Dose–Response Relationships between Levels of Alcohol Use and Risks of Mortality or Disease, for All People, by Age, Sex, and Specific Risk Factors
Abstract
1. Introduction
2. Materials and Methods
3. Results
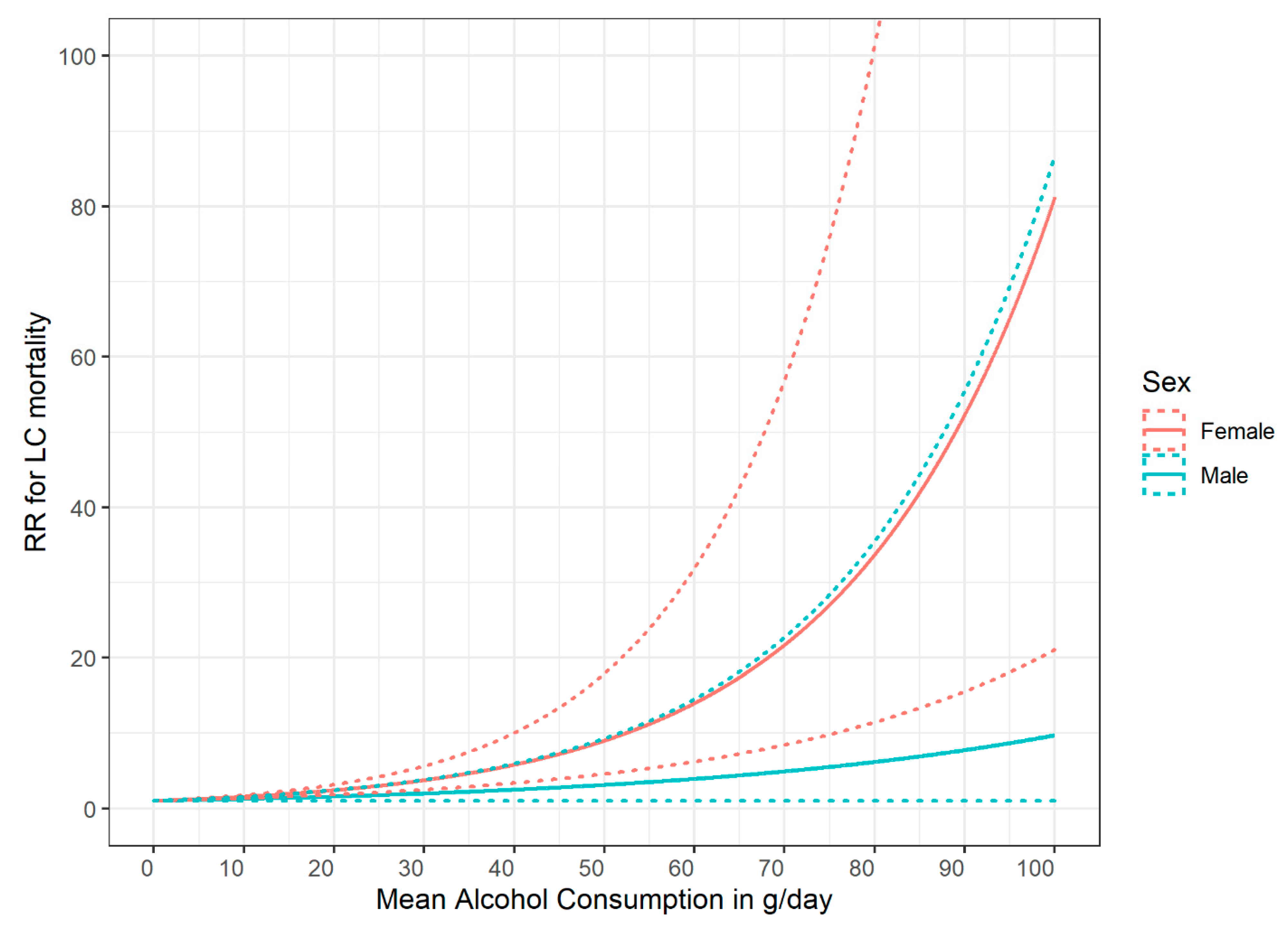
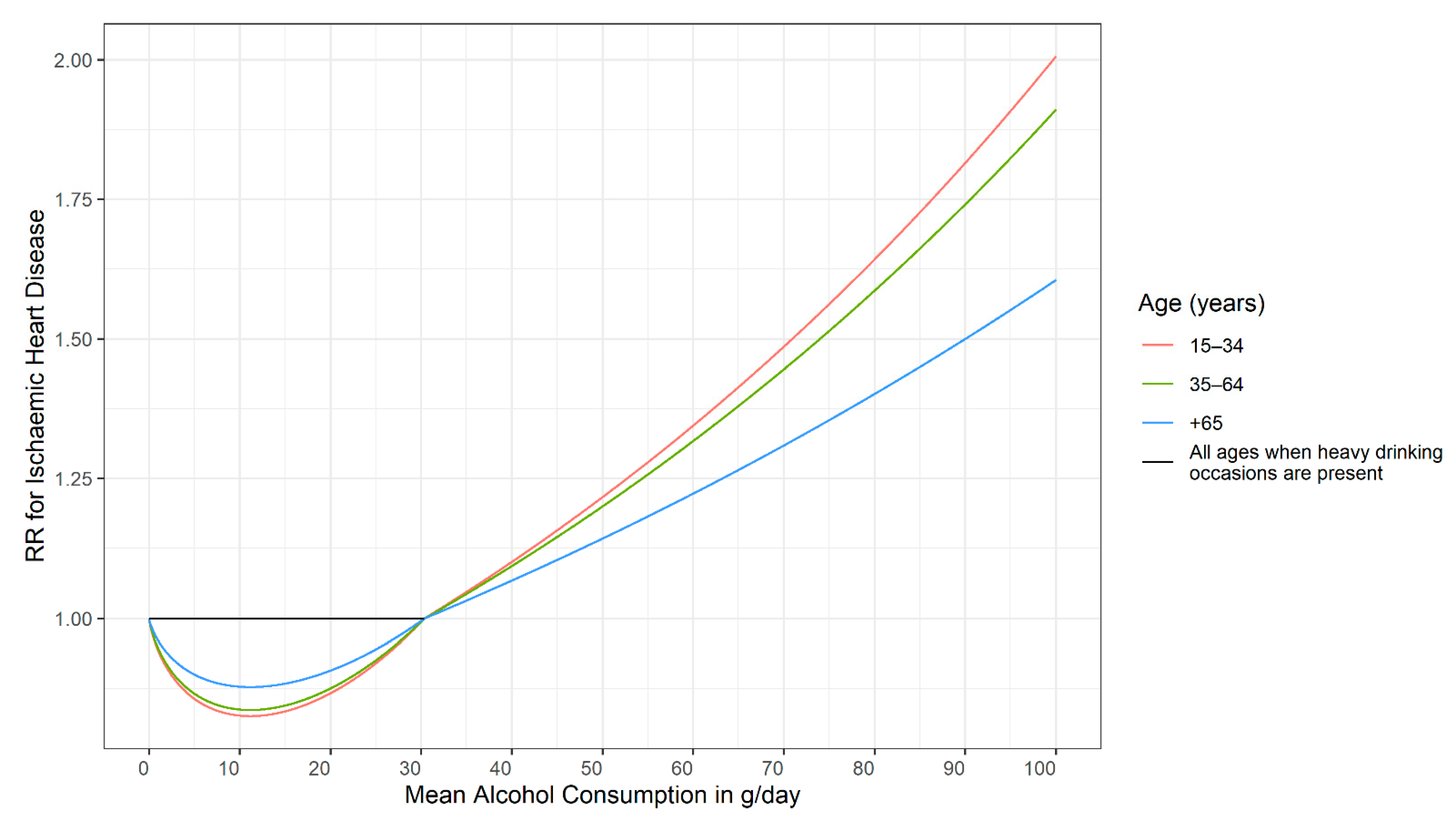
3.1. Basic Typologies of Dose–Response Relationships
3.2. Modifiers of Dose–Response Relationships
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef]
- Rehm, J.; Probst, C.; Shield, K.D.; Shuper, P.A. Does alcohol use have a causal effect on HIV incidence and disease progression? A review of the literature and a modeling strategy for quantifying the effect. Popul. Health Metr. 2017, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J.; Greenland, S.; Lash, T.L. Modern Epidemiology, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Rehm, J.; Ashley, M.J.; Room, R.; Single, E.; Bondy, S.; Ferrence, R.; Giesbrecht, N. On the emerging paradigm of drinking patterns and their social and health consequences. Addiction 1996, 91, 1615–1621. [Google Scholar] [CrossRef]
- Cherpitel, C.J.; Ye, Y.; Bond, J.; Borges, G.; Monteiro, M. Relative risk of injury from acute alcohol consumption: Modeling the dose-response relationship in emergency department data from 18 countries. Addiction 2015, 110, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.; Irving, H.; Kanteres, F.; Room, R.; Borges, G.; Cherpitel, C.; Greenfield, T.; Rehm, J. The more you drink, the harder you fall: A systematic review and meta-analysis of how acute alcohol consumption and injury or collision risk increase together. Drug Alcohol Depend. 2010, 110, 108–116. [Google Scholar] [CrossRef]
- Roerecke, M.; Rehm, J. Alcohol consumption, drinking patterns, and ischemic heart disease: A narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 2014, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Shield, K.; Manthey, J.; Rylett, M.; Probst, C.; Wettlaufer, A.; Parry, C.D.H.; Rehm, J. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: A comparative risk assessment study. Lancet Public Health 2020, 5, e51–e61. [Google Scholar] [CrossRef]
- Heilig, M.; MacKillop, J.; Martinez, D.; Rehm, J.; Leggio, L.; Vanderschuren, L.J. Addiction as a brain disease revised: Why it still matters, and the need for consilience. Neuropsychopharmacology 2021, 1–9. [Google Scholar] [CrossRef]
- Jiang, H.; Lange, S.; Tran, A.; Imtiaz, S.; Rehm, J. Determining the sex-specific distributions of average daily alcohol consumption using cluster analysis: Is there a separate distribution for people with alcohol dependence? Popul. Health Metr. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Rehm, J.; Gmel, G.E., Sr.; Gmel, G.; Hasan, O.S.M.; Imtiaz, S.; Popova, S.; Probst, C.; Roerecke, M.; Room, R.; Samokhvalov, A.V.; et al. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction 2017, 112, 968–1001. [Google Scholar] [CrossRef] [PubMed]
- Gmel, G.; Kuntsche, E.; Rehm, J. Risky single—Occasion drinking: Bingeing is not bingeing. Addiction 2011, 106, 1037–1045. [Google Scholar] [CrossRef]
- Roerecke, M.; Rehm, J. The cardioprotective association of average alcohol consumption and ischaemic heart disease: A systematic review and meta—Analysis. Addiction 2012, 107, 1246–1260. [Google Scholar] [CrossRef]
- Gmel, G.; Bissery, A.; Gammeter, R.; Givel, J.C.; Calmes, J.M.; Yersin, B.; Daeppen, J.B. Alcohol—Attributable injuries in admissions to a Swiss emergency room—An analysis of the link between volume of drinking, drinking patterns, and preattendance drinking. Alcohol. Clin. Exp. Res. 2006, 30, 501–509. [Google Scholar] [CrossRef]
- Krüger, H.P.; Kazenwadel, J.; Vollrath, M. Grand Rapids effects revisited: Accidents, alcohol and risk. In Alcohol, Drugs, and Traffic Safety, Proceedings of the 13th International Conference on Alcohol, Drugs and Traffic Safety, Adelaide, Australia, 13–18 August 1995; Kloeden, C.N., McLean, A.J., Eds.; NHMRC Road Accident Research Unit, University of Adelaide: Adelaide, Australia, 1995; pp. 222–230. [Google Scholar]
- Keyes, K.M.; Galea, S. Population Health Science; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Rose, G. Sick individuals and sick populations. Int. J. Epidemiol. 1985, 14, 32–38. [Google Scholar] [CrossRef]
- Nutt, D.J.; Rehm, J. Doing it by numbers: A simple approach to reducing the harms of alcohol. J. Psychopharmacol. 2014, 28, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.J.; Mulrow, C.D.; Haynes, R.B. Systematic reviews: Synthesis of best evidence for clinical decisions. Ann. Intern. Med. 1997, 126, 376–380. [Google Scholar] [CrossRef]
- Rehm, J.; Sherk, A.; Shield, K.D.; Gmel, G. Risk Relations between Alcohol Use and Non-Injury Causes of Death Toronto, ON: Centre for Addiction and Mental Health. 2017. Available online: https//www.camh.ca/-/media/files/pdfs---reports-and-books---research/camh-risk-relations-between-alcohol-use-and-non-injury-causes-of-death-sept2017-pdf.pdf?la=en&hash=393C0B60D26A218F2512C39C1B61EFCE0FCC5128 (accessed on 29 July 2021).
- GBD. 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Rehm, J.; Monteiro, M.; Room, R.; Gmel, G.; Jernigan, D.; Frick, U.; Graham, K. Steps towards constructing a global comparative risk analysis for alcohol consumption: Determining indicators and empirical weights for patterns of drinking, deciding about theoretical minimum, and dealing with different consequences. Eur. Addict. Res. 2001, 7, 138–147. [Google Scholar] [CrossRef]
- Rehm, J.; Irving, H.; Ye, Y.; Kerr, W.C.; Bond, J.; Greenfield, T.K. Are lifetime abstainers the best control group in alcohol epidemiology? On the stability and validity of reported lifetime abstention. Am. J. Epidemiol. 2008, 168, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Klatsky, A.L. Invited commentary: Never, or hardly ever? It could make a difference. Am. J. Epidemiol. 2008, 168, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Kehoe, T.; Gmel, G.; Stinson, F.; Grant, B.; Gmel, G. Statistical modeling of volume of alcohol exposure for epidemiological studies of population health: The US example. Popul. Health Metr. 2010, 8, 3. [Google Scholar] [CrossRef]
- Shield, K.; Rehm, J. Russia-specific relative risks and their effects on the estimated alcohol-attributable burden of disease. BMC Public Health 2015, 15, 482. [Google Scholar] [CrossRef] [PubMed]
- Zaridze, D.; Lewington, S.; Boroda, A.; Scélo, G.; Karpov, R.; Lazarev, A.; Konobeevskaya, I.; Igitov, V.; Terechova, T.; Boffetta, P. Alcohol and mortality in Russia: Prospective observational study of 151,000 adults. Lancet 2014, 383, 1465–1473. [Google Scholar] [CrossRef]
- Lönnroth, K.; Williams, B.; Stadlin, S.; Jaramillo, E.; Dye, C. Alcohol use as a risk factor for tuberculosis—A systematic review. BMC Public Health 2008, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, S.; Shield, K.D.; Roerecke, M.; Samokhvalov, A.V.; Lönnroth, K.; Rehm, J. Alcohol consumption as a risk factor for tuberculosis: Meta-analyses and burden of disease. Eur. Respir. J. 2017, 50, 1700216. [Google Scholar] [CrossRef] [PubMed]
- Llamosas-Falcón, L. Proposal: To Include Mortality and Morbidity for Sexually Transmitted Diseases (STDs) as Alcohol-Attributable Disease Conditions in Comparative Risk Assessments—Rationale and Procedures; Centre for Addiction and Mental Health: Toronto, ON, Canada, 2021. [Google Scholar]
- Larsson, S.C.; Wallin, A.; Wolk, A.; Markus, H.S. Differing association of alcohol consumption with different stroke types: A systematic review and meta-analysis. BMC Med. 2016, 14, 178. [Google Scholar] [CrossRef]
- Baliunas, D.; Taylor, B.; Irving, H.; Roerecke, M.; Patra, J.; Mohapatra, S.; Rehm, J. Alcohol as a risk factor for type 2 diabetes—A systematic review and meta-analysis. Diabetes Care 2009, 32, 2123–2132. [Google Scholar] [CrossRef]
- Knott, C.; Bell, S.; Britton, A. Alcohol consumption and the risk of type 2 diabetes: A systematic review and Dose-Response Meta-analysis of more than 1.9 million individuals from 38 observational studies. Diabetes Care 2015, 38, 1804–1812. [Google Scholar] [CrossRef]
- Wood, A.M.; Kaptoge, S.; Butterworth, A.S.; Willeit, P.; Warnakula, S.; Bolton, T.; Paige, E.; Paul, D.S.; Sweeting, M.; Burgess, S.; et al. Risk thresholds for alcohol consumption: Combined analysis of individual-participant data for 599,912 current drinkers in 83 prospective studies. Lancet 2018, 391, 1513–1523. [Google Scholar] [CrossRef]
- Zhao, J.; Stockwell, T.; Roemer, A.; Naimi, T.; Chikritzhs, T. Alcohol consumption and mortality from coronary heart disease: An updated meta-analysis of cohort studies. J. Stud. Alcohol Drugs 2017, 78, 375–386. [Google Scholar] [CrossRef]
- Brien, S.E.; Ronksley, P.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. Br. Med. J. 2011, 342, d636. [Google Scholar] [CrossRef]
- Rehm, J. On the limitations of observational studies. Addict. Res. Theory 2007, 15, 20–22. [Google Scholar]
- Roerecke, M.; Rehm, J. Ischemic heart disease mortality and morbidity in former drinkers: A meta-analysis. Am. J. Epidemiol. 2011, 73, 245–258. [Google Scholar] [CrossRef]
- Rehm, J.; Hasan, O.S.M.; Black, S.E.; Shield, K.D.; Schwarzinger, M. Alcohol use and dementia: A systematic scoping review. Alzheimers Res. Ther. 2019, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Schwarzinger, M.; Pollock, B.G.; Hasan, O.S.M.; Dufouil, C.; Rehm, J.; QalyDays Study Group. Contribution of alcohol use disorders to the burden of dementia in France 2008-13: A nationwide retrospective cohort study. Lancet Public Health 2018, 3, e124–e132. [Google Scholar] [CrossRef]
- Manthey, J.; Shield, K.D.; Rylett, M.; Hasan, O.S.M.; Probst, C.; Rehm, J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: A modelling study. Lancet 2019, 393, 2493–2502. [Google Scholar] [CrossRef]
- English, D.; Holman, C.; Milne, E.; Winter, M.; Hulse, G.; Codde, J.; Bower, C.; Corti, B.; De Klerk, N.; Knuiman, M.; et al. The Quantification of Drug caused Morbidity and Mortality in Australia 1995; Australian Government Publishing Service: Canberra, Australia, 1995.
- Roerecke, M.; Vafaei, A.; Hasan, O.S.M.; Chrystoja, B.R.; Cruz, M.; Lee, R.; Neuman, M.R.; Rehm, J. Alcohol Consumption and Risk of Liver Cirrhosis: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2019, 114, 1574–1586. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Gmel, G.; Sempos, C.T.; Trevisan, M. Alcohol-related morbidity and mortality. Alcohol Res. Health 2003, 27, 39. [Google Scholar]
- Klatsky, A.L.; Udaltsova, N. Alcohol drinking and total mortality risk. Ann. Epidemiol. 2007, 17, S63–S67. [Google Scholar] [CrossRef]
- Rehm, J.; Shield, K.D.; Roerecke, M.; Gmel, G. Modelling the impact of alcohol consumption on cardiovascular disease mortality for comparative risk assessments: An overview. BMC Public Health 2016, 16, 363. [Google Scholar] [CrossRef]
- Bond Huie, S.A.; Krueger, P.M.; Rogers, R.G.; Hummer, R.A. Wealth, race, and mortality. Soc. Sci. Q. 2003, 84, 667–684. [Google Scholar] [CrossRef]
- Feinstein, J.S. The relationship between socioeconomic status and health: A review of the literature. Milbank Q. 1993, 71, 279–322. [Google Scholar] [CrossRef]
- Mackenbach, J.P.; Stirbu, I.; Roskam, A.-J.R.; Schaap, M.M.; Menvielle, G.; Leinsalu, M.; Kunst, A.E. Socioeconomic inequalities in health in 22 European countries. N. Engl. J. Med. 2008, 358, 2468–2481. [Google Scholar] [CrossRef]
- Petrovic, D.; de Mestral, C.; Bochud, M.; Bartley, M.; Kivimäki, M.; Vineis, P.; Mackenbach, J.; Stringhini, S. The contribution of health behaviors to socioeconomic inequalities in health: A systematic review. Prev. Med. 2018, 113, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Simbayi, L.C.; Parry, C.D.; Shuper, P.A.; Rehm, J. Alcohol use, socioeconomic status and risk of HIV infections. AIDS Behav. 2017, 21, 1926–1937. [Google Scholar] [CrossRef]
- Probst, C.; Roerecke, M.; Behrendt, S.; Rehm, J. Socioeconomic differences in alcohol-attributable mortality compared with all-cause mortality: A systematic review and meta-analysis. Int. J. Epidemiol. 2014, 43, 1314–1327. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Kilian, C.; Sanchez, S.; Lange, S.; Rehm, J. The role of alcohol use and drinking patterns in socioeconomic inequalities in mortality: A systematic review. Lancet Public Health 2020, 5, e324–e332. [Google Scholar] [CrossRef]
- Boyd, J.; Sexton, O.; Angus, C.; Meier, P.; Purshouse, R.C.; Holmes, J. Causal mechanisms proposed for the alcohol harm paradox—A systematic review. Addiction 2021. [Google Scholar] [CrossRef]
- Shield, K.D.; Rehm, J. Societal development and the alcohol-attributable burden of disease. Addiction 2021. [Google Scholar] [CrossRef]
- Babor, T.F.; Casswell, S.; Graham, K.; Huckle, T.; Livingston, M.; Österberg, E.; Rehm, J.; Room, R.; Rossow, I.; Sornpaisarn, B. Alcohol: No Ordinary Commodity. Research and Public Policy, 3rd ed.; Oxford University Press: Oxford, UK, 2021; (forthcoming). [Google Scholar]
- Anonymous. Tackling poverty in tuberculosis control. Lancet 2005, 366, 2063. [Google Scholar] [CrossRef]
- Rehm, J.; Samokhvalov, A.V.; Neuman, M.G.; Room, R.; Parry, C.; Lönnroth, K.; Patra, J.; Poznyak, V.; Popova, S. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health 2009, 9, 1–12. [Google Scholar] [CrossRef]
- Llamosas-Falcón, L.; Shield, K.D.; Gelovany, M.; Hasan, O.S.; Manthey, J.; Monteiro, M.; Walsh, N.; Rehm, J. Impact of alcohol on the progression of HCV-related liver disease: A systematic review and meta-analysis. J. Hepatol. 2021. [Google Scholar] [CrossRef]
- Eng, M.Y.; Luczak, S.E.; Wall, T.L. ALDH2, ADH1B, and ADH1C genotypes in Asians: A literature review. Alcohol Res. Health 2007, 30, 22. [Google Scholar]
- International Agency for Research on Cancer. International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Volume 96—Alcohol Consumption and Ethyl Carbamate; International Agency for Research on Cancer: Lyon, France, 2010. [Google Scholar]
- Roerecke, M.; Shield, K.D.; Higuchi, S.; Yoshimura, A.; Larsen, E.; Rehm, M.X.; Rehm, J. Estimates of alcohol-related oesophageal cancer burden in Japan: Systematic review and meta-analyses. Bull. World Health Organ. 2015, 93, 329–338. [Google Scholar] [CrossRef]
- Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Altieri, A.; Cogliano, V.; WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007, 8, 292–293. [Google Scholar] [CrossRef]
- Brooks, P.J.; Enoch, M.-A.; Goldman, D.; Li, T.-K.; Yokoyama, A. The alcohol flushing response: An unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009, 6, e1000050. [Google Scholar] [CrossRef] [PubMed]
- Sarich, P.; Canfell, K.; Banks, E.; Paige, E.; Egger, S.; Joshy, G.; Korda, R.; Weber, M. A prospective study of health conditions related to alcohol consumption cessation among 97,852 drinkers aged 45 and over in Australia. Alcohol. Clin. Exp. Res. 2019, 43, 710–721. [Google Scholar] [CrossRef]
- Puddey, I.B.; Rakic, V.; Dimmitt, S.B.; Beilin, L.J. Influence of pattern of drinking on cardiovascular disease and cardiovascular risk factors--a review. Addiction 1999, 94, 649–663. [Google Scholar] [CrossRef]
- Rehm, J.; Roerecke, M. Cardiovascular effects of alcohol consumption. Trends Cardiovasc. Med. 2017, 27, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Fekjær, H.O. Alcohol—A universal preventive agent? A critical analysis. Addiction 2013, 108, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J. The role of the comparison group in epidemiology and general limitations. Addiction 2013, 108, 2058–2059. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Del Boca, F.K.; Darkes, J. The validity of self—Reports of alcohol consumption: State of the science and challenges for research. Addiction 2003, 98, 1–12. [Google Scholar] [CrossRef]
- Rehm, J.; Kilian, C.; Rovira, P.; Shield, K.D.; Manthey, J. The elusiveness of representativeness in general population surveys for alcohol. Drug Alcohol Rev. 2021, 40, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Shipley, M.; Lewington, S.; Youngman, L.; Collins, R.; Marmot, M.; Peto, R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am. J. Epidemiol. 1999, 150, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Marmet, S.; Anderson, P.; Gual, A.; Kraus, L.; Nutt, D.; Room, R.; Samokhvalov, A.V.; Scafato, E.; Trapencieris, M.; et al. Defining substance use disorders: Do we really need more than heavy use? Alcohol Alcohol. 2013, 48, 633–640. [Google Scholar] [CrossRef]
- Gmel, G.; Shield, K.D.; Kehoe-Chan, T.A.; Rehm, J. The effects of capping the alcohol consumption distribution and relative risk functions on the estimated number of deaths attributable to alcohol consumption in the European Union in 2004. BMC Med. Res. Methodol. 2013, 13, 1–9. [Google Scholar] [CrossRef]
- Shield, K.D.; Gmel, G.; Mäkelä, P.; Probst, C.; Room, R.; Gmel, G.; Rehm, J. Risk, individual perception of risk and population health. Addiction 2017, 112, 2272–2273. [Google Scholar] [CrossRef]
- Rossow, I.; Mäkelä, P. Public health thinking around alcohol-related harm: Why does per capita consumption matter? J. Stud. Alcohol Drugs 2021, 82, 9–17. [Google Scholar] [CrossRef]
- Bondy, S.J.; Rehm, J.; Ashley, M.J.; Walsh, G.; Single, E.; Room, R. Low-risk drinking guidelines: The scientific evidence. Can. J. Public Health 1999, 90, 264–270. [Google Scholar] [CrossRef]
| Global Health Estimate 2015 Cause Category | ICD-10 Coding | ||||
|---|---|---|---|---|---|
| I. | Communicable, maternal, perinatal and nutritional conditions | A00–B99, D50–53, D64.9, E00–02, E40–46, E50–64, G00–04, G14, H65–66, J00–22, N70–73, O00–99, P00–96, U04 | |||
| A. | Infectious and parasitic diseases | A00–B99, G00–04, G14, N70–73, P37.3, P37.4 | |||
| 1 | Tuberculosis | A15–19, B90 | |||
| 3 | HIV/AIDS | B20–24 | |||
| B. | Respiratory infections | H65–66, J00–22, P23, U04 | |||
| 1 | Lower respiratory infections | J09–22, P23, U04 | |||
| II. | Noncommunicable diseases | C00–97, D00–48, D55–64 (minus D64.9), D65–89, E03–07, E10–34, E65–88, F01–99, G06–98 (minus G14), H00–61, H68–93, I00–99, J30–98, K00–92, L00–98, M00–99, N00–64, N75–98, Q00–99, X41–42, X44, X45, R95 | |||
| A. | Malignant neoplasms | C00–97 | |||
| 1 | Mouth and oropharynx cancers | C00–14 | |||
| a. | Lip and oral cavity | C00–08 | |||
| c. | other pharyngeal cancers | C09–10, C12–14 | |||
| 2 | Oesophagus cancer | C15 | |||
| 4 | Colon and rectum cancers | C18–21 | |||
| 5 | Liver cancer | C22 | |||
| 9 | Breast cancer | C50 | |||
| 19 | Larynx cancer | C32 | |||
| C. | Diabetes mellitus | E10–14 (minus E10.2–10.29, E11.2–11.29, E12.2, E13.2–13.29, E14.2) | |||
| E. | Mental and substance use disorders | F04–99, G72.1, Q86.0, X41–42, X44, X45 | |||
| 4 | Alcohol use disorders | F10, G72.1, Q86.0, X45 | |||
| F. | Neurological conditions | F01–03, G06–98 (minus G14, G72.1) | |||
| 3 | Epilepsy | G40–41 | |||
| H. | Cardiovascular diseases | I00–99 | |||
| 2 | Hypertensive heart disease | I10–15 | |||
| 3 | Ischemic heart disease | I20–25 | |||
| 4 | Stroke | I60–69 | |||
| a. | Ischemic stroke | G45–46.8, I63–63.9, I65–66.9, I67.2–67.848, I69.3–69.4 | |||
| b. | Hemorrhagic stroke | I60–62.9, I67.0–67.1, I69.0–69.298 | |||
| 5 | Cardiomyopathy, myocarditis, endocarditis | I30–33, I38, I40, I42 | |||
| J. | Digestive diseases | K20–92 | |||
| 2 | Cirrhosis of the liver | K70, K74 | |||
| 8 | Pancreatitis | K85–86 | |||
| III. | Injuries | V01–Y89 (minus X41–42, X44, X45) | |||
| A. | Unintentional injuries | V01–X40, X43, X46–59, Y40–86, Y88, Y89 | |||
| 1 | Road injury | V01–04, V06, V09–80, V87, V89, V99 | |||
| 2 | Poisonings | X40, X43, X46–48, X49 | |||
| 3 | Falls | W00–19 | |||
| 4 | Fire, heat and hot substances | X00–19 | |||
| 5 | Drowning | W65–74 | |||
| 6 | Exposure to mechanical forces | W20–38, W40–43, W45, W46, W49–52, W75, W76 | |||
| 8 | Other unintentional injuries | Rest of V, W39, W44, W53–64, W77–99, X20–29, X50–59, Y40–86, Y88, Y89 | |||
| B. | Intentional injuries | X60–Y09, Y35–36, Y870, Y871 | |||
| 1 | Self-harm | X60–84, Y870 | |||
| 2 | Interpersonal violence | X85–Y09, Y871 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehm, J.; Rovira, P.; Llamosas-Falcón, L.; Shield, K.D. Dose–Response Relationships between Levels of Alcohol Use and Risks of Mortality or Disease, for All People, by Age, Sex, and Specific Risk Factors. Nutrients 2021, 13, 2652. https://doi.org/10.3390/nu13082652
Rehm J, Rovira P, Llamosas-Falcón L, Shield KD. Dose–Response Relationships between Levels of Alcohol Use and Risks of Mortality or Disease, for All People, by Age, Sex, and Specific Risk Factors. Nutrients. 2021; 13(8):2652. https://doi.org/10.3390/nu13082652
Chicago/Turabian StyleRehm, Jürgen, Pol Rovira, Laura Llamosas-Falcón, and Kevin D. Shield. 2021. "Dose–Response Relationships between Levels of Alcohol Use and Risks of Mortality or Disease, for All People, by Age, Sex, and Specific Risk Factors" Nutrients 13, no. 8: 2652. https://doi.org/10.3390/nu13082652
APA StyleRehm, J., Rovira, P., Llamosas-Falcón, L., & Shield, K. D. (2021). Dose–Response Relationships between Levels of Alcohol Use and Risks of Mortality or Disease, for All People, by Age, Sex, and Specific Risk Factors. Nutrients, 13(8), 2652. https://doi.org/10.3390/nu13082652






