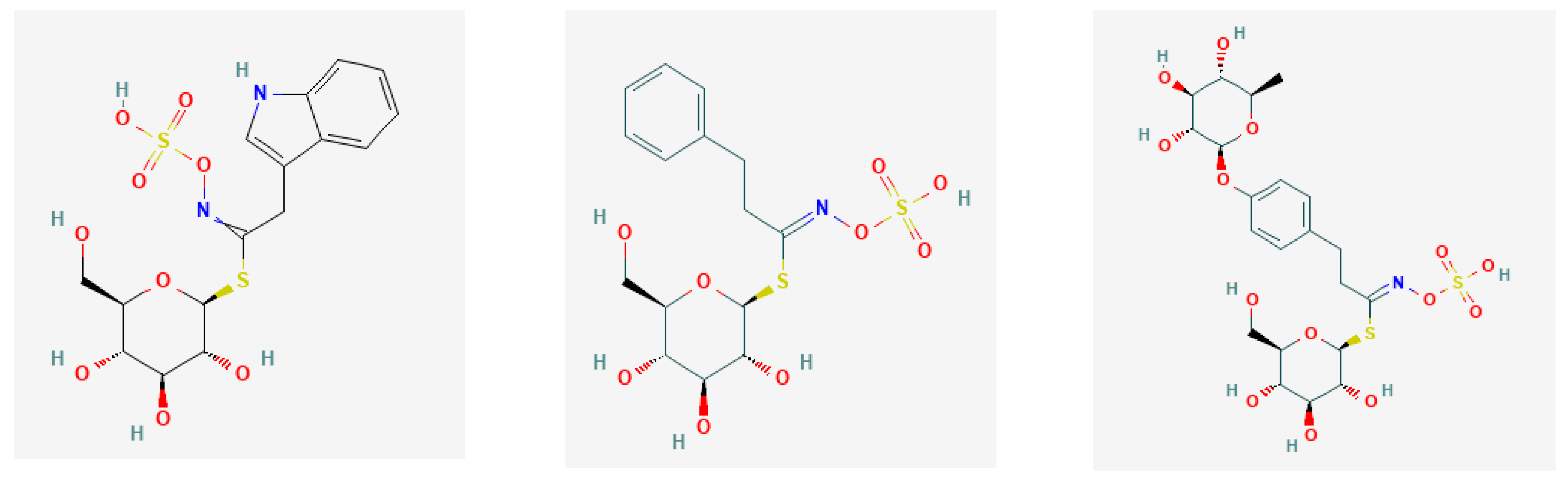

| Trivial Names/Abbreviation | Semisystemic Names * | Possible Derivatives * | Ref. |
|---|---|---|---|
| Aliphatic | |||
| Epiprogoitrin/EPI | 2(S)-2-Hydroxy-3-butenyl | (S)- and (R)-1-Cyano-2-hydroxy-3-butene | [29] |
| Glucoalyssin/GAL | 5-Methyl-sulfinyl-pentyl | no corresponding isothiocyanate (ITC)/indole | [30] |
| 5-Methylsulfinylpentyl | [31] | ||
| Glucobrassicanapin/GBN | 4-Pentenyl | 5-hexenenitrile | [32] |
| Pent-4-enyl | |||
| Glucoberteroin/GOP | 5-Methylthiopentyl | 6-(Methylsulfanyl) hexanenitrile | [31] |
| Glucoerucin/GER | 4-Methlythio-butyl | 1-isothiocyanato-4-methylsulfanylbutane; erucin | [33] |
| Glucoerysolin | 4-Methyl-sulfonyl-butyl | 4-(Methylsulfonyl)pentane nitrile | [34] |
| 4-Methylsulphonylbutyl | |||
| Glucoiberin/GIB | 3-Methyl-sulfinyl-propyl | 3-Methylsulfinylpropyl isothiocyanate; iberin; | [35] |
| Glucoibervirin/GIV | 3-Methylthio-propyl | 3-methylthiopropyl isothiocyanate | [36] |
| 3-Methylthiopropyl | |||
| Gluconapin/GNA | 3-Butenyl | 3-butenyl isothiocyanate | [37] |
| But-3-enyl | |||
| Gluconapoleiferin/GNP | 2(R)-2-Hydroxy-4-pentenyl | respective oxazolidinethiones | [38] |
| 2-Hydroxypent-4-enyl | |||
| 2-Hydroxy-pent-4-enyl | |||
| Glucoraphanin/GRA | 4-Methyl-sulfinyl-butyl | 1-Isothiocyanato-4-(methylsulfinyl)butane; sulphoraphane | [33] |
| 4-Methylsulfinylbutyl; | |||
| Glucoraphenin/GRE | 4-Methyl-sulfinyl-3-butenyl | 1-Isothiocyanato-4-(methylsulfinyl)butane; sulphoraphane | [31] |
| 4-Methylsulfinylbut-3-enyl; | |||
| Glucorapiferin/GRPProgoitrin/PRO | 2(R)-2-Hydroxy-3-butenyl | (S)- and (R)-1-Cyano-2-hydroxy-3-butene; crambene | [29] |
| Sinigrin/SIN | 2-Propenyl | 3-isothiocyanatoprop-1-ene; allyl isothiocyanate | [39] |
| Prop-2-enyl | |||
| Indolic | |||
| 1-hydroxy-3-indolyl methyl | |||
| 4-Hydroxyglucobrassicin/4OHBGS | 4-Hydroxy-3-indolyl-methyl; | 4-hydroxy-3-indoleacetonitrile | [40] |
| 4-Hydroxyindol-3-ylmethyl; | |||
| 4-Hydroxy-3-indolylmethyl | |||
| 4-Methoxyglucobrassicin/4MEGBS | 4-Methoxy-3-indolyl-methyl; | 4-methoxyindolyl-3-acetonitrile | [41] |
| 4-Methoxyindol-3-ylmethyl; | |||
| 4-Methoxy-3-indolylmethyl | |||
| Glucobrassicin/GBS | 3-Indolyl-methyl; | indole-3-carbinol | [33] |
| 3-Indolylmethyl; | |||
| Indol-3-ylmethyl | |||
| Neoglucobrassicin/NGBS | 1-Methoxy-3-indolyl-methyl; | N-methoxy indole- 3-carbinol | [42] |
| N-Methoxyindol-3-ylmethyl; | |||
| N-Methoxy-3-indolylmethyl | |||
| Aromatic | |||
| Glucobarbarin/GBA | 2(S)-2-Hydroxy-2-phenyl–ethyl | p-hydroxyepiglucobarbarin | [43] |
| (2S)-2-Hydroxy-2-phenethyl | (R)-barbarin; (R)-resedine; 3-Hydroxy-3-phenylpropanenitrile | [44] | |
| Gluconasturtiin/GNR | 2-Phenyl–ethyl | phenethyl isothiocyanate | [39] |
| Phenethyl | phenyl-3-propanenitrile | [32] | |
| 2-Phenethyl | |||
| Glucotropaeolin/GTL | Benzyl | isothiocyanatomethylbenzene; benzyl ITC | [31] |
| Phylum | Family | Genus | Species (Strain) | Substrate | Products | Cell-Free Protein Extract * | Reference | |
|---|---|---|---|---|---|---|---|---|
| GSL | ITC | NIT | ||||||
| Actinobacteria | Bifidobacteriaceae | Bifidobacterium | pseudocatenulatum | SIN, GTL | NT ‡ | NT | [87] | |
| adolescents | SIN | − | + | 1 | ||||
| adolescents | GTL | − | + | |||||
| longum | SIN, GTL | − | NT | |||||
| Bacteroidetes | Bacteroidaceae | Bacteroides | thetaiotaonicron (II8) | SIN | + | − | [22] | |
| Firmicutes | Bacillaceae | Bacillus | cereus | rape seed meal | + | NT | [88] | |
| sSubtilis | PRO | + | NT | [89] | ||||
| Bacillus (isolates) | spp. | SIN | NT | NT | [90] | |||
| Enterococcaceae | Enterococcus | casseliflavus CP1 | SIN | + | + | 2 | [91] | |
| GER | + | + | [85] | |||||
| GIB | Trace | − | ||||||
| GRA | − | Trace | ||||||
| GTL | + | + | [91] | |||||
| GNR | + | + | [91] | |||||
| Lactobacillaceae | Lactobacillus | spp. | SIN | NT | NT | [90] | ||
| plantarum KW30 | GRA, GIB | − | + | [29] | ||||
| gasseri | GRA | − | + | [86] | ||||
| acidophilus | GRA | − | + | |||||
| casei | GRA | − | + | |||||
| plantarum | GRA | − | + | |||||
| curvatus (various strains) | SIN | NT | NT | [92] | ||||
| plantarum (various strains) | SIN | NT | NT | |||||
| (LEM) | SIN | NT | NT | [93] | ||||
| (LEM) | PRO | NT | NT | |||||
| agilis R16 | SIN | + | + | 2 | [21,91] | |||
| GER | + | + | [85] | |||||
| GIB | − | − | ||||||
| GRA | − | − | ||||||
| GTL | + | + | [91] | |||||
| GNR | + | − | ||||||
| Streptococcaceae | Lactococcus | lactis subsp.lactis KF147 | GRA, GIB | − | + | [29] | ||
| Listeriaceae | Listeria | monocytogenes | SIN | + | NT | [94] | ||
| monocytogenes | SIN | + | NT | [95] | ||||
| Lactobacillaceae | Pediococcus | pentosaceus | SIN | NT | NT | [92] | ||
| acidilactici | SIN | NT | NT | |||||
| pentosaceus | SIN | + | NT | [94] | ||||
| Staphylococcaceae | Staphylococcus | carnosus (various strains) | SIN | NT | NT | [92] | ||
| spp. | SIN | NT | NT | [90] | ||||
| epidermis | PRO | + | NT | [89] | ||||
| aureus | SIN | + | NT | [94] | ||||
| carnosus | SIN | + | NT | |||||
| Streptomyces | (isolates) | SIN | NT | NT | [90] | |||
| Proteobacteria | Enterobacteriaceae | Aerobacter (Klebsiella) | aerogenes | PRO | + | NT | [89] | |
| Citrobacter | WYE1 | SIN | − | − | 3 | [16] | ||
| Enterobacter | cloacae | SIN | NT | NT | 4 | [15] | ||
| cloacae KS50 | SIN | NT | NT | 4 | [17] | |||
| Escherichia | coli VL8 | SIN | + | + | 2 | [91] | ||
| GER | + | + | [85] | |||||
| GIB | + | + | ||||||
| GRA | + | + | ||||||
| GTL | + | + | [91] | |||||
| GNR | + | + | ||||||
| Escherichia | coli Nissle 1917 | GRA, GIB | − | + | [29] | |||
| coli | PRO | + | NT | [89] | ||||
| coli O157:H7 | SIN | + | NT | [18] | ||||
| SIN | + | NT | [94] | |||||
| fecalis | SIN | + | NT | |||||
| Salmonella | typhimurium | SIN | + | NT | ||||
| spp. | SIN | + | NT | [95] | ||||
| Paracolobactrum * | aerogenoides | PRO | + | NT | 5 | [89] | ||
| Morganellaceae | Proteus | vulgaris | PRO | + | NT | |||
| Pseudomonadaceae | Pseudomonas | spp. | SIN | NT | NT | [90] | ||
| fluorescens | SIN | + | NT | [94] | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikorska-Zimny, K.; Beneduce, L. The Metabolism of Glucosinolates by Gut Microbiota. Nutrients 2021, 13, 2750. https://doi.org/10.3390/nu13082750
Sikorska-Zimny K, Beneduce L. The Metabolism of Glucosinolates by Gut Microbiota. Nutrients. 2021; 13(8):2750. https://doi.org/10.3390/nu13082750
Chicago/Turabian StyleSikorska-Zimny, Kalina, and Luciano Beneduce. 2021. "The Metabolism of Glucosinolates by Gut Microbiota" Nutrients 13, no. 8: 2750. https://doi.org/10.3390/nu13082750
APA StyleSikorska-Zimny, K., & Beneduce, L. (2021). The Metabolism of Glucosinolates by Gut Microbiota. Nutrients, 13(8), 2750. https://doi.org/10.3390/nu13082750