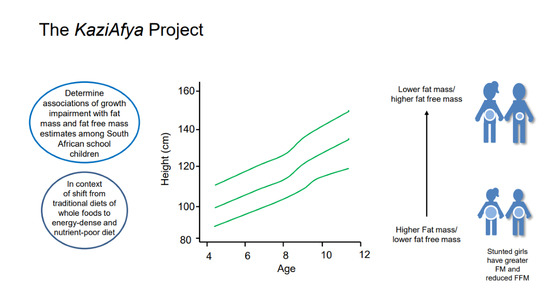
Associations of Growth Impairment and Body Composition among South African School-Aged Children Enrolled in the KaziAfya Project
Abstract
1. Introduction
2. Materials and Methods
2.1. Participating Schools and Subjects
2.2. Body Composition Estimates and Anthropometric Measures
2.3. Statistics
3. Results
3.1. Child Characteristics
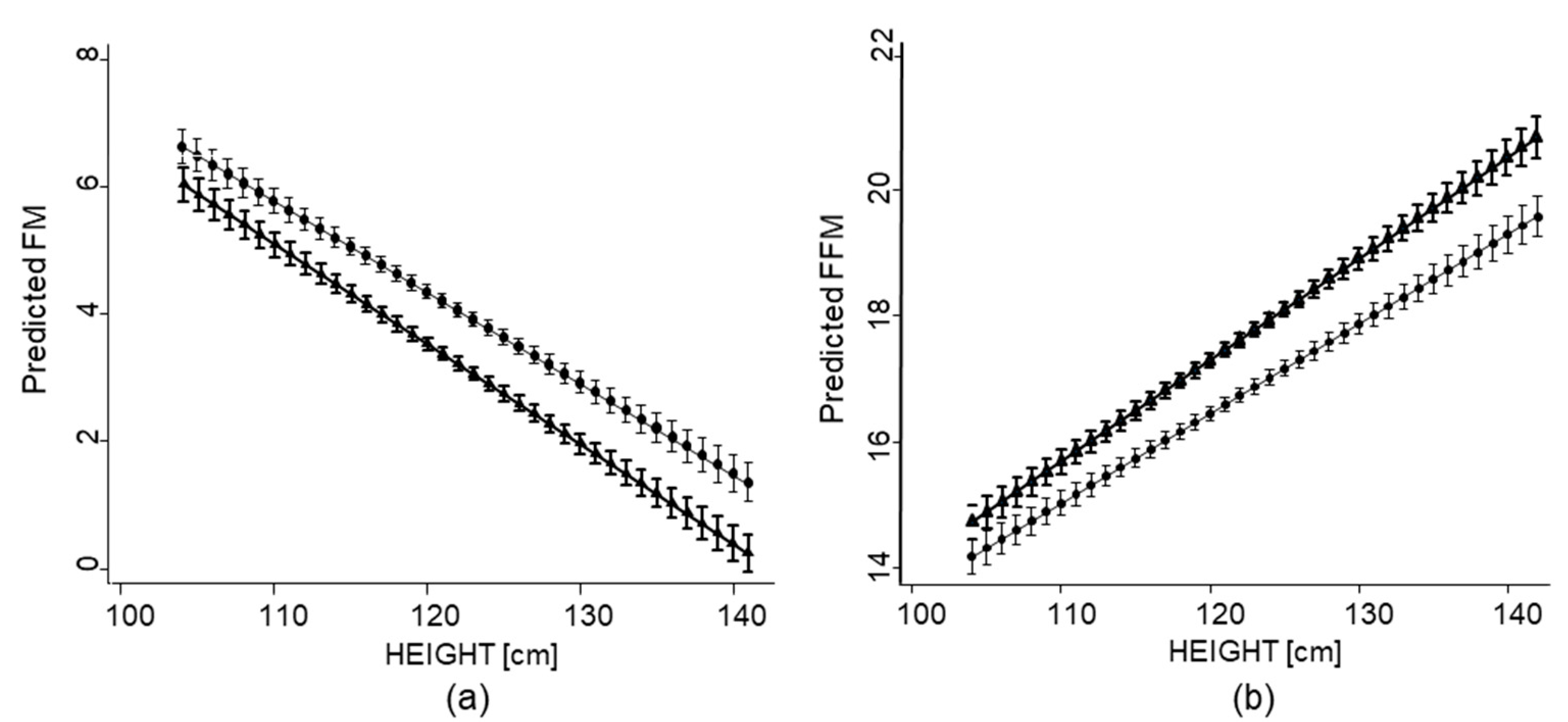
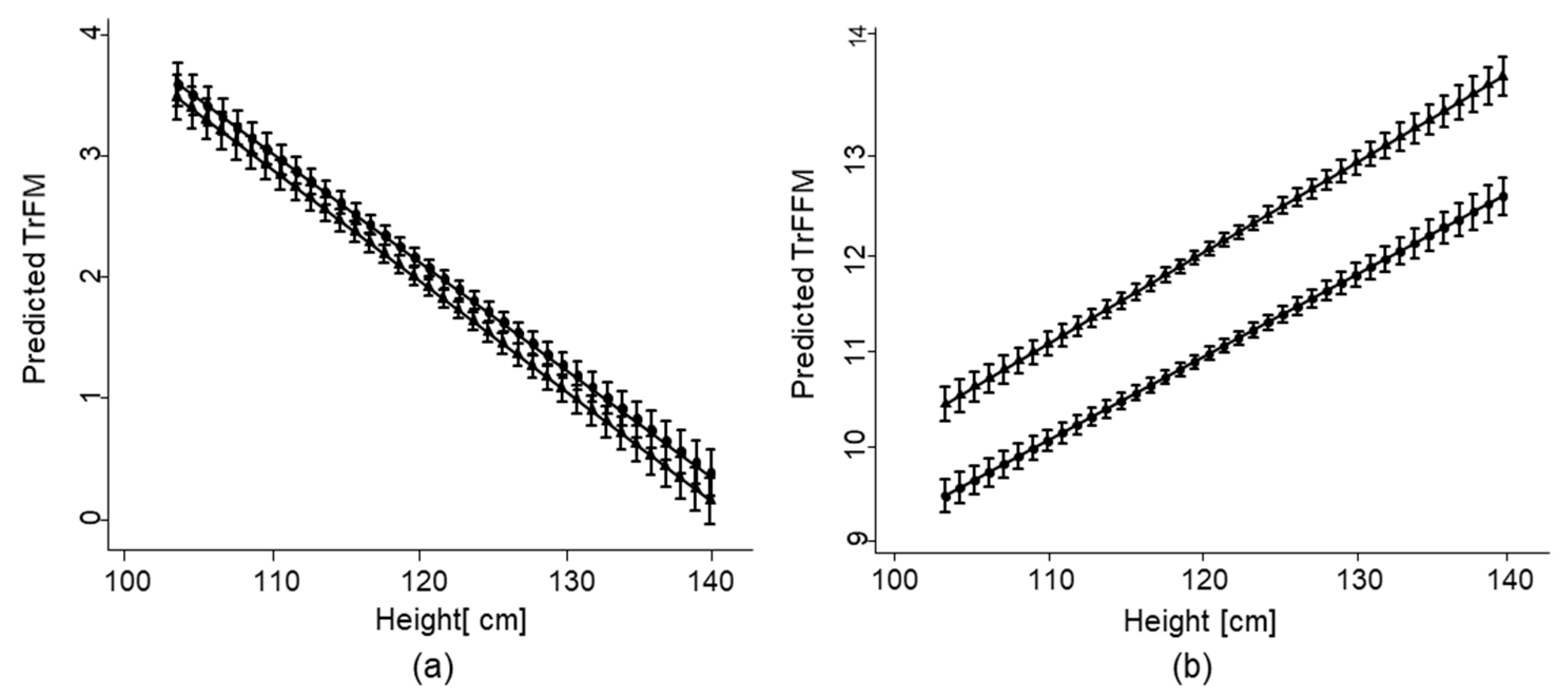
3.2. Associations of Growth Impairment with Overall FM and FFM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Steyn, K.; Damasceno, A. Lifestyle and related risk factors for chroic diseases. In Disease and Mortality in Sub-Saharan Africa; Jamison, D.T., Feachem, R.G., Makgoba, M.W., Bos, E.R., Baingana, F.K., Hoffman, K.J., Rogo, K.O., Eds.; World Bank: Washington, DC, USA, 2006. [Google Scholar]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Klingberg, S.; Draper, C.E.; Micklesfield, L.K.; Benjamin-Neelon, S.E.; van Sluijs, E.M.F. Childhood obesity prevention in Africa: A systematic review of intervention effectiveness and implementation. Int. J. Environ. Res. Public Health 2019, 16, 1212. [Google Scholar] [CrossRef]
- Doak, C.; Monteiro, C.; Popkin, B. The coexistence of obesity and undernutrition in the same households is an emerging phenomena in lower income countries. FASEB J. 1999, 13, 673. [Google Scholar]
- Popkin, B.M.; Richards, M.K.; Montiero, C.A. Stunting is associated with overweight in children of four nations that are undergoing the nutrition transition. J. Nutr. 1996, 126, 3009–3016. [Google Scholar] [CrossRef] [PubMed]
- Sawaya Al Grillo, L.P.; Verreschi, S.I.; da Silva, A.C.; Roberts, S.B. Mild stunting is associated with higher susceptibility to the effects of high fat diets: Studies in a shantytown population in São Paulo, Brazil. J. Nutr. 1998, 128 (Suppl. 2), 415S–420S. [Google Scholar] [CrossRef] [PubMed]
- Bénéfce, E.; Garnier, D.; Simondon, K.B.; Malina, R.M. Relationship between stunting in infancy and growth and fat distribution during adolescence in Senegalese girls. Eur. J. Clin. Nutr. 2001, 55, 50–58. [Google Scholar] [CrossRef]
- Schroeder, D.G.; Martorell, R.; Flores, R. Infant and child growth and fatness and fat distribution in Guatemalan adults. Am. J. Epidemiol. 1999, 149, 177–185. [Google Scholar] [CrossRef]
- Walker, S.P.; Gaskin, P.S.; Powell, C.A.; Bennett, F.I. The effects of birth weight and postnatal linear growth retardation on body mass index, fatness and fat distribution in mid and late childhood. Public Health Nutr. 2002, 5, 391–396. [Google Scholar] [CrossRef]
- Sawaya, A.L.; Roberts, S. Stunting and future risk of obesity: Principal physiological mechanisms. Cad. Saúde Pública 2003, 19 (Suppl. 1), S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Fjeld, C.R.; Schoeller, D.A.; Brown, K.H. Body composition of children recovering from severe protein-energy malnutrition at two rates of catch-up growth. Am. J. Clin. Nutr. 1989, 50, 1266–1275. [Google Scholar] [CrossRef]
- Hoffman, D.J.; Sawaya, A.L.; Verreschi, I.; Tucker, K.L.; Roberts, S.B. Why are nutritionally stunted children at increased risk of obesity? Studies of metabolic rate and fat oxidation in shantytown children from São Paulo, Brazil. Am. J. Clin. Nutr. 2000, 72, 702–707. [Google Scholar] [CrossRef]
- Smith, S.A. Central role of the adipocyte in the insulin-sensitizing and cardiovascular risk modifying actions of the thiazolidinediones. Biochemie 2003, 85, 1219–1230. [Google Scholar] [CrossRef]
- Daniels, S.R.; Morrison, J.A.; Sprecher, D.L.; Khoury, P.; Kimball, T.R. Association of body fat distribution and cardiovascular risk factors in children and adolescents. Circulation 1999, 99, 541–545. [Google Scholar] [CrossRef]
- Popkin, B.M.; Corvalan, C.; Grummer-Strawn, L.M. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 2020, 395, 65–74. [Google Scholar] [CrossRef]
- Wells, J.C.; Sawaya, A.L.; Wibaek, R.; Mwangome, M.; Poullas, M.S.; Yajnik, C.S.; Demaio, A. The double burden of malnutrition: Aetiological pathways and consequences for health. Lancet 2020, 395, 75–88. [Google Scholar] [CrossRef]
- Savanur, M.S.; Ghugre, P.S. BMI, body fat and waist-to-height ratio of stunted v. non-stunted Indian children: A case-control study. Public Health Nutr. 2016, 19, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.P.; Chang, S.M.; Powell, C.A. The association between early childhood stunting and weight status in late adolescence. Int. J. Obes. 2007, 31, 347–352. [Google Scholar] [CrossRef]
- Pomeroy, E.; Stock, J.T.; Stanojevic, S.; Miranda, J.J.; Cole, T.J.; Wells, J.C. Stunting, adiposity, and the individual-level “dual burden” among urban lowland and rural highland Peruvian children. Am. J. Hum. Biol. 2014, 26, 481–490. [Google Scholar] [CrossRef]
- Kagura, J.; Feeley, A.B.; Micklesfield, L.K.; Pettifor, J.M.; Norris, S.A. Association between infant nutrition and anthropometry, and pre-pubertal body composition in urban South African children. J. Dev. Orig. Health Dis. 2012, 3, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N.; Wright, M.M.; Griffiths, P.L.; Norris, S.A.; Pettifor, J.M. Stunting at 2 years in relation to body composition a 9 years in African urban children. Obes. Res. 2005, 13, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.A.; Hoffman, D.J.; Fernandes, M.T.; Nascimento, C.R.; Roberts, S.B.; Sesso, R.; Sawaya, A.L. Stunted children gain less lean body mass and more fat mass than their non-stunted counterparts: A prospective study. Br. J. Nutr. 2004, 92, 819–825. [Google Scholar] [CrossRef]
- Barrios, P.L.; Garcia-Feregrino, R.; Rivera, J.A.; Barraza-Villarreal, A.; Hernández-Cadena, L.; Romieu, I.; Gonzalez-Casanova, I.; Ramakrishnan, U.; Hoffman, D.J. Height trajectory during early childhood is inversely associated with fat mass in later childhood in Mexican boys. J. Nutr. 2019, 149, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodle, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Gerber, M.; Endes, K.; Herrmann, C.; Colledge, F.; Brand, S.; Donath, L.; Faude, O.; Pühse, U.; Hanssen, H.; Zahner, L. Fitness, stress, and body composition in primary schoolchildren. Med. Sci. Sports Exerc. 2017, 49, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.; Ayekoé, S.A.; Beckmann, J.; Bonfoh, B.; Coulibaly, J.T.; Daouda, D.; du Randt, R.; Finda, L.; Gall, S.; Mollel, G.J.; et al. Effects of school-based physical activity and multi-micronutrient supplementation intervention on growth, health and well-being of schoolchildren in three African countries: The KaziAfya cluster randomised controlled trial protocol with a 2 × 2 factorial design. Trials 2020, 21, 3883–3885. [Google Scholar]
- Barreira, T.V.; Staiano, A.E.; Katzmarzyk, P.T. Validity assessment of a portable bioimpedance scale to estimate body fat percentage in white and African-American children and adolescents. Pediatr. Obes. 2013, 8, e29–e32. [Google Scholar] [CrossRef]
- Ogden, C.L.; Kuczmarski, R.J.; Flegal, K.M.; Mei, Z.; Guo, S.; Wei, R.; Grummer-Strawn, L.M.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. Centers for Disease Control and Prevention 2000 growth charts for the United States: Improvements to the 1977 National Center for Health Statistics version. Pediatrics 2002, 109, 45–60. [Google Scholar] [CrossRef]
- Stevens, G.A.; Finucane, M.M.; Paciorek, C.J.; Flaxman, S.R.; White, R.A.; Donner, A.J.; Ezzati, M. Trends in mild, moderate, and severe stunting and underweight, and progress towards MDG 1 in 141 developing countries: A systematic analysis of population representative data. Lancet 2012, 380, 824–834. [Google Scholar] [CrossRef]
- De Lucia Rolfe, E.; de França, G.V.A.; Avila Vianna, C.; Gigante, D.P.; Miranda, J.J.; Yudkin, J.S.; Lessa Horta, B.; Ong, K.K. Associations of stunting in early childhood with cardiometabolic risk factors in adulthood. PLoS ONE 2018, 13, e0192196. [Google Scholar] [CrossRef]
- Sawaya, A.L.; Dallal, G.; Solymos, G.; de Sousa, M.H.; Ventura, M.L.; Roberts, S.B.; Sigulem, D.M. Obesity and malnutrition in a Shantytown population in the city of São Paulo, Brazil. Obes. Res. 1995, 3 (Suppl. 2), 107s–115s. [Google Scholar] [CrossRef] [PubMed]
- Motswagole, B.S.; Kruger, H.S.; Faber, M.; Monyeki, K.D. Body composition in stunted, compared to non-stunted, black South African children, from two rural communities. S. Afr. J. Clin. Nutr. 2012, 25, 62–66. [Google Scholar] [CrossRef]
- Wells, J.C.K.; Devakumar, D.; Manandhar, D.S.; Saville, N.; Chaube, S.S.; Costello, A.; Osrin, D. Associations of stunting at 2 years with body composition and blood pressure at 8 years of age: Longitudinal cohort analysis from lowland Nepal. Eur. J. Clin. Nutr. 2019, 73, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Vink, E.E.; van Coeverden, S.C.; van Mil, E.G.; Felius, B.A.; van Leerdam, F.J.; Delemarre-van de Waal, H.A. Changes and tracking of fat mass in pubertal girls. Obesity 2010, 18, 1247–1251. [Google Scholar] [CrossRef]
- Martorell, R.; Khan, L.K.; Schroeder, D.G. Reversibility of stunting: Epidemiological findings in children from developing countries. Eur. J. Clin. Nutr. 1994, 48 (Suppl. 1), 45–57. [Google Scholar]
- Hills, A.; Byrne, N.M. An overview of physical growth and maturation. Med. Sport Sci. 2010, 55, 1–13. [Google Scholar] [PubMed]
- Hoffman, D.J.; Roberts, S.B.; Verresch, I.; Martins, P.A.; de Nascimento, C.; Tucker, K.L.; Sawaya, A.L. Regulation of energy intake may be impaired in nutritionally stunted children from the shantytowns of São Paulo, Brazil. J. Nutr. 2000, 130, 2265–2270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Astrup, A.; Buemann, B.; Christensen, N.J.; Toubro, S. Failure to increase lipid oxidation in response to increasing dietary fat content in formerly obese women. Am. J. Physiol. 1994, 266, e592–e599. [Google Scholar]
- Seidell, J.C.; Muller, D.C.; Sorkin, J.D.; Andres, R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: The Baltimore Longitudinal Study on Aging. Int. J. Obes. Relat. Metab. Disord. 1992, 16, 667–674. [Google Scholar] [PubMed]
- Lee, S.K. North Korean children: Nutrition and growth. Ann. Pediatr. Endocrinol. Metab. 2017, 22, 231–239. [Google Scholar] [CrossRef]
- Popkin, B.M. Nutrition, agriculture and the global food system in low and middle income countries. Food Policy 2014, 47, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Ford, N.; Patel, S.A.; Narayan, K.M.V. Obesity in low-and middle-income countries: Burden, drivers, and emerging challenges. Annu. Rev. Public Health 2017, 38, 145–164. [Google Scholar] [CrossRef]
- Ness, A.R.; Leary, S.D.; Mattocks, C.; Blair, S.N.; Reilly, J.J.; Wells, J.; Ingle, S.; Tilling, K.; Smith, G.D.; Riddoch, C. Objectively measured physical activity and fat mass in a large cohort of children. PLoS Med. 2007, 4, e97. [Google Scholar] [CrossRef] [PubMed]
- Said-Mohamed, R.; Micklesfield, L.K.; Pettifor, J.M.; Norris, S.A. Has the prevalence of stunting in South African children changed in 40 years? A systematic review. BMC Public Health 2015, 15, 534. [Google Scholar] [CrossRef]
- Abrahams, Z.; McHiza, Z.; Steyn, N.P. Diet and mortality rates in Sub-Saharan Africa: Stages in the nutrition transition. BMC Public Health 2011, 11, 801. [Google Scholar] [CrossRef]
- Sartorius, B.; Sartorius, K.; Green, R.; Lutge, E.; Scheelbeek, P.; Tanser, F.; Dangour, A.D.; Slotow, R. Spatial-temporal trends and risk factors for undernutrition and obesity among children (<5 years) in South Africa, 2008–2017: Findings from a nationally representative longitudinal panel survey. BMJ Open 2020, 10, e034476. [Google Scholar] [CrossRef]
- McLachlan, M.; Landman, A.P. Nutrition-sensitive agriculture—A South African perspective. Food Secur. 2013, 5, 857–871. [Google Scholar] [CrossRef]
- De Vos, J.C.W.; Du Toit, D.; Coetzee, D. The types and levels of physical activity and sedentary behaviour of senior phase learners in Potchefstroom. Health S. Afr. Gesondheld 2016, 21, 372–380. [Google Scholar] [CrossRef]
- Gerber, M.; Müller, I.; Walter, C.; du Randt, R.; Adams, L.; Gall, S.; Joubert, N.; Nqweniso, S.; Smith, D.; Steinmann, P.; et al. Physical activity and dual disease burden among South African primary schoolchildren from disadvantaged neighbourhoods. Prev. Med. 2018, 112, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Micklesfield, L.K.; Pedro, T.M.; Kahn, K.; Kinsman, J.; Pettifor, J.M.; Tollman, S.; Norris, S.A. Physical activity and sedentary behavior among adolescents in rural South Africa: Levels, patterns and correlates. BMC Public Health 2014, 14, 40. [Google Scholar] [CrossRef]
- Jebb, S.A.; Cole, T.J.; Doman, D.; Murgatroyd, P.R.; Prentice, A.M. Evaluation of the novel Tanita body-fat analyser to measure body composition by comparison with a four-compartment model. Br. J. Nutr. 2000, 83, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, A.; White, Z.; Ferreira, J.; Wenhold, F.A.M. Developing an impedance based equation for fat-free mass of Black preadolescent South African children. Nutrients 2019, 11, 2021. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.; Ruel, M.T.; Salm, L.; Sinclair, S.; Branca, F. Double-duty actions: Seizing programme and policy opportunities to address malnutrition in all its forms. Lancet 2020, 395, 142–155. [Google Scholar] [CrossRef]
- WHO. Double-Duty Actions; Policy Brief; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]

 ; girl
; girl  .
.
 ; girl
; girl  .
.
 ; girl
; girl  .
.
 ; girl
; girl  .
.
| Total (n = 1287) | Boys (n = 668) | Girls (n = 619) | |
|---|---|---|---|
| Anthropometric measures | M (SD) a | M (SD) | M (SD) |
| Age (years) | 8.33 (1.5) | 8.20 (1.42) f | 8.37 (1.55) f |
| Height (cm) | 124.69 (9.24) | 125.17 (8.88) f | 124.02 (9.34) f |
| Weight—(kg) | 25.38 (6.87) | 25.38 (6.38) | 25.18 (7.05) |
| BMI (kg/m2) | 16.07 (2.61) | 16.01 (2.42) | 16.13 (2.80) |
| Categories of malnutrition | n (%) | n (%) | n (%) |
| Overweight b | 123 (10%) | 53 (8.5) f | 68 (11.6) |
| Obese c | 61 (4.5%) | 17 (2.7) f | 43 (7.3) f |
| Mild stunting d | 500 (36.7) | 249 (37.3) | 251 (40.7) |
| Moderate/severe stunting e | 112 (8.2) | 48 (7.2) f | 64 (10.4) f |
| Body composition | M (SD) | M (SD) | M (SD) |
| Overall FM (kg) | 5.94 (3.20) | 5.43 (2.88) f | 6.40 (3.13) f |
| Overall FFM (kg) | 19.44 (4.26) | 19.95 (4.07) f | 18.78 (4.2) f |
| Truncal FM (kg) | 2.81 (1.48) | 2.60 (1.28) f | 3.00 (1.60) f |
| Truncal FFM (kg) | 12.46 (2.05) | 12.9 (1.78) f | 11.96 (2.15) f |
| SES characteristics | |||
| Overall SES index | 3.01 (1.20) | 2.00 (1.24) | 3.04 (1.15) |
| Outcome | Overall (n = 1287) a | Girls (n = 619) | Boys (n = 668) b | |||
|---|---|---|---|---|---|---|
| B-Coefficient (SE) | p Value | B-Coefficient (SE) | p Value | B-Coefficient (SE) | p Value | |
| FM (kg) | ||||||
| Height | −0.16 (0.01) | <0.01 | −0.16 (0.01) | <0.01 | −0.14 (0.01) | <0.01 |
| FFM (kg) | ||||||
| Height | 0.15 (0.01) | <0.01 | 0.16 (0.06) | <0.01 | 0.15 (0.01) | <0.01 |
| TrFM (kg) | ||||||
| Height | −0.09 (0.05) | <0.01 | −0.10 (0.01) | <0.01 | −0.09 (0.05) | <0.01 |
| TrFFM (kg) | ||||||
| Height | 0.08 (0.01) | <0.01 | 0.08 (0.01) | <0.01 | 0.08 (0.01) | <0.01 |
| Overall (n = 1287) a | Girls (n = 619) b | Boys (n = 668) b | ||||
|---|---|---|---|---|---|---|
| Outcome | B-Coefficient (SE) | p Value | B-Coefficient (SE) | p Value | B-Coefficient (SE) | p Value |
| FM (kg) | ||||||
| Mild stunting c | −0.09 (0.09) | 0.38 | 0.27 (0.12) | 0.02 | 0.08 (0.12) | 0.94 |
| Stunted d | 0.70 (0.15) | <0.01 | 0.88 (0.18) | <0.01 | 0.31 (0.22) | 0.16 |
| FFM (kg) | ||||||
| Mild stunting | −0.08 (0.09) | 0.36 | −0.27 (0.12) | 0.02 | −0.10 (0.12) | 0.94 |
| Stunted | −0.70 (0.11) | <0.01 | −0.90 (0.18) | <0.01 | −0.31 (0.22) | 0.10 |
| TrFM (kg) | ||||||
| Mild stunting | 0.08 (0.05) | 0.11 | 0.16 (0.08) | 0.04 | 0.98 (0.07) | 0.20 |
| Stunted | 0.42 (0.09) | <0.01 | 0.54 (0.11) | <0.01 | 0.25 (0.12) | 0.04 |
| TrFFM (kg) | ||||||
| Mild stunting | 0.48 (0.01) | 0.35 | −0.22 (0.07) | <0.01 | −0.02 (0.07) | 0.80 |
| Stunted | 0.07 (0.74) | 0.93 | −0.57 (0.11) | ≤0.01 | −0.22 (0.12) | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, K.Z.; Beckmann, J.; Lang, C.; Seelig, H.; Nqweniso, S.; Probst-Hensch, N.; Müller, I.; Pühse, U.; Steinmann, P.; du Randt, R.; et al. Associations of Growth Impairment and Body Composition among South African School-Aged Children Enrolled in the KaziAfya Project. Nutrients 2021, 13, 2735. https://doi.org/10.3390/nu13082735
Long KZ, Beckmann J, Lang C, Seelig H, Nqweniso S, Probst-Hensch N, Müller I, Pühse U, Steinmann P, du Randt R, et al. Associations of Growth Impairment and Body Composition among South African School-Aged Children Enrolled in the KaziAfya Project. Nutrients. 2021; 13(8):2735. https://doi.org/10.3390/nu13082735
Chicago/Turabian StyleLong, Kurt Z., Johanna Beckmann, Christin Lang, Harald Seelig, Siphesihle Nqweniso, Nicole Probst-Hensch, Ivan Müller, Uwe Pühse, Peter Steinmann, Rosa du Randt, and et al. 2021. "Associations of Growth Impairment and Body Composition among South African School-Aged Children Enrolled in the KaziAfya Project" Nutrients 13, no. 8: 2735. https://doi.org/10.3390/nu13082735
APA StyleLong, K. Z., Beckmann, J., Lang, C., Seelig, H., Nqweniso, S., Probst-Hensch, N., Müller, I., Pühse, U., Steinmann, P., du Randt, R., Walter, C., Utzinger, J., & Gerber, M. (2021). Associations of Growth Impairment and Body Composition among South African School-Aged Children Enrolled in the KaziAfya Project. Nutrients, 13(8), 2735. https://doi.org/10.3390/nu13082735