Improvement of Cutaneous Wound Healing via Topical Application of Heat-Killed Lactococcus chungangensis CAU 1447 on Diabetic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Postbiotic
2.2. Bioactive Metabolite Analysis
2.3. Animals
2.4. Streptozotocin-Induced Diabetes Model and Experimental Design
2.5. Excisional Wound Procedures
2.6. Measuring the Wound Closure
2.7. Histological Analysis
2.8. Cytokines, Growth Factors, and Chemokines Expression Levels from Wounded Skin Tissue
2.9. Analysis of Myeloperoxidase Activity Assay
2.10. DNA Extraction in Cutaneous Skin Wounds
2.11. 16S rRNA Gene Amplicon Sequencing
2.12. Statistical Analysis
3. Results
3.1. Bioactive Postbiotic Metabolites for Wound Healing
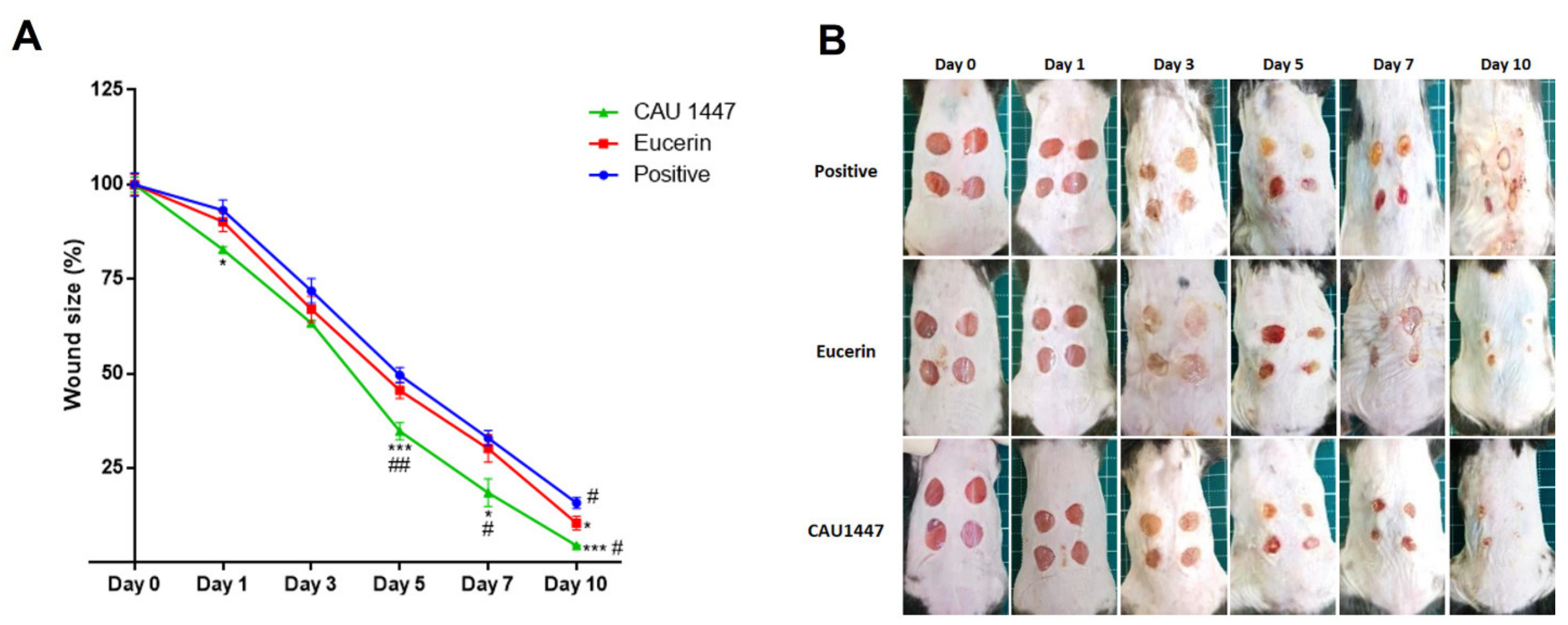
3.2. Postbiotics for Rapid Wound Closure
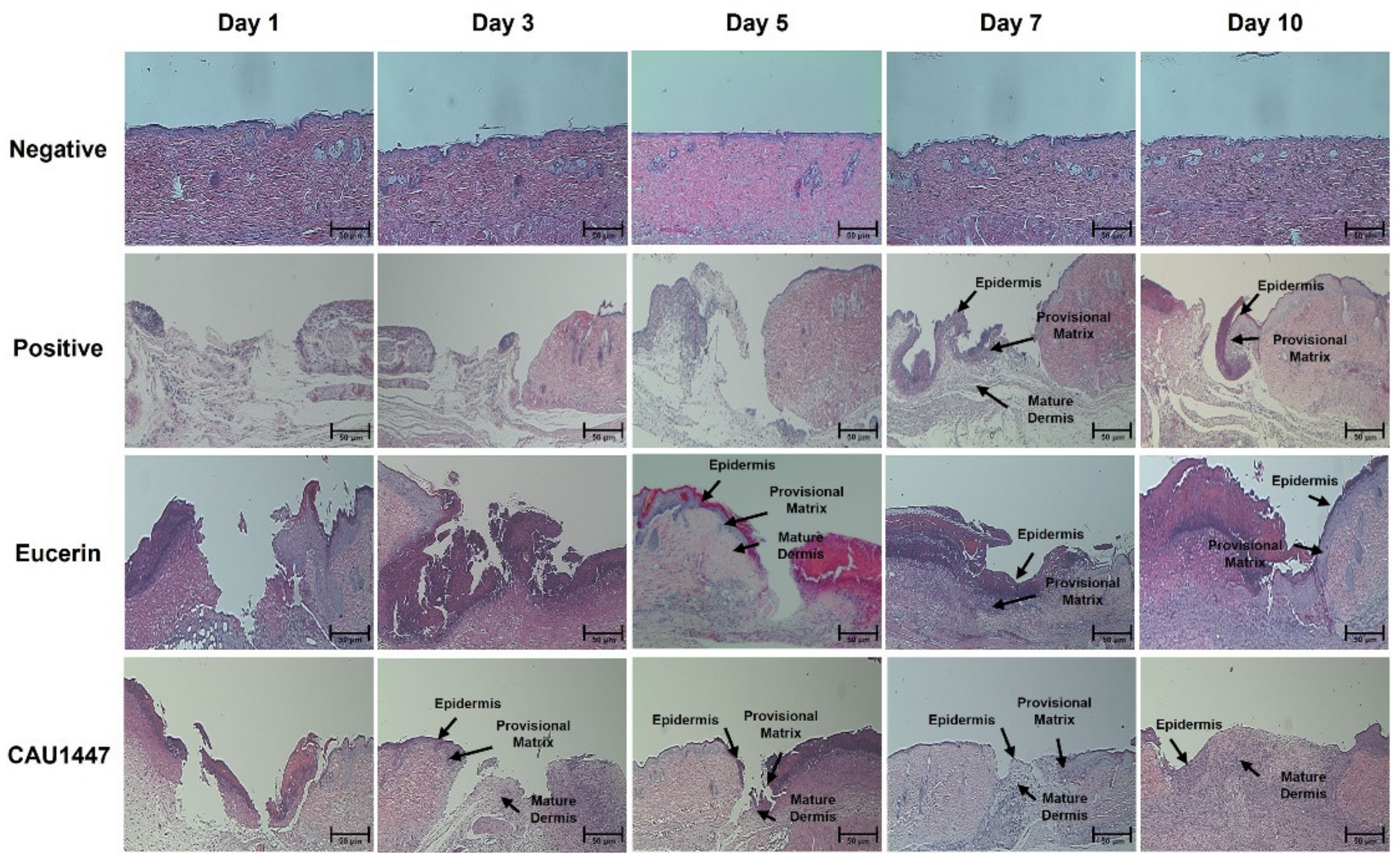
3.3. Effects of Postbiotic Administration on Histopathological Changes
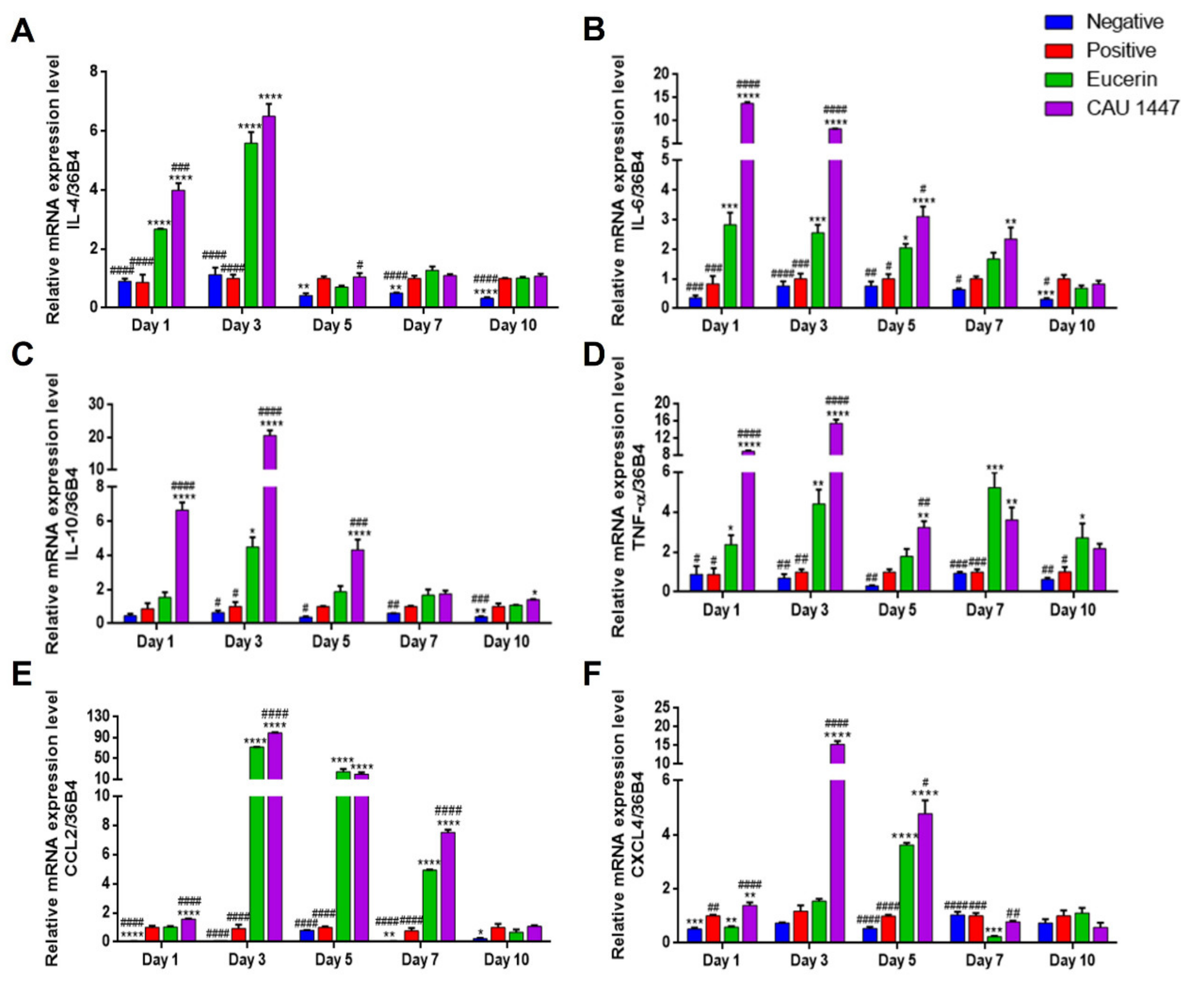
3.4. Effects of Postbiotic Administration on mRNA Expression of Cytokines and Chemokines in Wounded Skin
3.5. Effects of Postbiotic Administration on mRNA Expression of Growth Factors in Wounded Skin
3.6. Postbiotic Alleviated Myeloperoxidase Activity
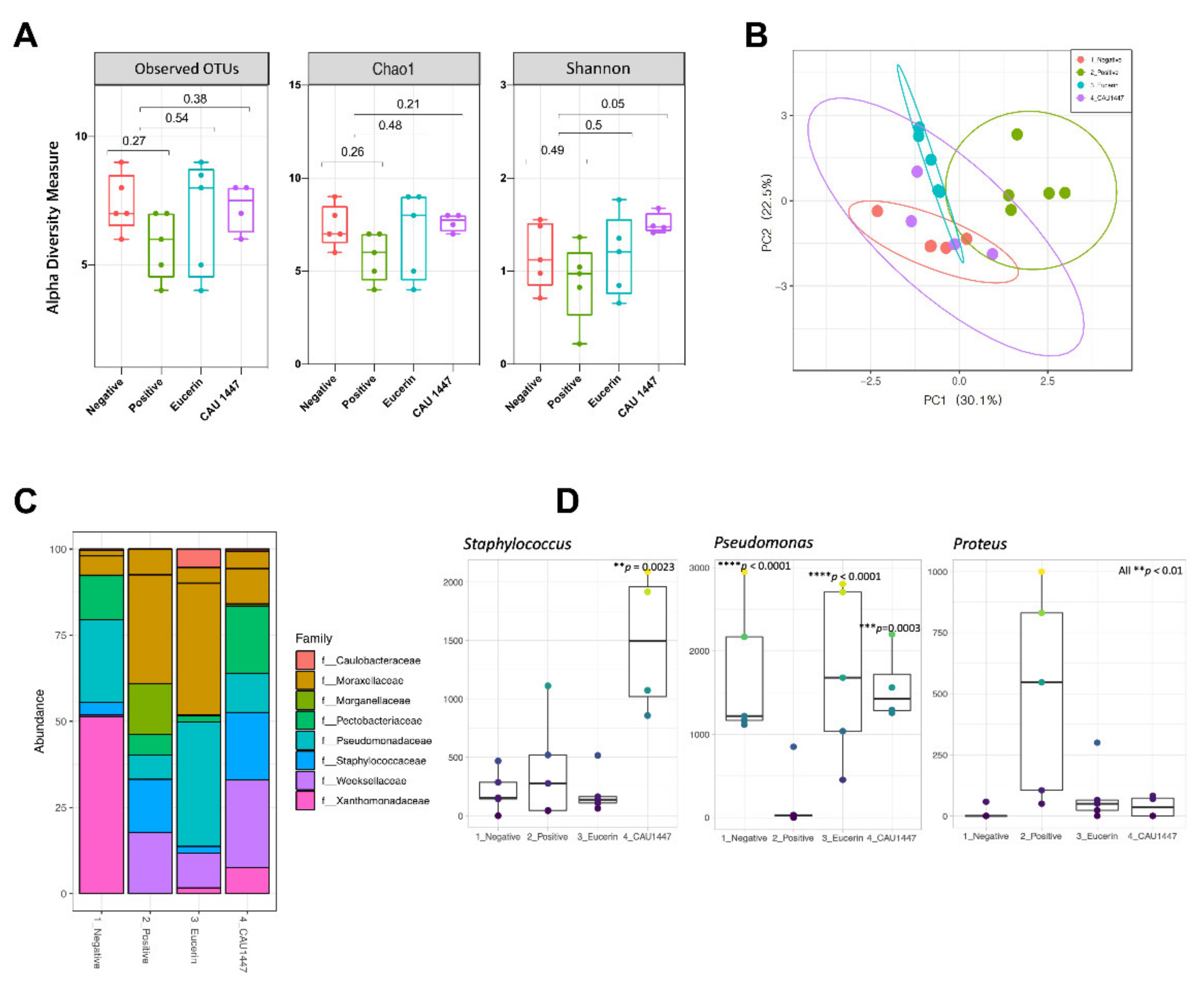
3.7. Microbial Diversity Displayed a Difference on Day 5 through Profiling of the Skin Microbiome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoso, C.R.; Favoreto, S., Jr.; Vancim, J.O.; Barban, G.B.; Ferraz, D.B.; Silva, J.S. Oleic acid modulation of the immune response in wound healing: A new approach for skin repair. Immunobiology 2011, 216, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Thu, H.E.; Zulfakar, M.H.; Ng, S.F. Alginate based bilayer hydrocolloid films as potential slow-release modern wound dressing. Int. J. Pharm. 2012, 434, 375–383. [Google Scholar] [CrossRef]
- Numata, Y.; Terui, T.; Okuyama, R.; Hirasawa, N.; Sugiura, Y.; Miyoshi, I.; Watanabe, T.; Kuramasu, A.; Tagami, A.; Tagami, H.; et al. The accelerating effect of histamine on the cutaneous wound-healing process through the action of basic fibroblast growth factor. J. Investig. Dermatol. 2006, 126, 1403–1409. [Google Scholar] [CrossRef][Green Version]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 1–4. [Google Scholar] [CrossRef]
- Martin, P. Wound healing–aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Tan, W.S.; Arulselvan, P.; Ng, S.F.; Taib, C.N.M.; Sarian, M.N.; Fakurazi, S. Improvement of diabetic wound healing by topical application of Vicenin-2 hydrocolloid film on Sprague Dawley rats. BMC Complement. Altern. Med. 2019, 19, 20–35. [Google Scholar]
- Tomic-Canic, M.; Burgess, J.L.; O’Neill, K.E.; Strbo, N.; Pastar, I. Skin microbiota and its interplay with wound healing. Am. J. Clin. Dermatol. 2020, 21, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Baek, S.R.; Lee, E.S.; Lee, S.H.; Moh, S.H.; Kim, S.Y.; Moh, J.H.; Kondo, C.; Cheon, Y.W. Wound healing effects of rose placenta in a mouse model of full-thickness wounds. Arch. Plast. Surg. 2015, 42, 686–694. [Google Scholar] [CrossRef]
- Siqueira, M.F.; Li, J.; Chehab, L.; Desta, T.; Chino, T.; Krothpali, N.; Behl, Y.; Alikhani, M.; Yang, J.; Braasch, C.; et al. Impaired wound healing in mouse models of diabetes is mediated by TNF-α dysregulation and associated with enhanced activation of forkhead box O1 (FOXO1). Diabetologia 2010, 53, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Valdéz, J.C.; Peral, M.C.; Rachid, M.; Santana, M.; Perdigón, G. Interference of L. plantarum with Pseudomonas aeruginosa in vitro and in infected burns: The potential use of probiotics in wound treatment. Clin. Microbiol. Infect. 2005, 11, 472–479. [Google Scholar] [CrossRef]
- Huseini, H.F.; Rahimzadeh, G.; Fazeli, M.R.; Mehrazma, M.; Salehi, M. Evaluation of wound healing activities of kefir products. Burns 2012, 38, 719–723. [Google Scholar] [CrossRef]
- Lee, Y.K.; Salminen, S. The coming of age of probiotics. Trends Food Sci. Technol. 1995, 6, 241–245. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Caputo, L.R.; Carvalho, J.C.; Evangelista, J.; Schneedorf, J.M. Antimicrobial and healing activity of kefir and kefiran extract. Int. J. Antimicrob. Agents 2005, 25, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.S.; Taylor, T.D.; Yong, C.C.; Khoo, B.Y.; Sasidharan, S.; Choi, S.B.; Ohno, H.; Liong, M.T. L. plantarum USM8613 aids in wound healing and suppresses Staphylococcus aureus infection at wound sites. Probiotics Antimicrob. 2019, 12, 125–137. [Google Scholar] [CrossRef]
- Wong, C.C.M.; Zhang, L.; Li, Z.J.; Wu, W.K.K.; Ren, S.X.; Chen, Y.C.; Ng, T.B.; Cho, C.H. Protective effects of cathelicidin-encoding Lactococcus lactis in murine ulcerative colitis. J. Gastroenterol. Hepatol. 2012, 27, 1205–1212. [Google Scholar] [CrossRef]
- Rossoni, R.D.; de Barros, P.P.; do Carmo, I.M.; Medina, R.P.; Silva, D.H.S.; Fuchs, B.B.; Junqueira, J.C.; Mylonakis, E. The postbiotic activity of Lactobacillus paracasei 28.4 against Candida auris. Front. Cell Infect Microbiol. 2020, 10, 397. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed]
- KFDA (Food and Drug Administration of Korea). Korean FDA Guidelines for Bioequivalence Test. KFDA 2011. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar]
- Callahan, B.J.; Sankaran, K.; Fukuyama, J.A.; McMurdie, P.J.; Holmes, S.P. Bioconductor workflow for microbiome data analysis: From raw reads to community analyses. F1000Research 2016, 5, 1492. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.C.L.; Eccles, R.M.; Baltrūnaitė, L. Species identity dominates over environment in shaping the microbiota of small mammals. Ecol. Lett. 2019, 22, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Heydari Nasrabadi, M.; Tajabadi Ebrahimi, M.; Dehghan Banadaki, S.H.; Torabi Kajousangi, M.; Zahedi, F. Study of cutaneous wound healing in rats treated with L. plantarum on days 1, 3, 7, 14 and 21. Afr. J. Pharm. Pharmacol. 2011, 5, 2395–2401. [Google Scholar] [CrossRef]
- Chuah, X.Q.; Okechukwu, P.N.; Amini, F.; Teo, S.S. Eicosane, pentadecane and palmitic acid: The effects in in vitro wound healing studies. Asian Pac. J. Trop. Bio. 2018, 8, 490–499. [Google Scholar]
- Bielawska-Pohl, A.; Blesson, S.; Benlalam, H.; Trenado, A.; Opolon, P.; Bawa, O.; Rouffiac, V.; Dus, D.; Kieda, C.; Chouaib, S. The anti-angiogenic activity of IL-12 is increased in iNOS-/-mice and involves NK cells. J. Mol. Med. 2010, 88, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Voest, E.E.; Kenyon, B.M.; O’Reilly, M.S.; Truitt, G.; D’Amato, R.J.; Folkman, J. Inhibition of angiogenesis in vivo by interleukin 12. J. Natl. Cancer Inst. 1995, 87, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Weimann, E.; Silva, M.B.B.; Murata, G.M.; Bortolon, J.R.; Dermargos, A.; Curi, R.; Hatanaka, E. Topical anti-inflammatory activity of palmitoleic acid improves wound healing. PLoS ONE 2018, 13, e0205338. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, J.C.; Belury, M.; Ahijevych, K.; Blakely, W. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008, 16, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, H.G.; Vinolo, M.A.R.; Sato, F.T.; Magdalon, J.; Kuhl, C.M.C.; Yamagata, A.S.; Pessoa, A.F.M.; Malheiros, G.; dos Santos, M.F.; Lima, C.; et al. Oral administration of linoleic acid induces new vessel formation and improves skin wound healing in diabetic rats. PLoS ONE 2016, 11, e0165115. [Google Scholar] [CrossRef]
- Majid, S.A.; Khorasgani, M.R.; Emami, S.H.; Talebi, A.; Gholami, A.M. Study of diabetic cutaneous wound healing in rats treated with L. casei and its exopolysaccharide. Int. J. Adv. Biotechnol. Res. 2016, 7, 2083–2092. [Google Scholar]
- Nguyen, V.L.; Truong, C.T.; Nguyen, B.C.Q.; Vo, T.N.V.; Dao, T.T.; Nguyen, V.D.; Trinh, D.T.T.; Huynh, H.K.; Bui, C.B. Anti-inflammatory and wound healing activities of calophyllolide isolated from Calophyllum inophyllum Linn. PLoS ONE 2017, 12, e0185674. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, E.; Morita, H.; Tanabe, S. L rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J. Dairy Sci. 2009, 92, 2400–2408. [Google Scholar] [CrossRef]
- Behm, B.; Babilas, P.; Landthaler, M.; Schreml, S. Cytokines, chemokines and growth factors in wound healing. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 812–820. [Google Scholar] [CrossRef]
- Foster, L.M.; Tompkins, T.A.; Dahl, W.J. A comprehensive post-market review of studies on a probiotic product containing L. helveticus R0052 and L. rhamnosus R0011. Benef. Microbes 2011, 2, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Khodaii, Z.; Afrasiabi, S.; Hashemi, S.A.; Ardeshirylajimi, A.; Natanzi, M.M. Accelerated wound healing process in rat by probiotic L. reuteri derived ointment. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 3. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, D.G.; Sprugel, K.H.; Murray, M.J.; Ross, R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am. J. Pathol. 1990, 136, 1235–1246. [Google Scholar]
- Yu, W.; Nairn, J.O.; Lanzafame, R.J. Expression of growth factors in early wound healing in rat skin. Lasers Surg. Med. 1994, 15, 281–289. [Google Scholar] [CrossRef]
- Mohammedsaeed, W.; Cruickshank, S.; McBain, A.J.; O’Neill, C.A. L. rhamnosus GG lysate increases re-epithelialization of keratinocyte scratch assays by promoting migration. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Han, N.; Jia, L.; Su, Y.; Du, J.; Guo, L.; Luo, Z.; Liu, Y. L. reuteri extracts promoted wound healing via PI3K/AKT/β-catenin/TGFβ1 pathway. Stem. Cell Res. Ther. 2019, 10, 243–253. [Google Scholar] [CrossRef]
- Lam, E.K.Y.; Yu, L.; Wong, H.P.S.; Wu, K.W.K.; Shin, V.Y.; Tai, E.K.K.; So, W.H.L.; Woo, P.C.Y.; Cho, C.H. Probiotic L. rhamnosus GG enhances gastric ulcer healing in rats. Eur. J. Pharmacol. 2007, 565, 171–179. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Paul Ehrlich, H.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Steven, B.L.; Deguzman, L.; Lee, W.P.; Xu, Y.; McFatridge, L.A.; Amento, E.P. TGF-β1 accelerates wound healing: Reversal of steroid-impaired healing in rats and rabbits. Growth Factors 1991, 5, 295–304. [Google Scholar]
- Wood, S.; Jayaraman, V.; Huelsmann, E.J.; Bonish, B.; Burgad, D.; Sivaramakrushnan, G.; Qin, S.; DiPietro, L.A.; Zloza, A.; Zhang, C.; et al. Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLoS ONE 2014, 9, e91574. [Google Scholar] [CrossRef]
- Bryan, D.; Walker, K.B.; Ferguson, M.; Thrope, R. Cytokine gene expression in a murine wound healing model. Cytokine 2005, 31, 429–438. [Google Scholar] [CrossRef]
- Holowacz, S.; Blondeau, C.; Guinobert, I.; Guilbot, A.; Hidalgo, S.; Bisson, J.F. L. salivarius LA307 and L. rhamnosus LA305 attenuate skin inflammation in mice. Benef. Microbes 2018, 9, 299–309. [Google Scholar] [CrossRef]
- Davis, P.A.; Corless, D.J.; Aspinall, R.; Wastell, C. Effect of CD4+ and CD8+ cell depletion on wound healing. Br. J. Surg. 2001, 88, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Lukic, J.; Chen, V.; Strahinic, I.; Begovic, J.; Lev-Tov, H.; Davis, S.C.; Tomic-Canic, M.; Pastar, I. Probiotics or pro-healers the role of beneficial bacteria in tissue repair. Wound Repair Regen. 2017, 25, 912–922. [Google Scholar] [CrossRef]
- Peranteau, W.H.; Zhang, L.; Muvarak, N.; Badillo, A.T.; Radu, A.; Zoltick, P.W.; Liechty, K.W. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J. Investig. Dermatol. 2008, 128, 1852–1860. [Google Scholar] [CrossRef]
- Sahin, H.; Wasmuth, H.E. Chemokines in tissue fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yang, B.; Tedesco, A.; Lebig, E.G.D.; Ruegger, P.M.; Xu, K.; Borneman, J.; Martins-Green, M. High levels of oxidative stress and skin microbiome are critical for initiation and development of chronic wounds in diabetic mice. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Johnson, T.R.; Gómez, B.I.; McIntyre, M.K.; Dubick, M.A.; Christy, R.J.; Nicholson, S.E.; Burmeister, D.M. The cutaneous microbiome and wounds: New molecular targets to promote wound healing. Int. J. Mol. Sci. 2018, 19, 2699. [Google Scholar] [CrossRef]
- SanMiguel, A.; Grice, E.A. Interactions between host factors and the skin microbiome. Cell. Mol. Life Sci. 2015, 72, 1499–1515. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Collier, A.; Townsend, E.M.; O’Donnell, L.E.; Bal, A.M.; Butcher, J.; Mackay, W.G.; Ramage, G.; Williams, C. One step closer to understanding the role of bacteria in diabetic foot ulcers: Characterising the microbiome of ulcers. BMC Microbiol. 2016, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sadeghpour Heravi, F.; Zakrzewski, M.; Vickery, K.; Armstrong, D.; Hu, H. Bacterial diversity of diabetic foot ulcers: Current status and future prospectives. J. Clin. Med. 2019, 8, 1935. [Google Scholar] [CrossRef]
- Frerejacques, M.; Rousselle, C.; Gauthier, L.; Cottet-Emard, S.; Derobert, L.; Roynette, A.; Lerch, T.Z.; Changey, F. Human skin bacterial community response to probiotic (Lactobacillus reuteri DSM 17938) introduction. Microorganisms 2020, 8, 1223. [Google Scholar] [CrossRef] [PubMed]
- Verbanic, S.; Shen, Y.; Lee, J.; Deacon, J.M.; Chen, I.A. Microbial predictors of healing and short-term effect of debridement on the microbiome of chronic wounds. NPJ Biofilms Microbiomes 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Tsai, W.H.; Chou, C.H.; Chiang, Y.J.; Lin, C.G.; Lee, C.H. Regulatory effects of Lactobacillus plantarum-GMNL6 on human skin health by improving skin microbiome. Int. J. Med. Sci. 2021, 18, 1114–1120. [Google Scholar] [CrossRef]


| Gene | Sequence | Primer |
|---|---|---|
| IL-4 | Sense (5′ to 3′) | GGCATTTTGAACGAGGTCAC |
| Anti-sense (3′ to 5′) | AAATATGCGAAGCACCTTGG | |
| IL-6 | Sense (5′ to 3′) | CTACCCCAATTTCCAATGCT |
| Anti-sense (3′ to 5′) | ACCACAGTGAGGAATGTCCA | |
| IL-10 | Sense (5′ to 3′) | TGAATTCCCTGGGTGAGAAG |
| Anti-sense (3′ to 5′) | TGGCCTTGTAGACACCTTGG | |
| TNF-α | Sense (5′ to 3′) | TCCCAGGTTCTCTTCAAGGGA |
| Anti-sense (3′ to 5′) | GGTGAGGAGCACGTAGTCGG | |
| TGF-β1 | Sense (5′ to 3′) | GCTACCATGCCAACTTCTGT |
| Anti-sense (3′ to 5′) | CGTAGTAGACGATGGGCAGT | |
| VEGF | Sense (5′ to 3′) | CCACGTCAGAGAGCAACATCA |
| Anti-sense (3′ to 5′) | TCATTCTCTCTATGTGCTGGCTTT | |
| PDGF | Sense (5′ to 3′) | TCAAGCTCGGGTGACCATTC |
| Anti-sense (3′ to 5′) | ACTTTCGGTGCTTGCCTTTG | |
| FGF | Sense (5′ to 3′) | CAGCACAATGGCAGGCAAAT |
| Anti-sense (3′ to 5′) | CATGGGGAGGAAGTGAGCAG | |
| CCL2/MCP-1 | Sense (5′ to 3′) | TTAAAAACCTGGATCGGAACCAA |
| Anti-sense (3′ to 5′) | GCATTAGCTTCAGATTTACGGGT | |
| CXCL4/PF4 | Sense (5′ to 3′) | CGATGGAGATCTTAGCTGTGTG |
| Anti-sense (3′ to 5′) | CATTCTTCAGGGTGGCTATGAG |
| Organic/Amino Acid | Concentration (mg/L) | Fatty Acid | Concentration (mg/L) |
|---|---|---|---|
| Lactic acid | 1138.34 | Caproic acid (hexanoic acid) | 0.094 |
| Acetic acid | 284.71 | Lauric acid (dodecanoic acid) | 0.087 |
| Aspartic acid | 77.19 | Myristic acid (tetradecanoic acid) | 0.307 |
| Glutamic acid | 272.43 | Palmitic acid (hexadecanoic acid) | 1.220 |
| Asparagine | 105.88 | Palmitoleic acid (omega-7) | 0.311 |
| Serine | 112.99 | Stearic acid (octadecanoic acid) | 1.038 |
| Glutamine | 30.72 | Oleic acid † (omega-9) | 0.435 |
| Histidine | 44.81 | Linoleic acid (omega-6) | 0.099 |
| Glycine | 178.60 | Alpha-linolenic acid (omega-3) | 0.764 |
| Alanine | 127.05 | ||
| GABA | 46.81 | ||
| Valine | 113.16 | ||
| Methionine | 61.57 | ||
| Tryptophane | 57.33 | ||
| Phenylalanine | 92.26 | ||
| Isoleucine | 61.15 | ||
| Leucine | 276.01 | ||
| Lysine | 397.66 | ||
| Proline | 16.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, Y.; Kim, J.; Baek, J.; Kim, W. Improvement of Cutaneous Wound Healing via Topical Application of Heat-Killed Lactococcus chungangensis CAU 1447 on Diabetic Mice. Nutrients 2021, 13, 2666. https://doi.org/10.3390/nu13082666
Nam Y, Kim J, Baek J, Kim W. Improvement of Cutaneous Wound Healing via Topical Application of Heat-Killed Lactococcus chungangensis CAU 1447 on Diabetic Mice. Nutrients. 2021; 13(8):2666. https://doi.org/10.3390/nu13082666
Chicago/Turabian StyleNam, Yohan, Jonghwa Kim, Jihye Baek, and Wonyong Kim. 2021. "Improvement of Cutaneous Wound Healing via Topical Application of Heat-Killed Lactococcus chungangensis CAU 1447 on Diabetic Mice" Nutrients 13, no. 8: 2666. https://doi.org/10.3390/nu13082666
APA StyleNam, Y., Kim, J., Baek, J., & Kim, W. (2021). Improvement of Cutaneous Wound Healing via Topical Application of Heat-Killed Lactococcus chungangensis CAU 1447 on Diabetic Mice. Nutrients, 13(8), 2666. https://doi.org/10.3390/nu13082666






