Oral Nutritional Supplements and Enteral Nutrition in Patients with Gastrointestinal Surgery
Abstract
:1. Introduction
- -
- Integration of nutrition and nutritional status in the overall management
- -
- Avoiding prolonged periods of sobriety preoperatively
- -
- Start nutritional therapy as soon as metabolic risk is apparent
- -
- Metabolic monitoring of blood sugar levels
- -
- Reduction of factors that trigger stress and catabolism or impair gastrointestinal motility and function
- -
- Early mobilization to stimulate protein synthesis and to maintain muscle function
2. Who Will Benefit from Perioperative Nutritional Supplementation?
Screening and Assessment of Nutritional Status
3. Preoperative Nutritional Therapy
4. Prehabilitation
5. Immunological Conditioning
6. Postoperative Nutrition
Early Oral Diet
- -
- Very low in fat (max. 30 g fat/day)
- -
- Use of easily digestible, lean protein carriers
- -
- High carbohydrate
- -
- Easily digestible (low fiber content)
7. Early Oral Delivery after Esophagectomy and Gastrectomy
8. Feeding Jejunostomy
9. Oral Food Intake after Prolonged Intensive Care Treatment
10. Post-Discharge Nutrition
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Williams, D.G.A.; Molinger, J.; Wischmeyer, P.E. The malnourished surgery patient: A silent epidemic in perioperative outcome? Curr. Opin. Anesthesiol. 2019, 32, 405–411. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, E.; Ferguson, M.; Banks, M.; Vivanti, A.; Batterham, B.; Bauer, J.; Capra, S.; Isenring, E. Malnutrition, poor food intake, and adverse healthcare outcomes in noncritically ill obese acute care hospital patients. Clin. Nutr. 2019, 38, 759–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, G.L.; Cederholm, T.; Correia, M.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barrazoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition: A consensus report from the global clinical nutrition community. J. Parenter. Enter. Nutr. 2019, 43, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Han, D.; Zhou, X.; Han, Y.; Zhang, Y.; Li, H. Effects of preoperative nutrition on postoperative outcomes in esophageal cancer: A systematic review and meta-analysis. Dis. Esophagus 2021. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Lobo, D.N.; Ljungqvist, O.; Martindale, R.; et al. ESPEN Guideline Clinical Nutrition in Surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenny, E.; Samavat, H.; Touger-Decker, R.; Parrott, J.S.; Byham-Gray, L.; Agust, D.A. Adverse perioperative outcomes among patients undergoing gastrointestinal cancer surgery: Quantifying attributable risk from malnutrition. J. Parenter. Enter. Nutr. 2021. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Wischmeyer, P.W.; Carli, F.; Evans, D.C.; Guilbert, S.; Kozar, R.; Pryor, A.; Thiele, R.H.; Everett, S.; Rocott, M.; Gan, T.J.; et al. American Society for Enhanced Recovery and Perioperative Quality Initiative joint consensus statement on nutritional screening and therapy within a surgical enhanced recovery pathway. Anesth. Analg. 2018, 126, 1883–1895. [Google Scholar] [CrossRef]
- Zhang, B.; Najarali, Z.; Ruo, L.; Alhusaini, A.; Solis, N.; Valencia, M.; Sanchez, M.I.P.; Serrano, P.E. Effect of Perioperative Nutritional Supplementation on Postoperative Complications-Systematic Review and Meta-Analysis. J. Gastrointest. Surg. 2019, 23, 1682–1693. [Google Scholar] [CrossRef]
- Simonsen, C.; de Heer, P.; Bjerre, E.D.; Suetta, C.; Hojman, P.; Pedersen, B.K.; Svendsen, L.B.; Christensen, J.F. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: A meta-analysis. Ann. Surg. 2018, 268, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F. Forcing the vicious circle: Sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann. Oncol. 2017, 28, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Caillet, P.; Liuu, E.; Raynaud Simon, A.; Bonnefoy, M.; Guerin, O.; Berrut, G.; Lesourd, B.; Jeandel, C.; Ferry, M.; Rolland, Y. Association between cachexia, chemotherapy and outcomes in older cancer patients: A systematic review. Clin. Nutr. 2017, 36, 1473–1482. [Google Scholar] [CrossRef]
- Olotu, C.; Weimann, A.; Bahrs, C.; Schwenk, W.; Scherer, M.; Kiefmann, R. The perioperative care of older patients—Time for a new, interdisciplinary approach. Dtsch. Ärzteblatt Int. 2019, 116, 63–69. [Google Scholar]
- Wobith, M.; Wehle, L.; Haberzettl, D.; Acikgöz, A.; Weimann, A. Needle catheter jejunostomy in patients undergoing surgery for upper gastrointestinal and pancreato-biliary cancer—Impact on nutritional and clinical outcome in the early and late postoperative period. Nutrients 2020, 12, 2564. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M.; Educational and Clinical Practice Committee; European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, F.; Bregenzer, T.; Braun, N.; et al. Individualized nutritional support in medical inpatients at nutritional risk: A randomized clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef]
- Martin, L.; Gillis, C.; Atkins, M.; Gillam, M.; Sheppard, C.; Buhler, S.; Hammond, C.B.; Nelson, G.; Gramlich, L. Implementation of an Enhanced Recovery After Surgery Program can change nutrition care practice: A multicenter experience in elective colorectal surgery. J. Parenter. Enter. Nutr. 2019, 43, 206–219. [Google Scholar] [CrossRef]
- Tamandl, D.; Paireder, M.; Asari, R.; Baltzer, P.A.; Schoppmann, S.F.; Ba-Ssalamah, A. Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur Radiol. 2016, 26, 1359–1367. [Google Scholar] [CrossRef]
- Runkel, M.; Diallo, T.D.; Lang, S.A.; Bamberg, F.; Benndorf, M.; Fichtner-Feigl, S. The Role of Visceral Obesity, Sarcopenia and Sarcopenic Obesity on Surgical Outcomes After Liver Resections for Colorectal Metastases. World J. Surg. 2021, 45, 2218–2226. [Google Scholar] [CrossRef]
- Pecorelli, N.; Capretti, G.; Sandini, M.; Damascelli, A.; Cristel, G.; De Cobelli, F.; Gianotti, L.; Zerbi, A.; Braga, M. Impact of Sarcopenic Obesity on Failure to Rescue from Major Complications Following Pancreaticoduodenectomy for Cancer: Results from a Multicenter Study. Ann. Surg. Oncol. 2018, 25, 308–317. [Google Scholar] [CrossRef]
- Feliciano, E.M.C.; Kroenke, C.H.; Meyerhardt, J.A.; Prado, C.M.; Bradshaw, P.T.; Kwan, M.L.; Xiao, J.; Alexeeff, S.; Douglas, D. Association of Systemic Inflammation and Sarcopenia with Survival in Nonmetastatic Colorectal Cancer. JAMA Oncol. 2017, 3, e172319. [Google Scholar] [CrossRef]
- Palmela, C.; Velho, S.; Agostinho, L.; Branco, F.; Santos, M.; Costa Santos, M.P.; Oliveira, M.H.; Strecht, J.; Maio, R.; Cravo, M.; et al. Body composition as a prognostic factor of neoadjuvant chemotherapy toxicity and outcome in patients with locally advanced gastric cancer. J. Gastric Cancer 2017, 17, 74–87. [Google Scholar] [CrossRef] [Green Version]
- Kroenke, C.H.; Prado, C.M.; Meyerhardt, J.A.; Weltzien, E.K.; Xiao, J.; Cespedes Feliciano, E.M.; Caan, B.J. Muscle radiodensity and mortality in patients with colorectal cancer. Cancer 2018, 124, 3008–3015. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Hopkins, J.; Malietzis, G.; Jenkins, J.T.; Sawyer, M.B.; Brisebois, R.; MacLean, A.; Nelson, G.; Gramlich, L.; Baracos, V.E. Assessment of Computed Tomography (CT)-Defined Muscle and Adipose Tissue Features in Relation to Short-Term Outcomes After Elective Surgery for Colorectal Cancer: A Multicenter Approach. Ann. Surg. Oncol. 2018, 25, 2669–2680. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Caan, B.J.; Cespedes Feliciano, E.M.; Meyerhardt, J.A.; Kroenke, C.H.; Baracos, V.E.; Weltzien, E.; Kwan, M.L.; Alexeeff, S.E.; Castillo, A.L.; et al. The association of medical and demographic characteristics with sarcopenia and low muscle radiodensity in patients with nonmetastatic colorectal cancer. Am. J. Clin. Nutr. 2019, 109, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Herrod, P.J.J.; Boyd-Carson, H.; Doleman, B.; Trotter, J.; Schlichtemeier, S.; Sathanapally, G.; Somerville, J.; Williams, J.P.; Lund, J.N. Quick and simple; psoas density measurement is an independent predictor of anastomotic leak and other complications after colorectal resection. Tech. Coloproctol. 2019, 23, 129–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linder, N.; Schaudinn, A.; Langenhan, K.; Krenzien, F.; Hau, H.-M.; Benzing, C.; Atanasov, G.; Schmelzle, M.; Kahn, T.; Busse, H.; et al. Power of computed-tomography-defined sarcopenia for prediction of morbidity after pancreaticoduodenectomy. BMC Med. Imaging 2019, 19, 32. [Google Scholar] [CrossRef]
- Yassaie, S.S.; Keane, C.; French, S.J.H.; Al-Herz, F.A.J.; Young, M.K.; Gordon, A.C. Decreased total psoas muscle area after neoadjuvant therapy is a predictor of increased mortality in patients undergoing oesophageal cancer resection. ANZ J. Surg. 2019, 89, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Gioulbasanis, I.; Senesse, P.; Baracos, V.E. Cancer-Associated Malnutrition and CT-Defined Sarcopenia and Myosteatosis are Endemic in Overweight and Obese Patients. J. Parenter. Enter. Nutr. 2020, 44, 227–238. [Google Scholar] [CrossRef]
- Xie, H.; Gong, Y.; Kuang, J.; Yan, L.; Ruan, G.; Tang, S.; Gao, F.; Gan, J. Computed Tomography-Determined Sarcopenia is a Useful Imaging Biomarker for Predicting Postoperative Outcomes in Elderly Colorectal Cancer Patients. Cancer Res. Treat. 2020, 52, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Gruber, E.S.; Jomrich, G.; Tamandl, D.; Gnant, M.; Schindl, M.; Sahora, K. Erratum: Sarcopenia and sarcopenic obesity are independent adverse prognostic factors in resectable pancreatic ductal adenocarcinoma. PLoS ONE 2020, 15, e0244896 1–18. [Google Scholar] [CrossRef]
- Ishida, T.; Makino, T.; Yamasaki, M.; Yamashita, K.; Tanaka, K.; Saito, T.; Yamamoto, K.; Takahashi, T.; Kurokawa, Y.; Motoori, M.; et al. Quantity and Quality of Skeletal Muscle as an Important Predictor of Clinical Outcomes in Patients with Esophageal Cancer Undergoing Esophagectomy after Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2021. [Google Scholar] [CrossRef]
- Argillander, T.E.; Spek, D.; van der Zaag-Loonen, H.J.; van Raamt, A.F.; van Duijvendijk, P.; van Munster, B.C. Association between postoperative muscle wasting and survival in older patients undergoing surgery for non-metastatic colorectal cancer. J. Geriatr. Oncol. 2021. [Google Scholar] [CrossRef]
- Gillis, C.; Ferton, T.; Sajobi, T.T.; Minnella, E.M.; Awasthi, R.; Loiselle, S.E.; Liberman, A.S.; Stein, B.; Charlebois, P.; Carli, F. Trimodal prehabilitation for colorectal surgery attemuates post-surgical losses in lean body mass: A pooled analysis of randomized controlled trials. Clin. Nutr. 2019, 38, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.J.; Hackney, R.J.; Lamb, P.J.; Wigmore, S.J.; Deans, D.C.; Skipworth, R.J.E. Prehabilitation Before Major Abdominal Surgery: A Systematic Review and Meta-analysis. World J. Surg. 2019, 43, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.L.; Lee, M.J.; George, J.; Kerr, K.; Moug, S.; Wilson, T.R.; Brown, S.R.; Wyld, L. Prehabilitation in elective abdominal cancer surgery in older patients: Systematic review and meta-analysis. BJS Open 2020, 4, 1022–1041. [Google Scholar] [CrossRef]
- Elia, M.; Normand, C.; Norman, K.; Laviano, A.; Norman, K. A systematic review of the cost and cost effectiveness of using standard oral nutritional supplements in the hospital setting. Clin. Nutr. 2016, 35, 370–380. [Google Scholar] [CrossRef] [Green Version]
- Gillis, C.; Buhler, K.; Bresee, L.; Carli, F.; Gramlich, l.; Culos-Reed, N.; Sajobi, T.T.; Fenton, T.R. Effects of nutritional prehabilitation with and without exercise, on outcomes of patients who undergo colorectal surgery: A systematic review and meta-analysis. Gastroenterol 2018, 155, 391–410. [Google Scholar] [CrossRef]
- Grass, F.; Bertrand, P.C.; Schäfer, M.; Ballabeni, P.; Cerantola, Y.; Demartines, N.; Hübner, M. Compliance with preoperative oral nutritional supplements in patients at nutritional risk-only a question of will? Eur. J. Clin. Nutr. 2015, 69, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Lidoriki, I.; Schizas, D.; Mylonas, K.S.; Frountzas, M.; Mastoraki, A.; Pikoulis, E.; Liakakos, T.; Karavokyros, I. Oral nutritional supplementation following upper gastrointestinal cancer surgery: A prospective analysis exploring potential barriers to compliance. J. Am. Coll. Nutr. 2010, 39, 650–656. [Google Scholar] [CrossRef]
- Davies, S.J.; West, M.A.; Rahman, S.A.; Underwood, T.J.; Marino, L.V. Oesophageal cancer: The effect of early nutrition support on clinical outcomes. Clin. Nutr. ESPEN 2021, 42, 117–123. [Google Scholar] [CrossRef]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 2016, 167, 829–842. [Google Scholar] [CrossRef] [Green Version]
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.D.; Warnke, I.; Weimann, A.; Calder, P.C. Expert Opinion on Benefits of Long-Chain Omega-3 Fatty Acids (DHA and EPA) in Aging and Clinical Nutrition. Nutrients 2020, 12, 2555. [Google Scholar] [CrossRef] [PubMed]
- Moya, P.; Soriano-Irigiaray, L.; Ramirez, J.M.; Garcea, A.; Blasco, O.; Blanco, F.; Brugiotti, C.; Miranda, E.; Arroyo, A. Perioperative standard oral nutrition supplements versus immunonutrition in patients undergoing colorectal resection in an enhanced recovery(ERAS) protocol: A multicenter randomized Clinical Trial (SONVI Study). Medicine 2016, 95, e3704. [Google Scholar] [CrossRef]
- Adiamah, A.; Skorepa, P.; Weimann, A.; Lobo, D.N. The impact of preoperative immune modulating nutrition on outcomes in patients undergoing durgery for gastrointestinal cancer: A systematic review and metaanalysis. Ann. Surg. 2019, 270, 247–256. [Google Scholar] [CrossRef]
- Burcharth, J.; Falkenberg, A.; Schack, A.; Ekeloef, S.; Gögenur, I. The effects of early enteral nutrition on mortality after major emergency abdominal surgery: A systematic review and meta-analysis with Trial Sequential Analysis. Clin. Nutr. 2021, 40, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.Q.; Chen, S.Y.; Jiang, Z.B.; Wu, B.; He, Y.; Wang, X.X.; Li, Y.; Gao, P.; Yang, X.J. Effect of postoperative early enteral nutrition on clinical outcomes and immune function of cholangiocarcinoma patients with malignant obstructive jaundice. World J. Gastroenterol. 2020, 26, 7405–7415. [Google Scholar] [CrossRef]
- Hogan, S.; Reece, L.; Solomon, M.; Rangan, A.; Carey, S. Early enteral feeding is beneficial for patients after pelvic exenteration surgery: A randomized controlled trial. J. Parenter. Enter. Nutr. 2021. [Google Scholar] [CrossRef]
- Berkelmans, G.H.K.; Fransen, L.F.C.; Dolmans-Zwartjes, A.C.P.; Kouwenhoven, E.A.; van Det, M.J.; Nilsson, M.; Nieuwenhuijzen, G.A.P.; Luyer, M.D.P. Direct oral feeding following minimally invasive esophagectomy (NUTRIENT II trial): An international, multicenter, open-label randomized controlled trial. Ann. Surg. 2020, 271, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Jeong, O. Early Postoperative Oral Feeding After Total Gastrectomy in Gastric Carcinoma Patients: A Retrospective Before-After Study Using Propensity Score Matching. J. Parenter. Enter. Nutr. 2019, 43, 649–657. [Google Scholar] [CrossRef]
- Constansia, R.D.; Hentzen, J.E.; Hogenbirk, R.N.; van der Plas, W.Y.; Campmans-Kuijpers, M.J.; Buis, C.I.; Kruijff, S.; Klaase, J.M. Actual postoperative protein and calorie intake in patients undergoing major open abdominal cancer surgery: A prospective, observational cohort study. Nutr. Clin. Pract. 2021. [Google Scholar] [CrossRef]
- Zhuang, W.; Wu, H.; Liu, H.; Huang, S.; Wu, Y.; Deng, C.; Tian, D.; Zhou, Z.; Shi, R.; Chen, G.; et al. Utility of feeding jejunostomy in patients with esophageal cancer undergoing esophagectomy with a high risk of anastomotic leakage. J. Gastrointest. Oncol. 2021, 12, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Li, H.N.; Chen, Y.; Dai, L.; Wang, Y.-Y.; Chen, M.-W.; Mei, L.-X. A Meta-analysis of Jejunostomy Versus Nasoenteral Tube for Enteral Nutrition Following Esophagectomy. J. Surg. Res. 2021, 264, 553–561. [Google Scholar] [CrossRef]
- Holmén, A.; Hayami, M.; Szabo, E.; Rouvelas, I.; Agustsson, T.; Klevebro, F. Nutritional jejunostomy in esophagectomy for cancer, a national register-based cohort study of associations with postoperative outcomes and survival. Langenbecks Arch. Surg. 2020. [Google Scholar] [CrossRef]
- Shen, X.; Zhuo, Z.-G.; Li, G.; Alai, G.-H.; Song, T.-N.; Xu, Z.-J.; Yao, P.; Lin, Y.-D. Is the routine placement of a feeding jejunostomy during esophagectomy worthwhile?—A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 4232–4241. [Google Scholar] [CrossRef]
- Grass, F.; Benoit, M.; Coti Bertrand, P.; Sola, J.; Schäfer, M.; Demartines, N.; Hübner, M. Nutritional status deteriorates postoperatively despite preoperative nutritional support. Ann. Nutr. Metab. 2016, 68, 291–297. [Google Scholar] [CrossRef]
- Ridley, E.J.; Parke, R.L.; Davies, A.R.; Bailey, M.; Hodgson, C.; Deane, A.M.; McGuinness, S.; Cooper, D.J. What happens to nutrition intake in the post-intensive care unit hospitalization period? An observational cohort study in critically ill adults. J. Parenter. Enter. Nutr. 2019, 43, 88–95. [Google Scholar] [CrossRef]
- Baker, M.; Halliday, V.; Williams, R.N.; Bowrey, D.J. A systematic review of the nutritional consequences of esophagectomy. Clin. Nutr. 2016, 35, 987–994. [Google Scholar] [CrossRef] [Green Version]
- Koterazawa, Y.; Oshikiri, T.; Takiguchi, G.; Urakawa, N.; Hasegawa, H.; Yamamoto, M.; Kanaji, S.; Yamashita, K.; Matsuda, T.; Nakamura, T.; et al. Severe weight loss after minimally invasive oesophagectomy is associated with poor survival in patients with oesophageal cancer at 5 years. BMC Gastroenterol. 2020, 20, 407. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, G.; Zhu, L. Home enteral nutrition for postoperative elderly patients with esophageal cancer. Ann. Palliat. Med. 2021, 10, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Xueting, H.; Li, L.; Meng, Y.; Yuqing, C.; Yutong, H.; Lihong, Q.; June, Z. Home enteral nutrition and oral nutritional supplements in postoperative patients with upper gastrointestinal malignancy: A systematic review and meta-analysis. Clin. Nutr. 2020, 40, 3082–3093. [Google Scholar] [CrossRef] [PubMed]
- Hatao, F.; Chen, K.Y.; Wu, J.M.; Wang, M.Y.; Aikou, S.; Onoyama, H.; Shimizu, N.; Fukatsu, K.; Seto, Y.; Lin, M.T. Randomized controlled clinical trial assessing the effects of oral nutritional supplements in postoperative gastric cancer patients. Langenbecks Arch. Surg. 2017, 402, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Tan, S.; Jiang, Y.; Han, J.; Xi, Q.; Zhuang, Q.; Wu, G. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: A randomized clinical trial. Clin. Nutr. 2020, 40, 40–46. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Omori, T.; Fujitani, K.; Fujita, J.; Kawabata, R.; Imamura, H.; Okada, K.; Moon, J.H.; Hirao, M.; Matsuyama, J.; et al. Oral nutritional supplements versus a regular diet alone for body weight loss after gastrectomy: A phase 3, multicenter, open-label randomized controlled trial. Gastric Cancer 2021. [Google Scholar] [CrossRef]
- Tropea, P.; Schlieter, H.; Sterpi, I.; Judica, E.; Gand, K.; Caprino, M.; Gabilondo, I.; Gomez-Esteban, J.C.; Busnatu, S.; Sinescu, C.; et al. Rehabilitation, the great absentee of virtual coaching in medical care: Scoping Review. J. Med. Internet Res. 2019, 21, e12805. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Nakazono, M.; Nagasawa, S.; Segami, K. Clinical Impact of Perioperative Oral Nutritional Treatment for Body Composition Changes in Gastrointestinal Cancer Treatment. Anticancer Res. 2021, 41, 1727–1732. [Google Scholar] [CrossRef]
| Year | Author | Cancer | N | Outcome |
|---|---|---|---|---|
| 2017 | Cespedes et al. [23] | Colorectal | 2470 | Higher mortality |
| 2017 | Palmela et al. [24] | Gastric | 48 | Higher mortality |
| 2018 | Kroenke et al. [25] | Colorectal | 3262 | Higher mortality |
| 2018 | Martin et al. [26] | Colorectal | 1139 | Longer LOS |
| 2018 | Pecorelli et al. [22] | Pancreas | 120 | Higher mortality |
| 2019 | Xiao et al. [27] | Colorectal | 3051 | Higher comorbidity |
| 2019 | Herrod et al. [28] | Colorectal | 169 | Higher comorbidity rate |
| 2019 | Lindner et al. [29] | Pancreas | 139 | Higher comorbidity rate |
| 2019 | Yassaie et al. [30] | Esophagus | 53 | Higher mortality |
| 2020 | Martin et al. [31] | Neck, Lung, GI | 1157 | Higher mortality |
| 2020 | Xie et al. [32] | Colorectal | 132 | Higher comorbidity rate, higher mortality |
| 2020 | Gruber et al. [33] | Pancreas | 133 | Higher comorbidity rate, higher mortality |
| 2021 | Ishida et al. [34] | Esophagus | 333 | Higher mortality |
| 2021 | Argillander et al. [35] | Colorectal | 233 | Higher mortality |
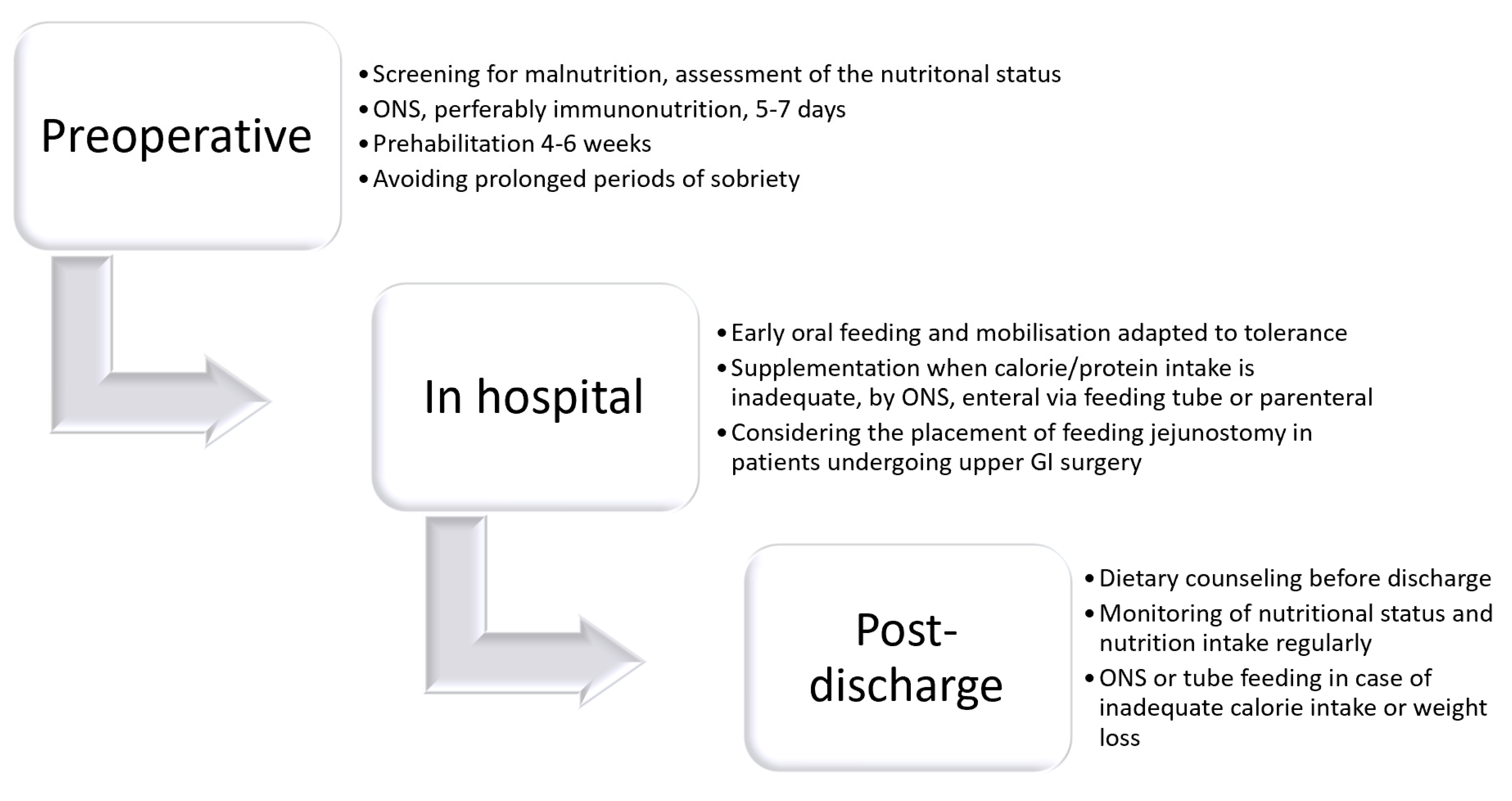
| Recommendations Summary |
|---|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wobith, M.; Weimann, A. Oral Nutritional Supplements and Enteral Nutrition in Patients with Gastrointestinal Surgery. Nutrients 2021, 13, 2655. https://doi.org/10.3390/nu13082655
Wobith M, Weimann A. Oral Nutritional Supplements and Enteral Nutrition in Patients with Gastrointestinal Surgery. Nutrients. 2021; 13(8):2655. https://doi.org/10.3390/nu13082655
Chicago/Turabian StyleWobith, Maria, and Arved Weimann. 2021. "Oral Nutritional Supplements and Enteral Nutrition in Patients with Gastrointestinal Surgery" Nutrients 13, no. 8: 2655. https://doi.org/10.3390/nu13082655
APA StyleWobith, M., & Weimann, A. (2021). Oral Nutritional Supplements and Enteral Nutrition in Patients with Gastrointestinal Surgery. Nutrients, 13(8), 2655. https://doi.org/10.3390/nu13082655






