Impact of Dietary Lipids on the Reverse Cholesterol Transport: What We Learned from Animal Studies
Abstract
1. Implications of the Reverse Cholesterol Transport in Cardiovascular Disease
2. General Aspects of Dietary Lipids: Chemistry, Sources, Intake, and Effect on CVD in Humans
2.1. Fatty Acids
2.2. Sterols
2.3. Other Lipids
3. Effects of SFA on RCT in Animal Models
4. Effects of MUFA on RCT in Animal Models
5. Effects of PUFA on RCT in Animal Models
6. Effects of TFA on RCT in Animal Models
7. Effects of Sterols on RCT in Animal Models
8. Effects of Other Lipids on RCT in Animal Models
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Dai, H.; Much, A.A.; Maor, E.; Asher, E.; Younis, A.; Xu, Y.; Lu, Y.; Liu, X.; Shu, J.; Bragazzi, N.L. Global, regional, and national burden of ischemic heart disease and its attributable risk factors, 1990–2017: Results from the global Burden of Disease Study 2017. Eur. Heart J. Qual. Care Clin. Outcomes 2020. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and reverse cholesterol transport: Basic mechanisms and their roles in vascular health and disease. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B.J.; Davidson, W.S.; Fayad, Z.A.; Fuster, V.; Goldstein, J.; Hellerstein, M.; Jiang, X.-C.; Phillips, M.C.; Rader, D.J.; et al. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation 2012, 125, 1905–1919. [Google Scholar] [CrossRef] [PubMed]
- Ossoli, A.; Pavanello, C.; Calabresi, L. High-Density Lipoprotein, Lecithin: Cholesterol Acyltransferase, and Atherosclerosis. Endocrinol. Metab. 2016, 31, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Chowaniec, Z.; Skoczyńska, A. Plasma lipid transfer proteins: The role of PLTP and CETP in atherogenesis. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2018, 27, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A. HDL and Reverse Remnant-Cholesterol Transport (RRT): Relevance to Cardiovascular Disease. Trends Mol. Med. 2020, 26, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Annema, W.; Tietge, U.J.F. Role of hepatic lipase and endothelial lipase in high-density lipoprotein-mediated reverse cholesterol transport. Curr. Atheroscler. Rep. 2011, 13, 257–265. [Google Scholar] [CrossRef]
- Zhang, Y.; Da Silva, J.R.; Reiliy, M.; Billheimer, J.T.; Rothblat, G.H.; Rader, D.J. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J. Clin. Investig. 2005, 115, 2870–2874. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.C.; VandenBroek, J.M.; Cooper, P.S. Lipoprotein cholesteryl ester production, transfer, and output in vivo in humans. J. Lipid Res. 2004, 45, 1594–1607. [Google Scholar] [CrossRef]
- Wang, J.; Mitsche, M.A.; Lütjohann, D.; Cohen, J.C.; Xie, X.-S.; Hobbs, H.H. Relative roles of ABCG5/ABCG8 in liver and intestine. J. Lipid Res. 2015, 56, 319–330. [Google Scholar] [CrossRef]
- Van der Velde, A.E.; Vrins, C.L.J.; van den Oever, K.; Kunne, C.; Oude Elferink, R.P.J.; Kuipers, F.; Groen, A.K. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology 2007, 133, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego, C.G.; Giannarelli, C.; Badimón, J.J. Experimental models for the investigation of high-density lipoprotein-mediated cholesterol efflux. Curr. Atheroscler. Rep. 2011, 13, 266–276. [Google Scholar] [CrossRef]
- Lee-Rueckert, M.; Escola-Gil, J.C.; Kovanen, P.T. HDL functionality in reverse cholesterol transport—Challenges in translating data emerging from mouse models to human disease. Biochim. Biophys. Acta 2016, 1861, 566–583. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef]
- Hisauchi, I.; Ishikawa, T.; Ayaori, M.; Uto-Kondo, H.; Koshikawa, Y.; Ukaji, T.; Nakamura, H.; Mizutani, Y.; Taguchi, I.; Nakajima, T.; et al. High-Density Lipoprotein Cholesterol Efflux Capacity as a Novel Prognostic Surrogate for Coronary Artery Disease. J. Atheroscler. Thromb. 2020. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Cuchel, M.; de la Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Kashif Jafri, B.A.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol Efflux Capacity, High-Density Lipoprotein Function, and Atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Ding, D.; Li, X.; Yang, Y.; Li, Q.; Zheng, Y.; Wang, D.; Ling, W. Cholesterol efflux capacity is an independent predictor of all-cause and cardiovascular mortality in patients with coronary artery disease: A prospective cohort study. Atherosclerosis 2016, 249, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Tang, W.H.W.; Mosior, M.K.; Huang, Y.; Wu, Y.; Matter, W.; Gao, V.; Schmitt, D.; DiDonato, J.A.; Fisher, E.A.; et al. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1696–1705. [Google Scholar] [CrossRef]
- Chindhy, S.; Joshi, P.; Khera, A.; Ayers, C.R.; Hedayati, S.S.; Rohatgi, A. Impaired Renal Function on Cholesterol Efflux Capacity, HDL Particle Number, and Cardiovascular Events. J. Am. Coll. Cardiol. 2018, 72, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Demler, O.; Adelman, S.J.; Collins, H.L.; Glynn, R.J.; Ridker, P.M.; Rader, D.J.; Mora, S. Cholesterol Efflux Capacity, HDL Particle Number, and Incident Cardiovascular Events. An Analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation 2017. [Google Scholar] [CrossRef] [PubMed]
- Josefs, T.; Wouters, K.; Tietge, U.J.F.; Annema, W.; Dullaart, R.P.F.; Vaisar, T.; Arts, I.C.W.; van der Kallen, C.J.H.; Stehouwer, C.D.A.; Schalkwijk, C.G.; et al. High-density lipoprotein cholesterol efflux capacity is not associated with atherosclerosis and prevalence of cardiovascular outcome: The CODAM study. J. Clin. Lipidol. 2020, 14, 122.e4–132.e4. [Google Scholar] [CrossRef]
- Cuchel, M.; Raper, A.C.; Conlon, D.M.; Pryma, D.A.; Freifelder, R.H.; Poria, R.; Cromley, D.; Li, X.; Dunbar, R.L.; French, B.; et al. A Novel Approach to Measuring Macrophage-specific Reverse Cholesterol Transport in Vivo in Humans. J. Lipid Res. 2017, 58, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Voogt, J.; Davidson, M.; Glass, A.; Killion, S.; Decaris, J.; Mohammed, H.; Minehira, K.; Boban, D.; Murphy, E.; et al. Measurement of reverse cholesterol transport pathways in humans: In vivo rates of free cholesterol efflux, esterification, and excretion. J. Am. Heart Assoc. 2012, 1, e001826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zanotti, I.; Reilly, M.P.; Glick, J.M.; Rothblat, G.H.; Rader, D.J. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation 2003, 108, 661–663. [Google Scholar] [CrossRef]
- Rader, D.J.; Alexander, E.T.; Weibel, G.L.; Billheimer, J.; Rothblat, G.H. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 2009, 50, S189–S194. [Google Scholar] [CrossRef]
- Escolà-Gil, J.C.; Llaverias, G.; Julve, J.; Jauhiainen, M.; Méndez-González, J.; Blanco-Vaca, F. The cholesterol content of western diets plays a major role in the paradoxical increase in high-density lipoprotein cholesterol and upregulates the macrophage reverse cholesterol transport pathway. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2493–2499. [Google Scholar] [CrossRef]
- Briand, F.; Thiéblemont, Q.; Muzotte, E.; Sulpice, T. High-fat and fructose intake induces insulin resistance, dyslipidemia, and liver steatosis and alters in vivo macrophage-to-feces reverse cholesterol transport in hamsters. J. Nutr. 2012, 142, 704–709. [Google Scholar] [CrossRef]
- Nishimoto, T.; Pellizzon, M.A.; Aihara, M.; Stylianou, I.M.; Billheimer, J.T.; Rothblat, G.; Rader, D.J. Fish oil promotes macrophage reverse cholesterol transport in mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1502–1508. [Google Scholar] [CrossRef]
- Cedó, L.; Fernández-Castillejo, S.; Rubió, L.; Metso, J.; Santos, D.; Muñoz-Aguayo, D.; Rivas-Urbina, A.; Tondo, M.; Méndez-Lara, K.A.; Farràs, M.; et al. Phenol-enriched virgin olive oil promotes macrophage-specific reverse cholesterol transport in vivo. Biomedicines 2020, 8, 266. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Lagiou, P. Worldwide patterns of dietary lipids intake and health implications. Am. J. Clin. Nutr. 1997, 66, 961S–964S. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S. Coconuts and Health: Different Chain Lengths of Saturated Fats Require Different Consideration. J. Cardiovasc. Dev. Dis. 2020, 7, 59. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Ascherio, A.; Colditz, G.A.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am. J. Clin. Nutr. 1999, 70, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Montoya, C.; Cochard, B.; Flori, A.; Cros, D.; Lopes, R.; Cuellar, T.; Espeout, S.; Syaputra, I.; Villeneuve, P.; Pina, M.; et al. Genetic architecture of palm oil fatty acid composition in cultivated oil palm (Elaeis guineensis Jacq.) compared to its wild relative E. oleifera (H.B.K) Cortés. PLoS ONE 2014, 9, e95412. [Google Scholar] [CrossRef] [PubMed]
- Degirolamo, C.; Rudel, L.L. Dietary monounsaturated fatty acids appear not to provide cardioprotection. Curr. Atheroscler. Rep. 2010, 12, 391–396. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Zong, G.; Li, Y.; Sampson, L.; Dougherty, L.W.; Willett, W.C.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Hu, F.B.; Sun, Q. Monounsaturated fats from plant and animal sources in relation to risk of coronary heart disease among US men and women. Am. J. Clin. Nutr. 2018, 107, 445–453. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Zong, G.; Willett, W.C.; Zock, P.L.; Wanders, A.J.; Hu, F.B.; Sun, Q. Associations of Monounsaturated Fatty Acids From Plant and Animal Sources With Total and Cause-Specific Mortality in Two US Prospective Cohort Studies. Circ. Res. 2019, 124, 1266–1275. [Google Scholar] [CrossRef]
- Nicklas, T.A.; Hampl, J.S.; Taylor, C.A.; Thompson, V.J.; Heird, W.C. Monounsaturated fatty acid intake by children and adults: Temporal trends and demographic differences. Nutr. Rev. 2004, 62, 132–141. [Google Scholar] [CrossRef]
- Schwab, U.; Lauritzen, L.; Tholstrup, T.; Haldorssoni, T.; Riserus, U.; Uusitupa, M.; Becker, W. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: A systematic review. Food Nutr. Res. 2014, 58. [Google Scholar] [CrossRef]
- Hunter, P.M.; Hegele, R.A. Functional foods and dietary supplements for the management of dyslipidaemia. Nat. Rev. Endocrinol. 2017, 13, 278–288. [Google Scholar] [CrossRef]
- Zhang, Z.; Fulgoni, V.L.; Kris-Etherton, P.M.; Mitmesser, S.H. Dietary Intakes of EPA and DHA Omega-3 Fatty Acids among US Childbearing-Age and Pregnant Women: An Analysis of NHANES 2001-2014. Nutrients 2018, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.; Fritsche, K. Linoleic Acid. Adv. Nutr. 2013, 4, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Remig, V.; Franklin, B.; Margolis, S.; Kostas, G.; Nece, T.; Street, J.C. Trans fats in America: A review of their use, consumption, health implications, and regulation. J. Am. Diet. Assoc. 2010, 110, 585–592. [Google Scholar] [CrossRef]
- Ganguly, R.; Pierce, G.N. The toxicity of dietary trans fats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 78, 170–176. [Google Scholar] [CrossRef]
- Song, J.; Park, J.; Jung, J.; Lee, C.; Gim, S.Y.; Ka, H.; Yi, B.; Kim, M.-J.; Kim, C.-I.; Lee, J. Analysis of Trans Fat in Edible Oils with Cooking Process. Toxicol. Res. 2015, 31, 307–312. [Google Scholar] [CrossRef]
- Djoussé, L.; Gaziano, J.M. Egg consumption in relation to cardiovascular disease and mortality: The Physicians’ Health Study. Am. J. Clin. Nutr. 2008, 87, 964–969. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Cholesterol and the Lack of Evidence in Cardiovascular Disease. Nutrients 2018, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.M.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Van Horn, L. Dietary Cholesterol and Cardiovascular Risk: A Science Advisory From the American Heart Association. Circulation 2020, 141, e39–e53. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Xue, L.; Zhang, L.; Wang, X.; Qi, X.; Jiang, J.; Yu, L.; Wang, X.; Zhang, W.; Zhang, Q.; et al. Phytosterol Contents of Edible Oils and Their Contributions to Estimated Phytosterol Intake in the Chinese Diet. Foods 2019, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.; Blomhoff, R. Retinol, Retinoic Acid, and Retinol-Binding Protein 4 are Differentially Associated with Cardiovascular Disease, Type 2 Diabetes, and Obesity: An Overview of Human Studies. Adv. Nutr. 2020, 11, 644–666. [Google Scholar] [CrossRef] [PubMed]
- Al Mheid, I.; Quyyumi, A.A. Vitamin D and Cardiovascular Disease: Controversy Unresolved. J. Am. Coll. Cardiol. 2017, 70, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Sozen, E.; Demirel, T.; Ozer, N.K. Vitamin E: Regulatory role in the cardiovascular system. IUBMB Life 2019, 71, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Kremmyda, L.S.; Tvrzicka, E.; Stankova, B.; Zak, A. Fatty acids as biocompounds: Their role in human metabolism, health and disease—A review. Part 2: Fatty acid physiological roles and applications in human health and disease. Biomed. Pap. 2011, 155, 195–218. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. S2), S33–S50. [Google Scholar]
- Pan, X.; Chen, F.; Wu, T.; Tang, H.; Zhao, Z. Prebiotic oligosaccharides change the concentrations of short-chain fatty acids and the microbial population of mouse bowel. J. Zhejiang Univ. Sci. B 2009, 10, 258–263. [Google Scholar] [CrossRef]
- Chen, X.-F.; Chen, X.; Tang, X. Short-chain fatty acid, acylation and cardiovascular diseases. Clin. Sci. 2020, 134, 657–676. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.A.; Jackson, J.; Stanton, M.; Rojas-Triana, A.; Bober, L.; Laverty, M.; Yang, X.; Zhu, F.; Liu, J.; Wang, S.; et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J. Gastroenterol. 2009, 15, 5549–5557. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef]
- Li, Q.; Wu, T.; Liu, R.; Zhang, M.; Wang, R. Soluble Dietary Fiber Reduces Trimethylamine Metabolism via Gut Microbiota and Co-Regulates Host AMPK Pathways. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.E.L.; Greenwood, D.C.; Threapleton, D.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.E.; Gale, C.P.; Burley, V.J. Effects of dietary fibre type on blood pressure: A systematic review and meta-analysis of randomized controlled trials of healthy individuals. J. Hypertens. 2015, 33, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, L.A.; Smith, N.J.; Milligan, G. International Union of Pharmacology. LXXI. Free fatty acid receptors FFA1, -2, and -3: Pharmacology and pathophysiological functions. Pharmacol. Rev. 2008, 60, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C. Health Effects of Coconut Oil-A Narrative Review of Current Evidence. J. Am. Coll. Nutr. 2019, 38, 97–107. [Google Scholar] [CrossRef]
- McCarty, M.F.; DiNicolantonio, J.J. Lauric acid-rich medium-chain triglycerides can substitute for other oils in cooking applications and may have limited pathogenicity. Open Heart 2016, 3, e000467. [Google Scholar] [CrossRef]
- Yuan, T.; Geng, Z.; Dai, X.; Zhang, X.; Wei, W.; Wang, X.; Jin, Q. Triacylglycerol Containing Medium-Chain Fatty Acids: Comparison of Human Milk and Infant Formulas on Lipolysis during In Vitro Digestion. J. Agric. Food Chem. 2020, 68, 4187–4195. [Google Scholar] [CrossRef]
- Hoppel, C.L. Carnitine and carnitine palmitoyltransferase in fatty acid oxidation and ketosis. Fed. Proc. 1982, 41, 2853–2857. [Google Scholar]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.M.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O.; Howell, S.; Earnest, C.P.; Teixeira, F.J. Coconut oil intake and its effects on the cardiometabolic profile—A structured literature review. Prog. Cardiovasc. Dis. 2019, 62, 436–443. [Google Scholar] [CrossRef]
- Tholstrup, T.; Vessby, B.; Sandstrom, B. Difference in effect of myristic and stearic acid on plasma HDL cholesterol within 24 h in young men. Eur. J. Clin. Nutr. 2003, 57, 735–742. [Google Scholar] [CrossRef]
- Flock, M.R.; Kris-Etherton, P.M. Diverse physiological effects of long-chain saturated fatty acids: Implications for cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Influence of stearic acid on cholesterol metabolism relative to other long-chain fatty acids. Am. J. Clin. Nutr. 1994, 60, 986S–990S. [Google Scholar] [CrossRef] [PubMed]
- Tholstrup, T. Influence of stearic acid on hemostatic risk factors in humans. Lipids 2005, 40, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Mikhailidis, D.P.; Sattar, N.; Toth, P.P.; Judd, S.; Blaha, M.J.; Hernandez, A.V.; Penson, P.E.; Banach, M. Association of types of dietary fats and all-cause and cause-specific mortality: A prospective cohort study and meta-analysis of prospective studies with 1,164,029 participants. Clin. Nutr. 2020, 39, 3677–3686. [Google Scholar] [CrossRef] [PubMed]
- Tvrzicka, E.; Kremmyda, L.-S.; Stankova, B.; Zak, A. Fatty acids as biocompounds: Their role in human metabolism, health and disease—A review. Part 1: Classification, dietary sources and biological functions. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2011, 155, 117–130. [Google Scholar] [CrossRef]
- Joris, P.J.; Mensink, R.P. Role of cis-Monounsaturated Fatty Acids in the Prevention of Coronary Heart Disease. Curr. Atheroscler. Rep. 2016, 18, 38. [Google Scholar] [CrossRef]
- Li, Y.; Hruby, A.; Bernstein, A.M.; Ley, S.H.; Wang, D.D.; Chiuve, S.E.; Sampson, L.; Rexrode, K.M.; Rimm, E.B.; Willett, W.C.; et al. Saturated Fats Compared With Unsaturated Fats and Sources of Carbohydrates in Relation to Risk of Coronary Heart Disease: A Prospective Cohort Study. J. Am. Coll. Cardiol. 2015, 66, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Linseisen, J.; Welch, A.A.; Ocké, M.; Amiano, P.; Agnoli, C.; Ferrari, P.; Sonestedt, E.; Chajès, V.; Bueno-de-Mesquita, H.B.; Kaaks, R.; et al. Dietary fat intake in the European Prospective Investigation into Cancer and Nutrition: Results from the 24-h dietary recalls. Eur. J. Clin. Nutr. 2009, 63 (Suppl. S4), S61–S80. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Monounsaturated fatty acids and cholesterol metabolism: Implications for dietary recommendations. J. Nutr. 1989, 119, 529–533. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; Calabriso, N.; Santarpino, G.; Verri, T.; De Caterina, R. Effects of Olive Oil on Blood Pressure: Epidemiological, Clinical, and Mechanistic Evidence. Nutrients 2020, 12, 1548. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, E.; Biel-Glesson, S.; Fernandez-Navarro, J.R.; Calleja, M.A.; Espejo-Calvo, J.A.; Gil-Extremera, B.; de la Torre, R.; Fito, M.; Covas, M.-I.; Vilchez, P.; et al. Effects of Virgin Olive Oils Differing in Their Bioactive Compound Contents on Biomarkers of Oxidative Stress and Inflammation in Healthy Adults: A Randomized Double-Blind Controlled Trial. Nutrients 2019, 11, 561. [Google Scholar] [CrossRef]
- Mazidi, M.; Katsiki, N.; Shekoohi, N.; Banach, M. Monounsaturated Fatty Acid Levels May Not Affect Cardiovascular Events: Results From a Mendelian Randomization Analysis. Front. Nutr. 2020, 7, 123. [Google Scholar] [CrossRef]
- Goldberg, I.J.; Trent, C.M.; Schulze, P.C. Lipid metabolism and toxicity in the heart. Cell Metab. 2012, 15, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Mag, T.K. Canola oil processing in Canada. J. Am. Oil Chem. Soc. 1983, 60, 380–384. [Google Scholar] [CrossRef]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: Their role in the determination of nutritional requirements and chronic disease risk. Exp. Biol. Med. 2010, 235, 785–795. [Google Scholar] [CrossRef]
- Dasilva, G.; Boller, M.; Medina, I.; Storch, J. Relative levels of dietary EPA and DHA impact gastric oxidation and essential fatty acid uptake. J. Nutr. Biochem. 2018, 55, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; De Caterina, R. Nutraceuticals and prevention of atherosclerosis: Focus on omega-3 polyunsaturated fatty acids and Mediterranean diet polyphenols. Cardiovasc. Ther. 2010, 28, e13–e19. [Google Scholar] [CrossRef]
- Marventano, S.; Kolacz, P.; Castellano, S.; Galvano, F.; Buscemi, S.; Mistretta, A.; Grosso, G. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: Does the ratio really matter? Int. J. Food Sci. Nutr. 2015, 66, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Wijendran, V.; Hayes, K.C. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu. Rev. Nutr. 2004, 24, 597–615. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.Y.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef] [PubMed]
- Montoya, M.T.; Porres, A.; Serrano, S.; Fruchart, J.C.; Mata, P.; Gerique, J.A.G.; Castro, G.R. Fatty acid saturation of the diet and plasma lipid concentrations, lipoprotein particle concentrations, and cholesterol efflux capacity. Am. J. Clin. Nutr. 2002, 75, 484–491. [Google Scholar] [CrossRef]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; AlAbdulghafoor, F.K.; Summerbell, C.D.; Worthington, H.V.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 11, CD003177. [Google Scholar] [CrossRef] [PubMed]
- Poorani, R.; Bhatt, A.N.; Dwarakanath, B.S.; Das, U.N. COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance. Eur. J. Pharmacol. 2016, 785, 116–132. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; Summerbell, C.D.; Worthington, H.V.; Song, F.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2020, 3, CD003177. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A.; Costeloe, K.; Ghebremeskel, K.; Phylactos, A.; Skirvin, L.; Stacey, F. Are deficits of arachidonic and docosahexaenoic acids responsible for the neural and vascular complications of preterm babies? Am. J. Clin. Nutr. 1997, 66, 1032S–1041S. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Willett, W.C. Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the nurses’ health study. Am. J. Epidemiol. 2005, 161, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, D.E.; Nyyssönen, K.; Niskanen, L.; Rissanen, T.H.; Salonen, J.T. Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch. Intern. Med. 2005, 165, 193–199. [Google Scholar] [CrossRef]
- Katan, M.B. Omega-6 polyunsaturated fatty acids and coronary heart disease. Am. J. Clin. Nutr. 2009, 89, 1283–1284. [Google Scholar] [CrossRef]
- Jakobsen, M.U.; O’Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Bälter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S.; et al. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Mensink, R.P. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Hooper, L.; Al-Khudairy, L.; Abdelhamid, A.S.; Rees, K.; Brainard, J.S.; Brown, T.J.; Ajabnoor, S.M.; O’Brien, A.T.; Winstanley, L.E.; Donaldson, D.H.; et al. Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 7, CD011094. [Google Scholar] [CrossRef]
- Maki, K.C.; Hasse, W.; Dicklin, M.R.; Bell, M.; Buggia, M.A.; Cassens, M.E.; Eren, F. Corn Oil Lowers Plasma Cholesterol Compared with Coconut Oil in Adults with Above-Desirable Levels of Cholesterol in a Randomized Crossover Trial. J. Nutr. 2018, 148, 1556–1563. [Google Scholar] [CrossRef]
- Maki, K.C.; Lawless, A.L.; Kelley, K.M.; Kaden, V.N.; Geiger, C.J.; Dicklin, M.R. Corn oil improves the plasma lipoprotein lipid profile compared with extra-virgin olive oil consumption in men and women with elevated cholesterol: Results from a randomized controlled feeding trial. J. Clin. Lipidol. 2015, 9, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Shimano, H.; Yahagi, N.; Ide, T.; Amemiya-Kudo, M.; Matsuzaka, T.; Nakakuki, M.; Tomita, S.; Okazaki, H.; Tamura, Y.; et al. Polyunsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. J. Biol. Chem. 2002, 277, 1705–1711. [Google Scholar] [CrossRef]
- Iacono, J.M.; Dougherty, R.M.; Puska, P. Reduction of blood pressure associated with dietary polyunsaturated fat. Hypertension 1982, 4, III34. [Google Scholar] [CrossRef] [PubMed]
- Bjermo, H.; Iggman, D.; Kullberg, J.; Dahlman, I.; Johansson, L.; Persson, L.; Berglund, J.; Pulkki, K.; Basu, S.; Uusitupa, M.; et al. Effects of n-6 PUFA compared with SFA on liver fat, lipoproteins, and inflammation in abdominal obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1003–1012. [Google Scholar] [CrossRef]
- Poudel-Tandukar, K.; Nanri, A.; Matsushita, Y.; Sasaki, S.; Ohta, M.; Sato, M.; Mizoue, T. Dietary intakes of alpha-linolenic and linoleic acids are inversely associated with serum C-reactive protein levels among Japanese men. Nutr. Res. 2009, 29, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Naughton, S.S.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Linoleic acid and the pathogenesis of obesity. Prostaglandins Other Lipid Mediat. 2016, 125, 90–99. [Google Scholar] [CrossRef]
- Valenzuela, C.A.; Baker, E.J.; Miles, E.A.; Calder, P.C. Eighteen-carbon trans fatty acids and inflammation in the context of atherosclerosis. Prog. Lipid Res. 2019, 76, 101009. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Katan, M.B.; Ascherio, A.; Stampfer, M.J.; Willett, W.C. Trans fatty acids and cardiovascular disease. N. Engl. J. Med. 2006, 354, 1601–1613. [Google Scholar] [CrossRef]
- De Roos, N.M.; Bots, M.L.; Katan, M.B. Replacement of dietary saturated fatty acids by trans fatty acids lowers serum HDL cholesterol and impairs endothelial function in healthy men and women. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1233–1237. [Google Scholar] [CrossRef]
- Bendsen, N.T.; Christensen, R.; Bartels, E.M.; Astrup, A. Consumption of industrial and ruminant trans fatty acids and risk of coronary heart disease: A systematic review and meta-analysis of cohort studies. Eur. J. Clin. Nutr. 2011, 65, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Smit, L.A.; Katan, M.B.; Wanders, A.J.; Basu, S.; Brouwer, I.A. A high intake of trans fatty acids has little effect on markers of inflammation and oxidative stress in humans. J. Nutr. 2011, 141, 1673–1678. [Google Scholar] [CrossRef]
- Micha, R.; Mozaffarian, D. Trans fatty acids: Effects on metabolic syndrome, heart disease and diabetes. Nat. Rev. Endocrinol. 2009, 5, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H. Dietary trans fatty acids and cardiovascular disease risk: Past and present. Curr. Atheroscler. Rep. 2014, 16, 433. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H.J.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef]
- Missimer, A.; DiMarco, D.M.; Andersen, C.J.; Murillo, A.G.; Vergara-Jimenez, M.; Fernandez, M.L. Consuming Two Eggs per Day, as Compared to an Oatmeal Breakfast, Decreases Plasma Ghrelin while Maintaining the LDL/HDL Ratio. Nutrients 2017, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.L.; Gnanaraj, J.; Treu, J.A.; Ma, Y.; Kavak, Y.; Njike, V.Y. Effects of egg ingestion on endothelial function in adults with coronary artery disease: A randomized, controlled, crossover trial. Am. Heart J. 2015, 169, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Mutungi, G.; Waters, D.; Ratliff, J.; Puglisi, M.; Clark, R.M.; Volek, J.S.; Fernandez, M.L. Eggs distinctly modulate plasma carotenoid and lipoprotein subclasses in adult men following a carbohydrate-restricted diet. J. Nutr. Biochem. 2010, 21, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Blesso, C.N.; Fernandez, M.L. Dietary Cholesterol, Serum Lipids, and Heart Disease: Are Eggs Working for or Against You? Nutrients 2018, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Poli, A. Phytosterols and cardiovascular health. Pharmacol. Res. 2010, 61, 193–199. [Google Scholar] [CrossRef]
- Klingberg, S.; Ellegård, L.; Johansson, I.; Hallmans, G.; Weinehall, L.; Andersson, H.; Winkvist, A. Inverse relation between dietary intake of naturally occurring plant sterols and serum cholesterol in northern Sweden. Am. J. Clin. Nutr. 2008, 87, 993–1001. [Google Scholar] [CrossRef]
- Andersson, S.W.; Skinner, J.; Ellegård, L.; Welch, A.A.; Bingham, S.; Mulligan, A.; Andersson, H.; Khaw, K.-T. Intake of dietary plant sterols is inversely related to serum cholesterol concentration in men and women in the EPIC Norfolk population: A cross-sectional study. Eur. J. Clin. Nutr. 2004, 58, 1378–1385. [Google Scholar] [CrossRef]
- Plat, J.; Mensink, R.P. Plant stanol and sterol esters in the control of blood cholesterol levels: Mechanism and safety aspects. Am. J. Cardiol. 2005, 96, 15D–22D. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.; Vinknes, K.J.; Svingen, G.F.; Pedersen, E.R.; Dhar, I.; Tell, G.S.; Blomhoff, R.; Ueland, P.M.; Midttun, Ø.; Refsum, H.; et al. The risk association of plasma total homocysteine with acute myocardial infarction is modified by serum vitamin A. Eur. J. Prev. Cardiol. 2018, 25, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Min, K.-B.; Min, J.-Y. Relation of serum vitamin A levels to all-cause and cause-specific mortality among older adults in the NHANES III population. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.-J.; Qiu, H.-C.; Zhang, Y.; Cao, J.-L.; Wang, H.; Zhao, J.-Z.; Liu, Q.; Zeng, X. Lower serum retinoic acid level for prediction of higher risk of mortality in ischemic stroke. Neurology 2019, 92, e1678–e1687. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; O’Keefe, J.H.; Bell, D.; Hensrud, D.D.; Holick, M.F. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J. Am. Coll. Cardiol. 2008, 52, 1949–1956. [Google Scholar] [CrossRef]
- De la Guía-Galipienso, F.; Martínez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galeano, H. Vitamin D and cardiovascular health. Clin. Nutr. 2021, 40, 2946–2957. [Google Scholar] [CrossRef] [PubMed]
- Scragg, R.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Sluyter, J.; Murphy, J.; Khaw, K.-T.; Camargo, C.A.J. Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study: A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Legarth, C.; Grimm, D.; Krüger, M.; Infanger, M.; Wehland, M. Potential Beneficial Effects of Vitamin D in Coronary Artery Disease. Nutrients 2019, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Dalan, R.; Liew, H.; Assam, P.N.; Chan, E.S.; Siddiqui, F.J.; Tan, A.W.; Chew, D.E.; Boehm, B.O.; Leow, M.K. A randomised controlled trial evaluating the impact of targeted vitamin D supplementation on endothelial function in type 2 diabetes mellitus: The DIMENSION trial. Diabetes Vasc. Dis. Res. 2016, 13, 192–200. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Huang, J.; Weinstein, S.J.; Yu, K.; Männistö, S.; Albanes, D. Relationship Between Serum Alpha-Tocopherol and Overall and Cause-Specific Mortality. Circ. Res. 2019, 125, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R., III; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef]
- Wallert, M.; Ziegler, M.; Wang, X.; Maluenda, A.; Xu, X.; Yap, M.L.; Witt, R.; Giles, C.; Kluge, S.; Hortmann, M.; et al. α-Tocopherol preserves cardiac function by reducing oxidative stress and inflammation in ischemia/reperfusion injury. Redox Biol. 2019, 26, 101292. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; Wallert, M.; Lorkowski, S.; Peter, K. Cardiovascular and Metabolic Protection by Vitamin E: A Matter of Treatment Strategy? Antioxidants 2020, 9, 935. [Google Scholar] [CrossRef]
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; de Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.; Dillon, E.; Guo, W.; Finucane, O.; McMorrow, A.; Murphy, A.; Lyons, C.; Jones, D.; Ryan, M.; Gibney, M.; et al. High-density lipoprotein proteomic composition, and not efflux capacity, reflects differential modulation of reverse cholesterol transport by saturated and monounsaturated fat diets. Circulation 2016, 133, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Hayek, T.; Ito, Y.; Azrolan, N.; Verdery, R.B.; Aalto-Setälä, K.; Walsh, A.; Breslow, J.L. Dietary fat increases high density lipoprotein (HDL) levels both by increasing the transport rates and decreasing the fractional catabolic rates of HDL cholesterol ester and apolipoprotein (Apo) A-I. Presentation of a new animal model and mechanistic stu. J. Clin. Investig. 1993, 91, 1665–1671. [Google Scholar] [CrossRef]
- Quig, D.W.; Zilversmit, D.B. High density lipoprotein metabolism in a rabbit model of hyperalphalipoproteinemia. Atherosclerosis 1989, 76, 9–19. [Google Scholar] [CrossRef]
- Dorfman, S.E.; Wang, S.; Vega-López, S.; Jauhiainen, M.; Lichtenstein, A.H. Dietary fatty acids and cholesterol differentially modulate HDL cholesterol metabolism in Golden-Syrian hamsters. J. Nutr. 2005, 135, 492–498. [Google Scholar] [CrossRef]
- Hatahet, W.; Soofi, A.; Ntekim, O.E.; Fungwe, T. Plasma lipid profiles of transgenic mice expressing the human apob100xcetp are altered differentially by diets enriched with defined fatty acids. Curr. Res. Nutr. Food Sci. 2015, 3, 207–218. [Google Scholar] [CrossRef]
- Cheema, S.K.; Agarwal-Mawal, A.; Murray, C.M.; Tucker, S. Lack of stimulation of cholesteryl ester transfer protein by cholesterol in the presence of a high-fat diet. J. Lipid Res. 2005, 46, 2356–2366. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.A. Saturated fatty acid, but not cholesterol, regulates apolipoprotein AI gene expression by posttranscriptional mechanism. Biochem. Mol. Biol. Int. 1994, 34, 393–402. [Google Scholar] [PubMed]
- Gillotte, K.L.; Lund-Katz, S.; de la Llera-Moya, M.; Parks, J.S.; Rudel, L.L.; Rothblat, G.H.; Phillips, M.C. Dietary modification of high density lipoprotein phospholipid and influence on cellular cholesterol efflux. J. Lipid Res. 1998, 39, 2065–2075. [Google Scholar] [CrossRef]
- Hatahet, W.; Cole, L.; Kudchodkar, B.J.; Fungwe, T. V Dietary fats differentially modulate the expression of lecithin:cholesterol acyltransferase, apoprotein-A1 and scavenger receptor b1 in rats. J. Nutr. 2003, 133, 689–694. [Google Scholar] [CrossRef][Green Version]
- Yang, Z.-H.; Gordon, S.M.; Sviridov, D.; Wang, S.; Danner, R.L.; Pryor, M.; Vaisman, B.; Shichijo, Y.; Doisaki, N.; Remaley, A.T. Dietary supplementation with long-chain monounsaturated fatty acid isomers decreases atherosclerosis and alters lipoprotein proteomes in LDLr(-/-) mice. Atherosclerosis 2017, 262, 31–38. [Google Scholar] [CrossRef]
- Le Morvan, V.; Dumon, M.-F.; Palos-Pinto, A.; Bérard, A.M. n-3 FA increase liver uptake of HDL-cholesterol in mice. Lipids 2002, 37, 767–772. [Google Scholar] [CrossRef]
- Morgado, N.; Rigotti, A.; Valenzuela, A. Comparative effect of fish oil feeding and other dietary fatty acids on plasma lipoproteins, biliary lipids, and hepatic expression of proteins involved in reverse cholesterol transport in the rat. Ann. Nutr. Metab. 2005, 49, 397–406. [Google Scholar] [CrossRef]
- Kasbi Chadli, F.; Nazih, H.; Krempf, M.; Nguyen, P.; Ouguerram, K. Omega 3 Fatty Acids Promote Macrophage Reverse Cholesterol Transport in Hamster Fed High Fat Diet. PLoS ONE 2013, 8, e61109. [Google Scholar] [CrossRef]
- Gatto, L.M.; Lyons, M.A.; Brown, A.J.; Samman, S. Trans fatty acids affect lipoprotein metabolism in rats. J. Nutr. 2002, 132, 1242–1248. [Google Scholar] [CrossRef]
- Cassagno, N.; Palos-Pinto, A.; Costet, P.; Breilh, D.; Darmon, M.; Bérard, A.M. Low amounts of trans 18:1 fatty acids elevate plasma triacylglycerols but not cholesterol and alter the cellular defence to oxidative stress in mice. Br. J. Nutr. 2005, 94, 346–352. [Google Scholar] [CrossRef]
- Jeyakumar, S.M.; Prashant, A.; Rani, K.S.; Laxmi, R.; Vani, A.; Kumar, P.U.; Vajreswari, A. Chronic consumption of trans-fat-rich diet increases hepatic cholesterol levels and impairs muscle insulin sensitivity without leading to hepatic steatosis and hypertriglyceridemia in female fischer rats. Ann. Nutr. Metab. 2011, 58, 272–280. [Google Scholar] [CrossRef]
- O’Reilly, M.E.; Lenighan, Y.M.; Dillon, E.; Kajani, S.; Curley, S.; Bruen, R.; Byrne, R.; Heslin, A.M.; Moloney, A.P.; Roche, H.M.; et al. Conjugated Linoleic Acid and Alpha Linolenic Acid Improve Cholesterol Homeostasis in Obesity by Modulating Distinct Hepatic Protein Pathways. Mol. Nutr. Food Res. 2020, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Valeille, K.; Férézou, J.; Parquet, M.; Amsler, G.; Gripois, D.; Annie Quignard-Boulangé, Y.; Martin, J.C. The natural concentration of the conjugated linoleic acid, cis-9,trans-11, in milk fat has antiatherogenic effects in hyperlipidemic hamsters. J. Nutr. 2006, 136, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Stein, O.; Dabach, Y.; Hollander, G.; Ben-Naim, M.; Halperin, G.; Stein, Y. Effect of atherogenic diet on reverse cholesterol transport in vivo in atherosclerosis susceptible (C57BL/6) and resistant (C3H) mice. Atherosclerosis 2001, 156, 307–313. [Google Scholar] [CrossRef]
- Fan, N.; Meng, K.; Zhang, Y.; Hu, Y.; Li, D.; Gao, Q.; Wang, J.; Li, Y.; Wu, S.; Cui, Y. The effect of ursodeoxycholic acid on the relative expression of the lipid metabolism genes in mouse cholesterol gallstone models. Lipids Health Dis. 2020, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kamisako, T.; Ogawa, H.; Yamamoto, K. Effect of cholesterol, cholic acid and cholestyramine administration on the intestinal mRNA expressions related to cholesterol and bile acid metabolism in the rat. J. Gastroenterol. Hepatol. 2007, 22, 1832–1837. [Google Scholar] [CrossRef]
- Tréguier, M.; Briand, F.; Boubacar, A.; André, A.; Magot, T.; Nguyen, P.; Krempf, M.; Sulpice, T.; Ouguerram, K. Diet-induced dyslipidemia impairs reverse cholesterol transport in hamsters. Eur. J. Clin. Investig. 2011, 41, 921–928. [Google Scholar] [CrossRef]
- Calpe-Berdiel, L.; Escolà-Gil, J.C.; Ribas, V.; Navarro-Sastre, A.; Garcés-Garcés, J.; Blanco-Vaca, F. Changes in intestinal and liver global gene expression in response to a phytosterol-enriched diet. Atherosclerosis 2005, 181, 75–85. [Google Scholar] [CrossRef]
- Lifsey, H.C.; Kaur, R.; Thompson, B.H.; Bennett, L.; Temel, R.E.; Graf, G.A. Stigmasterol stimulates transintestinal cholesterol excretion independent of liver X receptor activation in the small intestine. J. Nutr. Biochem. 2020, 76, 108263. [Google Scholar] [CrossRef]
- Prashanth, A.; Jeyakumar, S.M.; Giridharan, N.V.; Vajreswari, A. Vitamin a-enriched diet modulates reverse cholesterol transport in hypercholesterolemic obese rats of the WNIN/OB strain. J. Atheroscler. Thromb. 2014, 21, 1197–1207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zou, T.-B.; Zhu, S.S.; Luo, F.; Li, W.Q.; Sun, X.R.; Wu, H.F. Effects of Astaxanthin on Reverse Cholesterol Transport and Atherosclerosis in Mice. BioMed Res. Int. 2017, 2017, 4625932. [Google Scholar] [CrossRef] [PubMed]
- Witt, W.; Kolleck, I.; Fechner, H.; Sinha, P.; Rüstow, B. Regulation by vitamin E of the scavenger receptor BI in rat liver and HepG2 cells. J. Lipid Res. 2000, 41, 2009–2016. [Google Scholar] [CrossRef]
- Tang, F.; Lu, M.; Zhang, S.; Mei, M.; Wang, T.; Liu, P.; Wang, H. Vitamin E conditionally inhibits atherosclerosis in ApoE knockout mice by anti-oxidation and regulation of vasculature gene expressions. Lipids 2014, 49, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Bozaykut, P.; Karademir, B.; Yazgan, B.; Sozen, E.; Siow, R.C.M.; Mann, G.E.; Ozer, N.K. Effects of vitamin E on peroxisome proliferator-activated receptor γ and nuclear factor-erythroid 2-related factor 2 in hypercholesterolemia-induced atherosclerosis. Free Radic. Biol. Med. 2014, 70, 174–181. [Google Scholar] [CrossRef]
- Yin, K.; You, Y.; Swier, V.; Tang, L.; Radwan, M.M.; Pandya, A.N.; Agrawal, D.K. Vitamin D Protects Against Atherosclerosis via Regulation of Cholesterol Efflux and Macrophage Polarization in Hypercholesterolemic Swine. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2432–2442. [Google Scholar] [CrossRef]
- Spady, D.K.; Kearney, D.M.; Hobbs, H.H. Polyunsaturated fatty acids up-regulate hepatic scavenger receptor B1 (SR-BI) expression and HDL cholesteryl ester uptake in the hamster. J. Lipid Res. 1999, 40, 1384–1394. [Google Scholar] [CrossRef]
- Cedó, L.; Metso, J.; Santos, D.; Sánchez-Quesada, J.L.; Julve, J.; García-León, A.; Mora-Brugués, J.; Jauhiainen, M.; Blanco-Vaca, F.; Escolà-Gil, J.C. Consumption of polyunsaturated fat improves the saturated fatty acid-mediated impairment of HDL antioxidant potential. Mol. Nutr. Food Res. 2015, 59, 1987–1996. [Google Scholar] [CrossRef]
- Calpe-Berdiel, L.; Escolà-Gil, J.C.; Blanco-Vaca, F. Phytosterol-mediated inhibition of intestinal cholesterol absorption is independent of ATP-binding cassette transporter A1. Br. J. Nutr. 2006, 95, 618–622. [Google Scholar] [CrossRef]
- Igel, M.; Giesa, U.; Lütjohann, D.; Von Bergmann, K. Comparison of the intestinal uptake of cholesterol, plant sterols, and stanols in mice. J. Lipid Res. 2003, 44, 533–538. [Google Scholar] [CrossRef]
- Morton, R.E.; Izem, L. Cholesteryl ester transfer proteins from different species do not have equivalent activities. J. Lipid Res. 2014, 55, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Surdu, A.M.; Pînzariu, O.; Ciobanu, D.-M.; Negru, A.-G.; Căinap, S.-S.; Lazea, C.; Iacob, D.; Săraci, G.; Tirinescu, D.; Borda, I.M.; et al. Vitamin D and Its Role in the Lipid Metabolism and the Development of Atherosclerosis. Biomedicines 2021, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.P.; Coronel, J.; Amengual, J. The role of β-carotene and vitamin A in atherogenesis: Evidences from preclinical and clinical studies. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158635. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tanigawa, H.; Li, X.; Komaru, Y.; Billheimer, J.T.; Rader, D.J. Pharmacologic suppression of hepatic ATP-binding cassette transporter 1 activity in mice reduces high-density lipoprotein cholesterol levels but promotes reverse cholesterol transport. Circulation 2011, 124, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Katsuyama, H.; Hamasaki, H.; Abe, S.; Tada, N.; Sako, A. Effects of Dietary Fat Intake on HDL Metabolism. J. Clin. Med. Res. 2015, 7, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Erkkilä, A.; de Mello, V.D.F.; Risérus, U.; Laaksonen, D.E. Dietary fatty acids and cardiovascular disease: An epidemiological approach. Prog. Lipid Res. 2008, 47, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Zamora, D.; Leelarthaepin, B.; Majchrzak-Hong, S.F.; Faurot, K.R.; Suchindran, C.M.; Ringel, A.; Davis, J.M.; Hibbeln, J.R. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: Evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 2013, 346, e8707. [Google Scholar] [CrossRef]
- Khaw, K.-T.; Friesen, M.D.; Riboli, E.; Luben, R.; Wareham, N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: The EPIC-Norfolk prospective study. PLoS Med. 2012, 9, e1001255. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Babio, N.; Martínez-González, M.A.; Corella, D.; Ros, E.; Martín-Peláez, S.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Verschuren, W.M.; Jacobs, D.R.; Bloemberg, B.P.; Kromhout, D.; Menotti, A.; Aravanis, C.; Blackburn, H.; Buzina, R.; Dontas, A.S.; Fidanza, F. Serum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five-year follow-up of the seven countries study. JAMA 1995, 274, 131–136. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef] [PubMed]
- Prentice, R.L.; Aragaki, A.K.; Van Horn, L.; Thomson, C.A.; Beresford, S.A.A.; Robinson, J.; Snetselaar, L.; Anderson, G.L.; Manson, J.A.E.; Allison, M.A.; et al. Low-fat dietary pattern and cardiovascular disease: Results from the Women’s Health Initiative randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 35–43. [Google Scholar] [CrossRef]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef]
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. PREPARE: Guidelines for planning animal research and testing. Lab. Anim. 2018, 52, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.; Rousseau-Blass, F.; Beauchamp, G.; Pang, D.S.J. Arrive has not arrived: Support for the arrive (animal research: Reporting of in vivo experiments) guidelines does not improve the reporting quality of papers in animal welfare, analgesia or anesthesia. PLoS ONE 2018, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Minniti, M.E.; Pedrelli, M.; Vedin, L.-L.; Delbès, A.-S.; Denis, R.G.P.; Öörni, K.; Sala, C.; Pirazzini, C.; Thiagarajan, D.; Nurmi, H.J.; et al. Insights From Liver-Humanized Mice on Cholesterol Lipoprotein Metabolism and LXR-Agonist Pharmacodynamics in Humans. Hepatology 2020, 72, 656–670. [Google Scholar] [CrossRef]
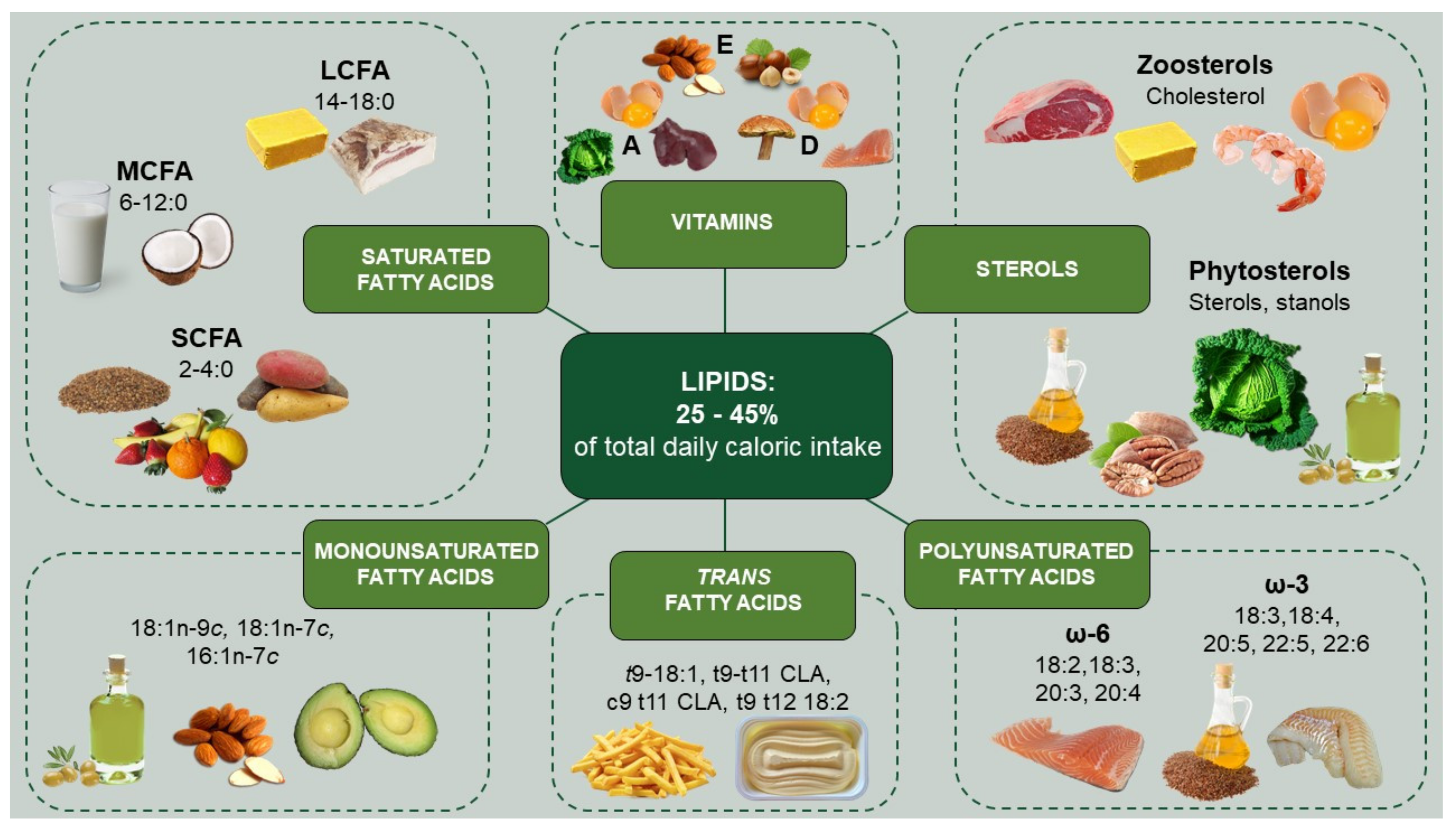
| Lipid Class | Daily Intake | Main Dietary Sources | Main Molecular Content | References |
|---|---|---|---|---|
| SFA SCFAs: acetic acid (2:0), propionic acid (3:0), butyric acid (4:0) MCFA: caproic acid (6:0), caprylic acid (8:0), lauric acid (10:0) LCSFA: myristic acid (14:0), palmitic acid (16:0), stearic acid (18:0) | 7–10% | Refined coconut oil, Virgin coconut oil | Lauric acid (50%) | [33] |
| 7–10% | Buttermilk | Palmitic acid (28%), Stearic acid (12%), Myristic acid (10%) | [34] | |
| 9 g | Palm oil | Palmitic acid (44%), Stearic acid (5%) | [35] | |
| MUFA oleic acid (18:1 n-9c), vaccenic acid (18:1 n-7c), palmitoleic acid (16:1 n-7c), myristoleic acid (14:1 n-5c), erucic acid (22:1 n-9c) | 15% | Canola Oil Olive oil Meat Peanut oil Sunflower oil | Oleic acid (57%) Oleic acid (68%) Oleic acid (30%) Oleic acid (50%) Oleic acid (45%) | [36,37,38,39,40] |
| ω-3 PUFA ALA (18:3), stearidonic acid (18:4), EPA (20:5), DPA (22:5), DHA (22:6) | 1.5 g | Flaxseeds Chia seeds Canola oil Soybean oil | ALA (57%) ALA (20%) ALA (10%) ALA (5%) | [41,42] |
| 250–500 mg | Cod liver oil Salmon Tuna | DHA + EPA (8.3 g + 10.8 g *) DHA + EPA (1.19 g + 0.89 g *) DHA + EPA (2.15 g + 0.8 g *) | [43] | |
| ω-6 PUFA LA (18:2), GLA (18:3), DHGLA (20:3), AA (20:4) | 10 g | Soybean oil Sunflower oil Corn oil Nuts Peanut butter Seeds | LA (67.7 g *) LA (50 g *) LA (50 g *) LA (34 g *) LA (13,45 g *) LA (4 g *) | [44,45] |
| TFA Elaidic acid (t9 18:1), trans vaccenic acid (t11 18:1), CLA, rumenic acid (c9 t11 CLA), t10 c12 CLA, linoelaidic acid (t9 t12 CLA) | 1% | Industrial bakery Meat, dairy Meat, dairy | t9,t11-CLA c9,t11-CLA t10,c12-CLA | [46,47,48] |
| 1% | Industrial bakery | Elaidic acid t-18:1 | ||
| Zoosterols Cholesterol | 300 mg | Egg yolk Shrimp Cod liver oil Buttermilk Bovine liver Lard | 1337 mg * 150 mg * 570 mg * 250 mg * 194 mg * 95 mg * | [49,50,51] |
| Phytosterols Campesterol, sitosterol, campestanol, sitostanol, stigmasterol, stigmastanol, brassicasterol | 150–400 mg | Canola oil Olive Chickpeas Sunflower oil | Campesterol (156 mg *) Sitosterol (158 mg *) Sitosterol (42.3 mg *) Sitosterol (171 mg *) | [49,52] |
| Liposoluble Vitamins | 700–900 µg | Bovine liver Buttermilk Egg yolk Carrots Yellow pumpkin | Vit. A (16 mg *) Vit. A (906 µg *) Vit. A (607 µg *) Vit. A (1.15 mg *) Vit. A (599 µg *) | [53] |
| 20 µg | Herring Tuna Sardines Salmon Egg yolk | Vit. D (30 µg *) Vit. D (16 µg *) Vit. D (11 µg *) Vit. D (8 µg *) Vit. D (5 µg *) | [54] | |
| 15 mg | Wheat germ oil Sunflower seed oil Hazelnut EVO Oil | Vit. E (136 µg *) Vit. E (49 µg *) Vit. E (25 µg *) Vit. E (21 µg *) | [55] |
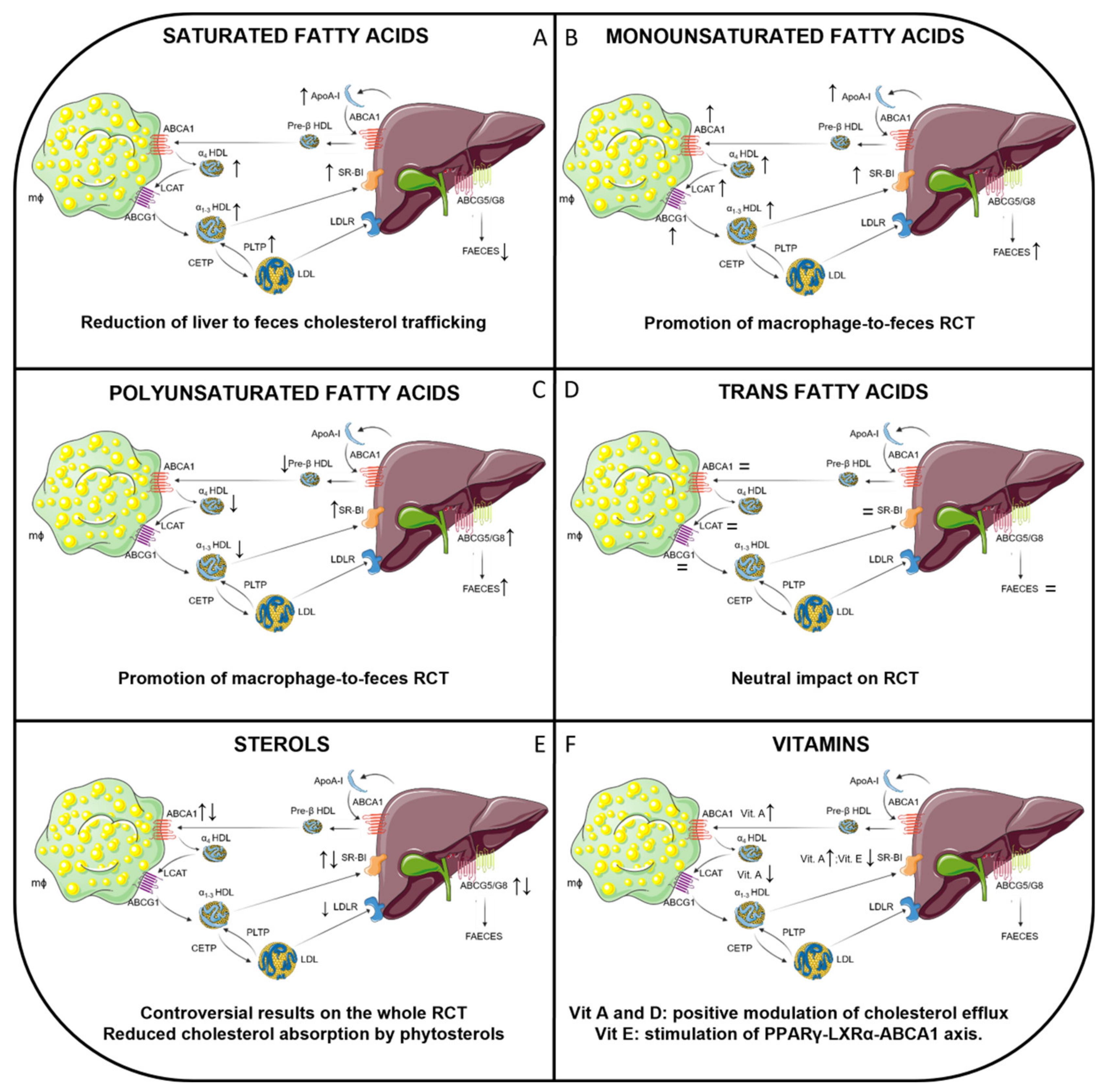
| Lipid Class | Animal Model | Dietary Treatment and Duration | Effect on Lipid Profile | Effect on RCT | Reference |
|---|---|---|---|---|---|
| SFA | Mice (C57BL/6 and human CETP transgenic; males and females) | LFLC diet with high SFA content (saturated fat/total fat ratio 0.64) for 8, 16, 24 weeks | ↑ HDL-C; ↑ ApoA-I | ↑ m-RCT | [28] |
| Mice (C57BL/6J; males) | SFA-HFD (45% kCal from palm oil) vs. micronutrient-matched LFD for 24 weeks | ↑ HDL-C | ↑ cholesterol levels in liver and feces; ↑ total cholesterol efflux to plasma and HDL | [149] | |
| Mice (C57BL/6 and ApoA-I Tg; males and females) | HFD with 27% w/w SFA for 5 weeks | ↑ HDL-C; ↑ ApoA-I | Not evaluated | [150] | |
| Rabbits (New Zealand White; females) | Chow diet + 15% w/w hydrogenated coconut oil for 3 months | ↑ HDL-C | ↑ HDL-ApoA-I transport rate | [151] | |
| Hamsters (Golden Syrian; males) | Chow diet supplemented with 16.5% w/w SFA (coconut oil and butter) for 6 weeks | ↑ HDL-C; ↑ ApoA-I; ↑ PLTP activity | Not evaluated | [153] | |
| Mice (Double Tg expressing human CETP and apoB100; males) | Chow diet enriched with 15% w/w palmitic acid for 4 weeks | = TC; = LDL-C | ↑ Hepatic SR-BI expression | [153] | |
| MUFA | Rats (Sprague-Dawley; males) | Chow diet enriched with 15% w/w trioleate for 20 days | ↑ HDL-C; ↑ ApoA-I | ↑ LCAT mRNA and activity ↑ SR-BI hepatic expression | [157] |
| Hamsters (Golden Syrian; males) | Chow diet supplemented with 10% w/w MUFA (canola oil) for 6 weeks | ↑ TC; ↑ non HDL-C; ↑ HDL-C | Not evaluated | [154] | |
| Mice (double Tg expressing both human CETP and apoB100; males) | Chow diet enriched with oleic acid for 4 weeks | ↓ TC; ↑ HDL-C; ↑ LDL-C | ↑ LCAT mRNA; ↑ SR-BI hepatic expression | [153] | |
| Mice (CETP-Tg; Sex n.s.) | low-fat (5%) or high-fat (20%) diets containing olive oil (enriched in MUFA) for 2 weeks | = TC; ↑ HDL-C | ↓ CETP expression and mass | [154] | |
| Mice (C57BL/6J; males) | MUFA-HFD (45% kcal from sunflower oil) for 24 weeks | ↑ TC; ↑ HDL-C | ↑ m-RCT; ↑ ABCA1-independent cholesterol efflux to HDL from C57BL/6J mice | [149] | |
| Mice (LDLr−/−; females) | Western diet supplemented with 5% w/w LC- MUFA for 12 weeks | = TC; = HDL-C; = LDL-C; = TG | ↑ ABCA1-mediated cholesterol efflux to ApoB-depleted plasma | [158] | |
| PUFA | Mice (C57BL/6; females) | Regular diet enriched in ω -3 FA for 16 weeks | ↓ TC; ↓ TG; ↓ HDL-C; | ↑ Hepatic SR-BI expression; ↑ Hepatic uptake of HDL-CE | [159] |
| Mice (C57BL/6J; females) | Diet supplemented with either low SO, high SO, CO, or FO for 6 weeks | Not reported | ↑ m-RCT (FO diet compared to high and low SO diet ↑ Hepatic ABCG5/ABCG8 expression induced by FO diet | [30] | |
| Rats (Wistar; males) | Diet supplemented with sunflower oil (ω–6) or fish oil (ω –3); duration n.s. | ↓ TC; ↓ TG; ↓ HDL-C | ↑ Biliary cholesterol secretion = biliary phospholipids = bile salts | [160] | |
| Hamsters (Golden Syrian; males) | HFD enriched in ω-3 for 20 weeks | ↓ TC; ↓ TG ↓ HDL-C; | ↑ m-RCT ↑Hepatic ABCA1, ABCG1, SR-BI, ABCG5/ABCG8 expression ↓LCAT activity | [161] | |
| TFA | Rats (Sprague-Dawley, males) | Various isomers of C18:1 TFA versus equal amounts of SFA or MUFA for 4 weeks. | ↓ TC; ↓ LDL-C; = HDL-C ↑ of TFA in HDL phospholipids | ↓ Hepatic [3H]-cholesterol | [162] |
| Mice (C57BL/6J, males) | Low amount of TFA (3% total daily energy intake as trans 18:1 fatty acid) for 7 weeks | = TC = HDL-C ↑ TG | = Cholesterol efflux to plasma from mice = LCAT activity; = Transfer of CE to liver by HDL | [163] | |
| Rats (Fischer, females) | High amount of TFA (4.2% total daily energy intake) versus MUFA/PUFA-containing diets for 52 weeks | ↑ of TFA in plasma phospholipids | = Hepatic SR-BI, LDLr, ABCA1 expression | [164] | |
| Mice (apoE3Leiden-hCETP, males) | HFD supplemented ± CLA or ALA for 12 weeks | = plasma lipids | = m-RCT | [165] | |
| Hamsters (Golden Syrian, males) | Milk fat diets ± rumenic acid | ↑ HDL-C ↓ TG | ↑ Aortic ABCA1 expression | [166] | |
| STEROLS | Mice (C57BL/6J and C3H, males and females) | HFD + 1.25% cholesterol + 0.5% cholate for 4 weeks | ↑ TC ↓ HDL-C | ↓ RCT (fecal elimination of cholesterol from a muscle depot) | [167] |
| Mice (C57BL/6J, males and females; other transgenic strains were also used in this study) | HFHC diet versus different control diets for 8 weeks | ↑ TC ↑ HDL-C ↑ non-HDL-C =TG | ↑ m-RCT | [28] | |
| Mice (C57BL/6J, males) | Lithogenic diet (1.25% cholesterol, 0.5% sodium cholate, 16% fat, 2% corn oil) for 8 weeks | Not evaluated | ↑ RCT ↑Hepatic and intestinal ABCG5/G8 expression | [168] | |
| Rats (Wistar, males) | Cholesterol rich diet (2% w/w) for 2 weeks | ↑ TC ↑ VLDL-C ↑ LDL-C ↑ HDL-C | ↑ Intestinal ABCG8, LXRα, SHP, SREBP-1c ↑ Hepatic CYP7A1 | [169] | |
| Hamsters (Golden Syrian, males) | Cholesterol enriched diet (0.3% w/w) for 4 weeks | ↑ TC ↑ HDL-C ↑ non-HDL-C ↑ TG | ↓ m-RCT ↓ cholesterol efflux capacity of chol-fed animal plasma ↑ Hepatic Abca1, Abcg1, Abcg5 ↓ Hepatic Scarb-1, Ldlr | [170] | |
| Hamsters (Golden Syrian, males) | HFD + 0.5% cholesterol + 0.25% deoxycholate + 10% fructose in drinking water for 4 weeks | ↑ TC ↑ HDL-C ↑ non-HDL-C ↑ TG | ↑ m-RCT * * controversial, because of the impaired hepatic cholesterol flux ↓ Hepatic Abcg1, Abcg5, Ldlr, Acat2 expression | [29] | |
| Mice (ApoE -/-, females | Western type diet ± 0.5, 1% or 2% phytosterols (mainly β-sitosterol, and equal amounts of campesterol and stigmasterol) for 4 weeks | ↓ TC ↓ VLDL-C ↓ IDL-C ↓ LDL-C (in the 2% phytosterol group) | ↓ biliary cholesterol | [171] | |
| Mice (C57BL/6J, males and females) | Standard diet ± 0.3% stigmasterol for 4 days | =TC | ↑ transintestinal cholesterol secretion | [172] | |
| Vitamin A | Rats (leand and obese WNIN/ob; males) | Diet supplemented with low (52mg/kg) or high (129 mg/kg) doses of Vitamin A for 20 weeks | ↓ TC; ↓ HDL-C | ↑LXRα, RXRα hepatic expression ↑ABCA1, SR-BI, HL hepatic expression only in obese rats | [173] |
| Mice (C57BL/6 and ApoE -/-; males) | AIN-93G diet supplemented with astaxanthin (0.05%, w/w) for 2 weeks | ↑ HDL-C ↓ non-HDL-C | ↑ m-RCT | [174] | |
| Vitamin E | Rats (Wistar; males) | Chow diet depleted of α-tocopherol for 28–40 days, followed by 400 mg/kg refeeding of vitamin E for 48 h | = TC; = HDL-C | ↓ Hepatic SR-BI expression | [175] |
| Mice (ApoE -/-; males) | Chow diet supplemented with vitamin E; 4–8 weeks | = TC; = TG | ↓ Aortic CD36 expression ↑ Aortic PPARγ, LXRα, ABCA1 expression | [176] | |
| Rabbits (albino; males) | Vitamin E-poor diet, vitamin E-poor diet with 2% cholesterol, or vitamin E-poor diet containing 2% cholesterol with daily intramuscular injections of vitamin E (50 mg/kg) for 4 weeks | ↑ TC in rabbits fed with diet supplemented with 2% cholesterol compared to controls | ↑ PPARγ, ABCA1 expression in rabbits that underwent intamuscolar injection of Vitamin E | [177] | |
| Vitamin D | Hypercholesterolemic miniswine | HCD supplemented with 1000 IU/day or 3000 IU/day Vitamin D vs. controls for 48 weeks | Not reported | ↑Aortic ABCA1 and ABCG1 expression | [178] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papotti, B.; Escolà-Gil, J.C.; Julve, J.; Potì, F.; Zanotti, I. Impact of Dietary Lipids on the Reverse Cholesterol Transport: What We Learned from Animal Studies. Nutrients 2021, 13, 2643. https://doi.org/10.3390/nu13082643
Papotti B, Escolà-Gil JC, Julve J, Potì F, Zanotti I. Impact of Dietary Lipids on the Reverse Cholesterol Transport: What We Learned from Animal Studies. Nutrients. 2021; 13(8):2643. https://doi.org/10.3390/nu13082643
Chicago/Turabian StylePapotti, Bianca, Joan Carles Escolà-Gil, Josep Julve, Francesco Potì, and Ilaria Zanotti. 2021. "Impact of Dietary Lipids on the Reverse Cholesterol Transport: What We Learned from Animal Studies" Nutrients 13, no. 8: 2643. https://doi.org/10.3390/nu13082643
APA StylePapotti, B., Escolà-Gil, J. C., Julve, J., Potì, F., & Zanotti, I. (2021). Impact of Dietary Lipids on the Reverse Cholesterol Transport: What We Learned from Animal Studies. Nutrients, 13(8), 2643. https://doi.org/10.3390/nu13082643









