The Use of Natural Fiber-Rich Food Product Is Safe and Reduces Aberrant Crypt Foci in a Pre-Clinical Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Agent
2.2. Preparation of Natural Fiber-Rich Food Product
2.3. Animals and Environmental Conditions
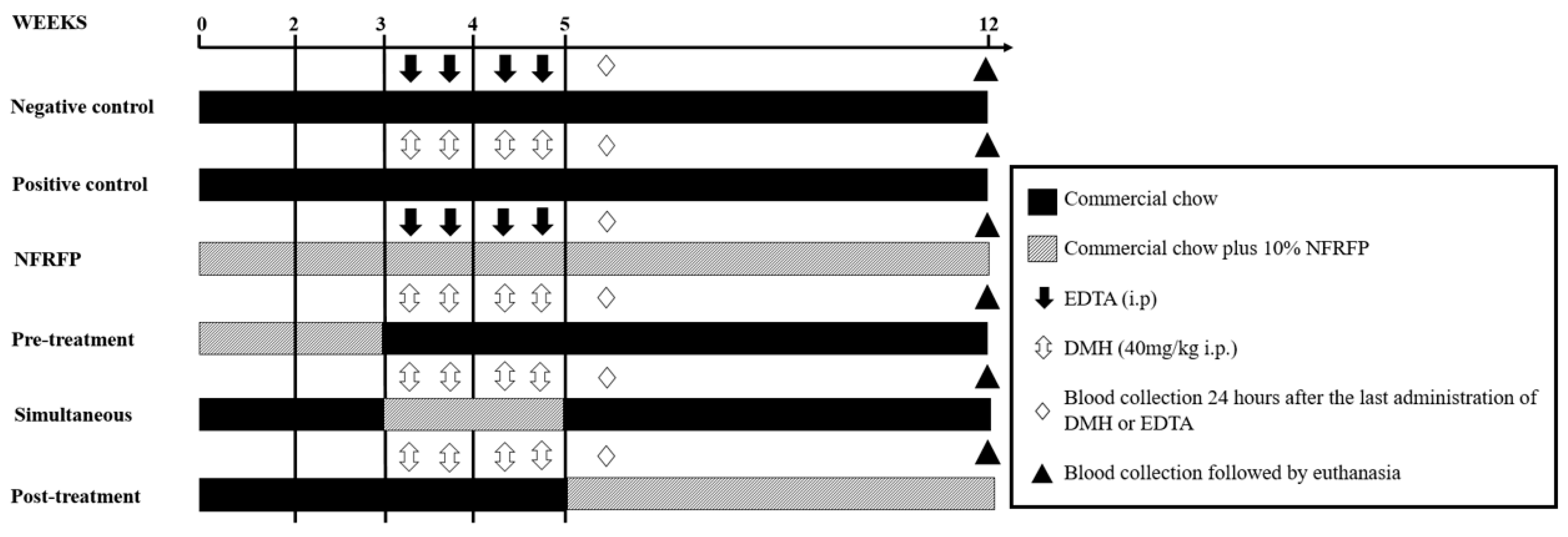
2.4. Experimental Design
2.5. Chemical Analysis of Feed and Natural Fiber-Rich Food Product
2.6. Biological Tests
2.6.1. Evaluation of Genotoxicity and Antigenotoxicity
Comet Assay
Peripheral Blood Micronucleus Assay
2.6.2. Testing of Aberrant Crypt Foci
2.6.3. Calculation of Damage Reduction Percentage (% DR)
2.6.4. Hematological and Biochemical Parameters
2.6.5. Quantification of IFN-γ, IL-6, IL-10, IL-12p70, MCP-1 and TNF-α Cytokine Expression
2.7. Statistical Analysis
3. Results
3.1. Chemical Analysis of Diets and Evaluation of Food Intake
3.2. Effects of Feed Plus 10% NFRFP and Feeding Protocols on Biometric Parameters
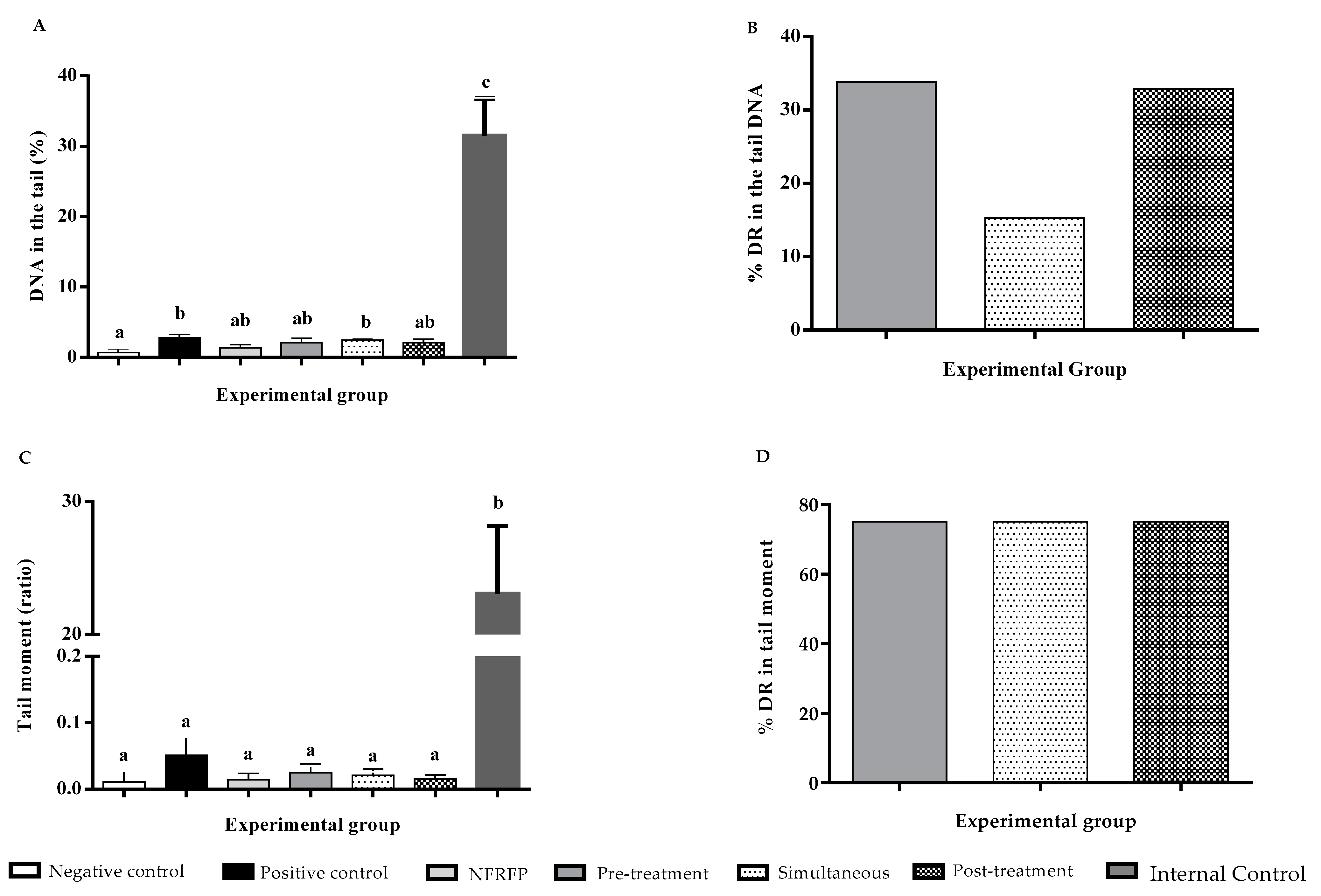
3.3. Genotoxicity Tests
3.4. Aberrant Crypt Foci
3.5. Evaluation of Biochemical and Hematological Parameters
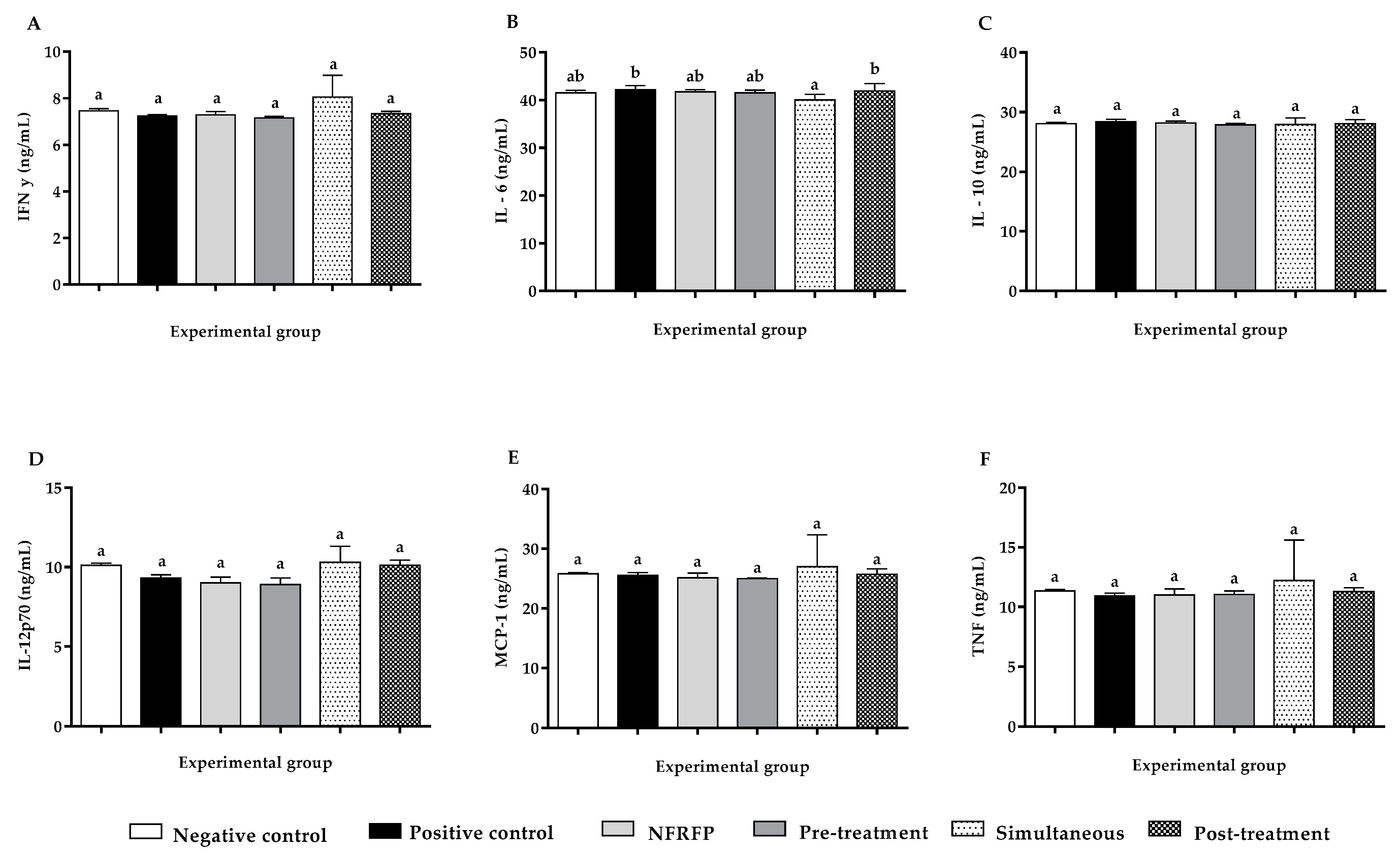
3.6. Quantification of IFN-y, IL-6, IL-10, IL-12p70, MCP-1 and TNF-α Cytokine Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Park, S.Y.; Boushey, C.J.; Wilkens, L.R.; Haiman, C.A.; Le Marchand, L. High-Quality Diets Associate With Reduced Risk of Colorectal Cancer: Analyses of Diet Quality Indexes in the Multiethnic Cohort. Gastroenterology 2017, 153, 386–394.e2. [Google Scholar] [CrossRef]
- Farinetti, A.; Zurlo, V.; Manenti, A.; Coppi, F.; Mattioli, A.V. Mediterranean diet and colorectal cancer: A systematic review. Nutrition 2017, 43–44, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Hold, G.L. Gastrointestinal Microbiota and Colon Cancer. Dig. Dis. 2016, 34, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Haslam, A.; Robb, S.W.; Hébert, J.R.; Huang, H.; Ebell, M.H. Greater adherence to a Mediterranean diet is associated with lower prevalence of colorectal adenomas in men of all races. Nutr. Res. 2017, 48, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.J.; Chau, C.F. Classification and regulatory perspectives of dietary fiber. J. Food Drug Anal. 2017. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012. [Google Scholar] [CrossRef]
- Pesarini, J.R.; Zaninetti, P.T.; Mauro, M.O.; Carreira, C.M.; Dichi, J.B.; Ribeiro, L.R.; Mantovani, M.S.; Oliveira, R.J. Antimutagenic and anticarcinogenic effects of wheat bran in vivo. Genet. Mol. Res. 2013, 12, 1646–1659. [Google Scholar] [CrossRef]
- Pamplona-Silva, M.T.; Morandi, W.V.; Bernardi, L.; Tura, B.B.; de Oliveira, D.D.M.; Antoniolli-Silva, A.C.M.B.; de Oliveira Mauro, M.; Oliveira, R. Brown Flaxseed Prevents DNA Damage Induced by 1,2-Dimethylhydrazine in a Pre-Clinical Model. Braz. Arch. Biol. Technol. 2018. [Google Scholar] [CrossRef]
- Tamargo, A.; Cueva, C.; Laguna, L.; Moreno-Arribas, M.V.; Muñoz, L.A. Understanding the impact of chia seed mucilage on human gut microbiota by using the dynamic gastrointestinal model simgi®. J. Funct. Foods 2018, 50, 104–111. [Google Scholar] [CrossRef]
- Haenen, D.; Zhang, J.; Souza da Silva, C.; Bosch, G.; van der Meer, I.M.; van Arkel, J.; van den Borne, J.J.G.C.; Pérez Gutiérrez, O.; Smidt, H.; Kemp, B.; et al. A Diet High in Resistant Starch Modulates Microbiota Composition, SCFA Concentrations, and Gene Expression in Pig Intestine. J. Nutr. 2013. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.D.; Mauro, M.O.; Pesarini, J.R.; Ogo, F.M.; Oliveira, R.J. Resistant starch: A functional food that prevents DNA damage and chemical carcinogenesis. Genet. Mol. Res. 2015, 14, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lin, Q.; Yang, T.; Zeng, L.; Shi, L.; Chen, Y.; Luo, F. Oat β-glucan ameliorates dextran sulfate sodium (DSS)-induced ulcerative colitis in mice. Food Funct. 2015, 6, 3454–3463. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, Y.; Qiu, B.; Fan, S.; Ding, H.; Liu, Z. Quinoa whole grain diet compromises the changes of gut microbiota and colonic colitis induced by dextran Sulfate sodium in C57BL/6 mice. Sci. Rep. 2018, 8, 14916. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Kuda, T.; Yazaki, T.; Takahashi, H.; Kimura, B. Caecal fermentation, putrefaction and microbiotas in rats fed milk casein, soy protein or fish meal. Appl. Microbiol. Biotechnol. 2014, 98, 2779–2787. [Google Scholar] [CrossRef]
- Park, H.S.; Goodlad, R.A.; Wright, N.A. The incidence of aberrant crypt foci and colonic carcinoma in dimethylhydrazine-treated rats varies in a site-specific manner and depends on tumor histology. Cancer Res. 1997, 57, 4507–4510. [Google Scholar]
- Limeiras, S.M.A.; Oliveira, B.C.; Pessatto, L.R.; Pesarini, J.R.; Kassuya, C.A.L.; Monreal, A.C.D.; Cantero, W.B.; Antoniolli-Silva, R.; Antoniolli-Silva, A.C.M.B.; Oliveira, M.E.A.; et al. Effects of Moquiniastrum polymorphum ssp floccosum ethnolic extract on colorectal carcinogenesis induced by 1,2-dimethylhydrazine. Genet. Mol. Res. 2017, 16. [Google Scholar] [CrossRef]
- Caetano, B.F.R.; Tablas, M.B.; Pereira, N.E.F.; de Moura, N.A.; Carvalho, R.F.; Rodrigues, M.A.M.; Barbisan, L.F. Capsaicin reduces genotoxicity, colonic cell proliferation and preneoplastic lesions induced by 1,2-dimethylhydrazine in rats. Toxicol. Appl. Pharmacol. 2018, 338, 93–102. [Google Scholar] [CrossRef]
- Ramos Caetano, B.F.; Baptista Tablas, M.; Ribeiro Romualdo, G.; Marchesan Rodrigues, M.A.; Barbisan, L.F. Early molecular events associated with liver and colon sub-acute responses to 1,2-dimethylhydrazine: Potential implications on preneoplastic and neoplastic lesion development. Toxicol. Lett. 2020, 329, 67–79. [Google Scholar] [CrossRef] [PubMed]
- de Moura, N.A.; Caetano, B.F.R.; Bidinotto, L.T.; Rodrigues, M.A.M.; Barbisan, L.F. Dietary hemin promotes colonic preneoplastic lesions and DNA damage but not tumor development in a medium-term model of colon carcinogenesis in rats. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 846, 403076. [Google Scholar] [CrossRef] [PubMed]
- de Moura, N.A.; Caetano, B.F.R.; de Moraes, L.N.; Carvalho, R.F.; Rodrigues, M.A.M.; Barbisan, L.F. Enhancement of colon carcinogenesis by the combination of indole-3 carbinol and synbiotics in hemin-fed rats. Food Chem. Toxicol. 2018, 112, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Limeiras, S.M.A.; Ogo, F.M.; Genez, L.A.L.; Carreira, C.M.; Oliveira, E.J.T.; Pessatto, L.R.; Neves, S.C.; Pesarini, J.R.; Schweich, L.C.; Silva, R.A.; et al. Prevention of DNA damage and anticarcinogenic activity of activia® in a preclinical model. Genet. Mol. Res. 2017, 16. [Google Scholar] [CrossRef]
- Bazo, A.P.; Rodrigues, M.A.M.; Sforcin, J.M.; de Camargo, J.L.V.; Ribeiro, L.R.; Salvadori, D.M.F. Protective action of propolis on the rat colon carcinogenesis. Teratog. Carcinog. Mutagen. 2002, 22, 183–194. [Google Scholar] [CrossRef] [PubMed]
- da Silva Almeida, A.P.; Avi, C.M.; Barbisan, L.F.; de Moura, N.A.; Caetano, B.F.R.; Romualdo, G.R.; Sivieri, K. Yacon (Smallanthus sonchifolius) and Lactobacillus acidophilus CRL 1014 reduce the early phases of colon carcinogenesis in male Wistar rats. Food Res. Int. 2015, 74, 48–54. [Google Scholar] [CrossRef]
- Kada, T.; Shimoi, K. Desmutagens and bio-antimutagens—Their modes of action. BioEssays 1987, 7, 113–116. [Google Scholar] [CrossRef]
- Oliveira, R.J. Mecanismos de Ação e Efeito Protetor de Danos no DNA do Polissacarídeo β-Glucana em Testes In Vitro e In Vivo; Universidade Estadual de Londrina: Londrina, Brasil, 2006. [Google Scholar]
- Oliveira, R.J.; Baise, É.; de Oliveira Mauro, M.; Pesarini, J.R.; Matuo, R.; da Silva, A.F.; Ribeiro, L.R.; Mantovani, M.S. Evaluation of chemopreventive activity of glutamine by the comet and the micronucleus assay in mice’s peripheral blood. Environ. Toxicol. Pharmacol. 2009, 28, 120–124. [Google Scholar] [CrossRef]
- Waters, M.D.; Brady, A.L.; Stack, H.F.; Brockman, H.E. Antimutagenicity profiles for some model compounds. Mutat. Res. Genet. Toxicol. 1990, 238, 57–85. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists International; AOAC: Rockville, MD, USA, 2011. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959. [Google Scholar] [CrossRef]
- Merrill, A.L.; Watt, B.K. Energy values of food: Basis and derivation. In Agriculture Handboook No. 74; Human Nutrition Research Branch, Agricultural Research Service, US Department of Agriculture: Beltsville, MD, USA, 1973. [Google Scholar]
- Navarro, S.D.; Beatriz, A.; Meza, A.; Pesarini, J.R.; Gomes, R.D.S.; Karaziack, C.B.; Cunha-Laura, A.L.; Monreal, A.C.D.; Romão, W.; Lacerda Júnior, V.; et al. A new synthetic resorcinolic lipid 3-Heptyl-3,4,6-trimethoxy-3H- isobenzofuran-1-one: Evaluation of toxicology and ability to potentiate the mutagenic and apoptotic effects of cyclophosphamide. Eur. J. Med. Chem. 2014. [Google Scholar] [CrossRef]
- Navarro, S.D.; Pessatto, L.R.; Meza, A.; de Oliveira, E.J.T.; Auharek, S.A.; Vilela, L.C.; de Lima, D.P.; de Azevedo, R.B.; Kassuya, C.A.L.; Cáceres, O.I.A.; et al. Resorcinolic lipid 3-heptyl-3,4,6-trimethoxy-3H-isobenzofuran-1-one is a strategy for melanoma treatment. Life Sci. 2018, 209, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Morita, T.; Kodama, Y.; Sofuni, T.; Ishidate, M. The micronucleus assay with mouse peripheral blood reticulocytes using acridine orange-coated slides. Mutat. Res. Lett. 1990. [Google Scholar] [CrossRef]
- Bird, R.P. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: Preliminary findings. Cancer Lett. 1987. [Google Scholar] [CrossRef]
- Bird, R.P.; Good, C.K. The significance of aberrant crypt foci in understanding the pathogenesis of colon cancer. Toxicol. Lett. 2000, 112–113, 395–402. [Google Scholar] [CrossRef]
- Manoharan, K.; Banerjee, M.R. β-Carotene reduces sister chromatid exchanges induced by chemical carcinogens in mouse mammary cells in organ culture. Cell Biol. Int. Rep. 1985. [Google Scholar] [CrossRef]
- Bain, B.J.; Bates, I.; Laffan, M.A.; Lewis, S.M. Dacie and Lewis Practical Haematology; Elsevier Health Sciences: Oxford, UK, 2016; ISBN 9780702050251. [Google Scholar]
- ANVISA. Dispõe sobre o Regulamento Técnico Sobre Informação Nutricional Complementar (Resolução no 54, de 12 de Novembro de 2012); Agência Nacional de Vigilância Sanitária, Ed.; Diário Oficial da República Federativa do Brasil: Brasilia, Brasil, 2012.
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients); National Academies Press (US): Washington, DC, USA, 2005; ISBN 030908525X. [Google Scholar]
- Elias, M.C.; Lopes, V.; Gutkoski, L.C.; Oliveira, M.; Mazzutti, S.; Dias, A.R.G. Umidade de colheita, métodos de secagem e tempo de armazenamento na qualidade tecnológica de grãos de trigo (cv. ‘Embrapa 16′). Cienc. Rural 2009, 39, 25–30. [Google Scholar] [CrossRef][Green Version]
- ANVISA. Aprova o Regulamento Técnico para Produtos de Cereais, Amidos, Farinhas e Farelos (Resolução no 263, de 22 de Setembro de 2005); Agência Nacional de Vigilância Sanitária, Ed.; Diário Oficial da República Federativa do Brasil: Brasilia, Brasil, 2005.
- Seid, H.; Rosenbaum, M. Low carbohydrate and low-fat diets: What we don’t know and why we should know it. Nutrients 2019, 11, 2749. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, R.M.; Seidell, J.C. Carbohydrate intake and obesity. Eur. J. Clin. Nutr. 2007, 61, S75–S99. [Google Scholar] [CrossRef] [PubMed]
- Doaei, S.; Gholamalizadeh, M.; Akbari, M.E.; Akbari, S.; Feradova, H.; Rahimzadeh, G.; Jarrahi, A.M. Dietary carbohydrate promotes cell survival in cancer via the up-regulation of fat mass and obesity-associated gene expression level. Malays. J. Med. Sci. 2019, 26, 8–17. [Google Scholar] [CrossRef]
- Sartorius, B.; Sartorius, K.; Aldous, C.; Madiba, T.E.; Stefan, C.; Noakes, T. Carbohydrate intake, obesity, metabolic syndrome and cancer risk? A two-part systematic review and meta-analysis protocol to estimate attributability. BMJ Open 2016, 6. [Google Scholar] [CrossRef][Green Version]
- Melo, M.G.D.; Dória, G.A.A.; Serafini, M.R.; Araújo, A.A.S. Valores de referência hematológicos e bioquímicos de ratos (Rattus novergicus linhagem Wistar) provenientes do biotério central da Universidade Federal de Sergipe. Sci. Plena 2014, 10, 2014. [Google Scholar]
- Charles River Laboratories Wistar Rats Biochemistry. Available online: https://www.criver.com/sites/default/files/resources/rm_d_Wistar_Rat.pdf (accessed on 8 September 2020).
- Dantas, J.A.; Ambiel, C.R.; Cuman, R.K.N.; Baroni, S.; Bersani-Amado, C.A. Valores de referência de alguns parâmetros fisiológicos de ratos do Biotério Central da Universidade Estadual de Maringá, estado do Paraná. Acta Sci. 2006, 28, 633–636. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Sima, P.; Vannucci, L.; Vetvicka, V. β-glucans and cholesterol (Review). Int. J. Mol. Med. 2018, 41, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M. Functional food concept and its application to prebiotics. Dig. Liver Dis. 2002, 34. [Google Scholar] [CrossRef]
- Liljeberg Elmståhl, H. Resistant starch content in a selection of starchy foods on the Swedish market. Eur. J. Clin. Nutr. 2002. [Google Scholar] [CrossRef] [PubMed]
- Wächtershäuser, A.; Stein, J. Rationale for the luminal provision of butyrate in intestinal diseases. Eur. J. Nutr. 2000. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Baird, P.; Davis, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009. [Google Scholar] [CrossRef] [PubMed]
- Perše, M.; Cerar, A. Morphological and molecular alterations in 1,2 dimethylhydrazine and azoxymethane induced colon carcinogenesis in rats. J. Biomed. Biotechnol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Serrano Campelo DE-SOUZA, A.; Andrade COSTA-CASAGRANDE, T. MODELOS ANIMAIS DE CARCINOGÊNESE COLORRETAL Animal models for colorectal cancer. ABCD Arq Bras. Cir. Dig. 2018, 31, 1369. [Google Scholar] [CrossRef]
- Ribeiro, L.R.; Salvadori, D.M.F.; Marques, E.K. Mutagênese Ambiental; ULBRA: Rio Grande do Sul, Brazil, 2003. [Google Scholar]
- Kirkland, D.J.; Henderson, L.; Marzin, D.; Müller, L.; Parry, J.M.; Speit, G.; Tweats, D.J.; Williams, G.M. Testing strategies in mutagenicity and genetic toxicology: An appraisal of the guidelines of the European Scientific Committee for Cosmetics and Non-Food Products for the evaluation of hair dyes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2005, 588, 88–105. [Google Scholar] [CrossRef]
- Richards, T.C. The effects of the carcinogen, 1,2-dimethylhydrazine, on turnover 3H-thymidine labeled cells from mucosal glands of mouse colon. Anat. Rec. 1981, 200, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Rabello-Gay, M.; Rodrigues, M.A.L.R.; Monteleone-Neto, R. Mutagênese, Teratogênese e Carcinogênese: Métodos e critérios de Avaliação; Sociedade Brasileira de Genetica: Ribeirão Preto, Brazil, 1991. [Google Scholar]
- Ferguson, L.R. Antimutagens as cancer chemopreventive agents in the diet. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1994, 307, 395–410. [Google Scholar] [CrossRef]
- Hartman, P.E.; Shankel, D.M. Antimutagens and anticarcinogens: A survey of putative interceptor molecules. Environ. Mol. Mutagen. 1990, 15, 145–182. [Google Scholar] [CrossRef]
- Kada, T.; Inoue, T.; Namiki, M. Environmental mutagenesis and plant biology. In Environmental Desmutagens and Antimutagens; Kiekowski, E.J., Jr., Ed.; Praeger: New York, NY, USA, 1982. [Google Scholar]
- Liskova, A.; Stefanicka, P.; Samec, M.; Smejkal, K.; Zubor, P.; Bielik, T.; Biskupska-Bodova, K.; Kwon, T.K.; Danko, J.; Büsselberg, D.; et al. Dietary phytochemicals as the potential protectors against carcinogenesis and their role in cancer chemoprevention. Clin. Exp. Med. 2020, 20, 173–190. [Google Scholar] [CrossRef]
- LaMont, T.; Gorman, T. Experimental colon cancer. Gastroenterology 1978, 75, 1157–1169. [Google Scholar] [CrossRef]
- Krutovskikh, V.; Turosov, V. Tumors of intestines. In Pathology of Tumors in Laboratory Animals; Yarc: Lyon, France, 1994. [Google Scholar]
- Li, S.C.; Lin, H.P.; Chang, J.S.; Shih, C.K. Lactobacillus acidophilus-fermented germinated brown rice suppresses preneoplastic lesions of the colon in rats. Nutrients 2019, 11, 2718. [Google Scholar] [CrossRef]

| Parameters | Commercial Chow Nuvilab® | Commercial Chow Plus 10% NFRFP | NFRFP |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Humidity (g/100 g) | 9.47 ± 0.16 b | 7.14 ± 0.17 a | 7.60 ± 0.16 |
| Ashes (g/100 g) | 7.00 ± 0.07 a | 6.96 ± 0.18 a | 3.44 ± 0.06 |
| Protein (g/100 g) | 20.14 ± 0.08 a | 21.19 ± 0.49 a | 17.65 ± 0.30 |
| Lipids (g/100 g) | 3.80 ± 0.04 a | 4.74 ± 0.47 a | 11.81 ± 0.13 |
| Carbohydrates (g/100 g) * | 42.20 ± 0.07 b | 38.95 ± 0.68 a | 40.76 ± 0.76 |
| Calories (kcal/100 g) | 281.57 ± 0.47 a | 282.06 ± 0.55 a | 337.72 ± 1.27 |
| Total food fiber (g/100 g) | 17.38 ± 0.04 a | 21.02 ± 0.88 b | 18.75 ± 0.62 |
| Group/Treatment | ACF Number | Total ACF Number | %DR | ||
|---|---|---|---|---|---|
| 1–3 Crypts | 4–9 Crypts | ≥9 Crypts | |||
| Negative Control | 1.00 ± 0.46 a | 0.25 ± 0.18 a | 0.00 ± 0.00 a | 1.25 ± 0.52 a | – |
| Positive Control | 50.27 ± 10.42 c | 36.82 ± 5.65 b | 3.91 ± 1.15 b | 91.00 ± 15.82 c | – |
| NFRFP | 3.08 ± 1.04 a | 1.69 ± 0.58 a | 0.15 ± 0.15 a | 4.92 ± 1.62 a | – |
| Pre-treatment | 28.20 ± 4.61 b | 37.30 ± 7.30 b | 2.90 ± 1.32 ab | 58.40 ± 11.45 bc | 25.18 |
| Simultaneous | 33.44 ± 4.49 bc | 27.33 ± 5.11 b | 1.22 ± 0.43 ab | 62.00 ± 9.07 bc | 32.31 |
| Post-treatment | 24.20 ± 3.67 b | 21.50 ± 3.18 b | 1.00 ± 0.39 ab | 46.70 ± 5.70 b | 49.36 |
| Parameters | Experimental Groups | Reference Value | |||||
|---|---|---|---|---|---|---|---|
| Negative Control | Positive Control | NFRFP | Pre-Treatment | Simultaneous | Pos-Treatment | ||
| Leucocytes (103/µL) | 8.34 ± 0.78 a | 10.94 ± 1.11 a | 9.11 ± 0.45 a | 9.82 ± 0.90 a | 9.87 ± 0.74 a | 10.32 ± 1.00 a | 3.41–13.7 1 |
| Erythrocytes (106/µL) | 5.91 ± 0.51 a | 6.20 ± 0.27 a | 6.59 ± 0.08 ab | 6.43 ± 0.20 ab | 7.74 ± 0.30 b | 7.20 ± 0.40 ab | 5.4–8.5 2 |
| Hemoglobin (g/dL) | 11.59 ± 0.82 a | 11.94 ± 0.58 a | 12.75 ± 0.09 a | 12.37 ± 0.31 a | 13.57 ± 0.97 a | 12.35 ± 1.16 a | 10.2–17.8 1 |
| Hematocrit (%) | 34.37 ± 2.48 a | 35.84 ± 1.12 ab | 38.05 ± 0.34 abc | 36.66 ± 0.94 abc | 43.00 ± 1.37 c | 40.58 ± 1.52 bc | 23.8–51.9 1 |
| Platelets (103µL) | 635.40 ± 59.47 a | 786.80 ± 38.78 ab | 712.00 ± 16.33 a | 684.20 ± 42.43 a | 981.60 ± 58.60 b | 938.90 ± 89.64 b | 727–1351 1 |
| Neutrophils (%) | 12.92 ± 1.55 ab | 16.18 ± 2.43 ab | 9.77 ± 0.44 a | 16.30 ± 1.67 ab | 13.11 ± 2.15 ab | 19.10 ± 3.24 b | NF |
| Lymphocytes (%) | 83.45 ± 1.43 b | 79.73 ± 2.59 ab | 86.35 ± 0.47 b | 79.33 ± 1.79 ab | 81.99 ± 2.27 ab | 75.12 ± 2.71 a | 43.1–93.7 1 |
| Monocytes (%) | 2.08 ± 0.29 a | 2.64 ± 0.31 ab | 2.39 ± 0.14 ab | 3.00 ± 0.21 ab | 3.33 ± 0.24 b | 3.40 ± 0.31 b | 1–15.2 1 |
| Eosinophils (%) | 1.08 ± 0.15 a | 1.46 ± 0.28 a | 1.62 ± 0.14 a | 1.40 ± 0.22 a | 1.78 ± 0.28 a | 1.50 ± 0.31 a | 0–3.6 1 |
| Basophils (%) | 0.58 ± 0.45 a | 0.20 ± 0.09 a | 0.05 ± 0.04 a | 0.13 ± 0.05 a | 0.03 ± 0.02 a | 1.07 ± 0.99 a | 0–3 1 |
| RDW (%) | 13.84 ± 0.99 a | 14.86 ± 2.02 a | 13.14 ± 0.21 a | 12.80 ± 0.30 a | 13.31 ± 0.17 a | 15.22 ± 1.33 a | 13–18.4 3 |
| Parameters | Experimental Groups | Reference Value | |||||
|---|---|---|---|---|---|---|---|
| Negative Control | Positive Control | NFRFP | Pre-Treatment | Simultaneous | Pos-Treatment | ||
| AST (U/L) 1 | 127.90 ± 8.89 b | 108.00 ± 6.22 ab | 97.08 ± 3.45 a | 110.80 ± 9.10 ab | 111.50 ± 5.63 ab | 89.45 ± 4.56 a | 18–267 a |
| ALT (U/L) 1 | 45.50 ± 3.52 a | 54.48 ± 4.75 a | 42.29 ± 2.94 a | 57.33 ± 5.15 a | 58.29 ± 5.56 a | 46.27 ± 4.21 a | 34–83 a |
| Total protein (g/dL) 1 | 6.26 ± 0.15 a | 5.91 ± 0.27 a | 6.42 ± 0.16 a | 6.39 ± 0.10 a | 6.25 ± 0.16 a | 6.14 ± 0.19 a | 5.5–10.4 b |
| Albumine (g/dL) 2 | 4.19 ± 0.09 ab | 3.85 ± 0.23 a | 4.36 ± 0.11 ab | 4.34 ± 0.05 ab | 4.19 ± 0.11 ab | 4.11 ± 0.18 b | 3.5–4.2 a |
| Serum urea (mg/dL) 1 | 40.40 ± 1.31 a | 38.23 ± 1.39 a | 39.15 ± 1.86 a | 39.95 ± 1.46 a | 39.61 ± 2.46 a | 36.56 ± 1.69 a | 26–58 c |
| Creatinine (mg/dL) 1 | 0.42 ± 0.03 a | 0.38 ± 0.02 a | 0.35 ± 0.02 a | 0.43 ± 0.05 a | 0.35 ± 0.04 a | 0.45 ± 0.03 a | 0.24–1.2 b |
| Uric acid (mg/dL) 1 | 2.04 ± 0.17 a | 1.61 ± 0.08 a | 1.92 ± 0.17 a | 1.83 ± 0.18 a | 1.98 ± 0.26 a | 1.71 ± 0.13 a | 1–3.2 b |
| Glicose (mg/dL) 1 | 133.0 ± 10.83 a | 109.7 ± 8.01 a | 124.3 ± 6.96 a | 121.6 ± 7.58 a | 129.2 ± 10.08 a | 119.5 ± 7.27 a | 72–193 b |
| α—amilase (U/L) 1 | 1972 ± 87.57 a | 2110 ± 74.16 a | 1968 ± 75.71 a | 1997 ± 74.98 a | 2029 ± 114.00 a | 1852 ± 87.03 a | NF |
| Cholesterol (mg/dL) 1 | 68.95 ± 4.59 b | 66.10 ± 3.78 ab | 52.28 ± 2.69 a | 70.41 ± 1.90 b | 62.51 ± 4.97 ab | 56.51 ± 4.53 ab | 68.9–105.1 c |
| HDL cholesterol (mg/dL) 1 | 65.93 ± 4.10 ab | 57.92 ± 4.92 ab | 52.71 ± 2.42 a | 68.92 ± 2.28 b | 60.25 ± 3.97 ab | 51.72 ± 3.22 a | 36.6–59.4 c |
| Triglycerides (mg/dL) 1 | 65.56 ± 7.93 a | 96.83 ± 34.67 a | 38.88 ± 3.11 a | 50.51 ± 5.41 a | 52.88 ± 8.44 a | 65.25 ± 27.08 a | 57.27–106.7 c |
| Lipase (U/L) 2 | 23.64 ± 15.34 a | 29.51 ± 19.36 a | 10.26 ± 3.48 a | 17.01 ± 10.92 a | 2.99 ± 0.22 a | 4.21 ± 0.91 a | NF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Amaral, L.A.; da Silva Fleming de Almeida, T.; Oliveira de Souza, G.H.; Baranoski, A.; Souza Maris, R.; Bittencourt Junior, F.F.; Murino Rafacho, B.P.; Duenhas Monreal, A.C.; Leite Kassuya, C.A.; Milan Brochado Antoniolli-Silva, A.C.; et al. The Use of Natural Fiber-Rich Food Product Is Safe and Reduces Aberrant Crypt Foci in a Pre-Clinical Model. Nutrients 2021, 13, 2708. https://doi.org/10.3390/nu13082708
do Amaral LA, da Silva Fleming de Almeida T, Oliveira de Souza GH, Baranoski A, Souza Maris R, Bittencourt Junior FF, Murino Rafacho BP, Duenhas Monreal AC, Leite Kassuya CA, Milan Brochado Antoniolli-Silva AC, et al. The Use of Natural Fiber-Rich Food Product Is Safe and Reduces Aberrant Crypt Foci in a Pre-Clinical Model. Nutrients. 2021; 13(8):2708. https://doi.org/10.3390/nu13082708
Chicago/Turabian Styledo Amaral, Luane Aparecida, Taina da Silva Fleming de Almeida, Gabriel Henrique Oliveira de Souza, Adrivanio Baranoski, Rafael Souza Maris, Felipe Francisco Bittencourt Junior, Bruna Paola Murino Rafacho, Antonio Carlos Duenhas Monreal, Cândida Aparecida Leite Kassuya, Andréia Conceição Milan Brochado Antoniolli-Silva, and et al. 2021. "The Use of Natural Fiber-Rich Food Product Is Safe and Reduces Aberrant Crypt Foci in a Pre-Clinical Model" Nutrients 13, no. 8: 2708. https://doi.org/10.3390/nu13082708
APA Styledo Amaral, L. A., da Silva Fleming de Almeida, T., Oliveira de Souza, G. H., Baranoski, A., Souza Maris, R., Bittencourt Junior, F. F., Murino Rafacho, B. P., Duenhas Monreal, A. C., Leite Kassuya, C. A., Milan Brochado Antoniolli-Silva, A. C., Freitas dos Santos, E., & Oliveira, R. J. (2021). The Use of Natural Fiber-Rich Food Product Is Safe and Reduces Aberrant Crypt Foci in a Pre-Clinical Model. Nutrients, 13(8), 2708. https://doi.org/10.3390/nu13082708






