Curcumin, an Inhibitor of p300-HAT Activity, Suppresses the Development of Hypertension-Induced Left Ventricular Hypertrophy with Preserved Ejection Fraction in Dahl Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dahl Salt-Sensitive Rats
2.2. Treatment
2.3. Echocardiography
2.4. Histological Analysis
2.5. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.6. Immunoprecipitation and Immuno-Blotting
2.7. Statistics
3. Results
3.1. The Effect of Curcumin on Hemodynamics
3.2. The Effect of Curcumin on Echocardiographic Parameters and Cardiac Function
3.3. The Effects of Curcumin on Myocardial Cell Hypertrophy and Fibrosis
3.4. The Effects of Curcumin on the Transcriptions of Hypertrophy-Response Genes
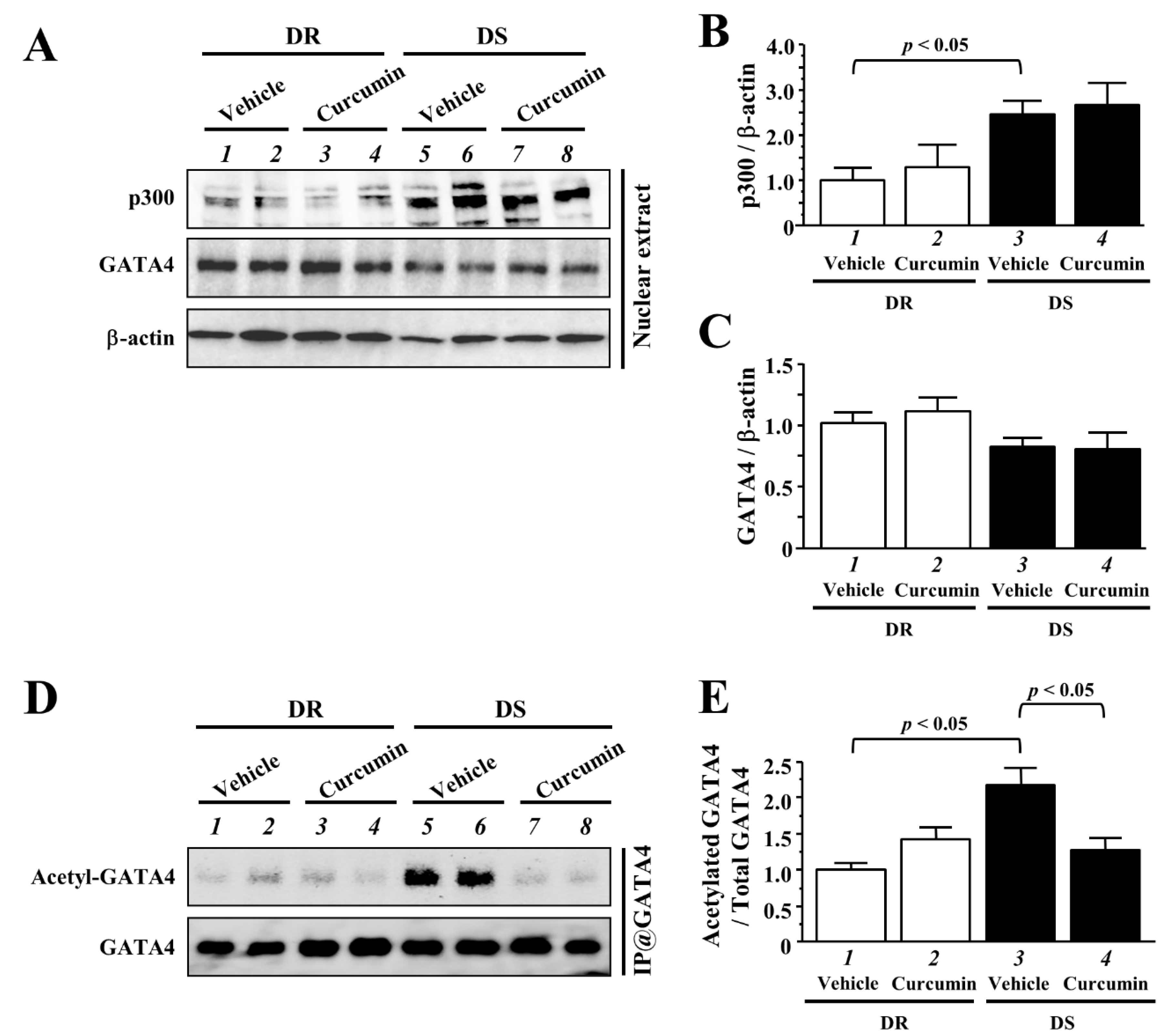
3.5. The Effects of Curcumin on the Acetylated Levels of GATA4
4. Discussions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stokes, J., 3rd; Kannel, W.B.; Wolf, P.A.; D’Agostino, R.B.; Cupples, L.A. Blood Pressure as a Risk Factor for Cardiovascular Disease. The Framingham Study—30 Years of Follow-Up. Hypertension 1989, 13, I13–I18. [Google Scholar] [CrossRef] [Green Version]
- Levy, D.; Garrison, R.J.; Savage, D.D.; Kannel, W.B.; Castelli, W.P. Prognostic Implications of Echocardiographically Determined Left Ventricular Mass in the Framingham Heart Study. N. Engl. J. Med. 1990, 322, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.; Olson, E.N. Cardiac Hypertrophy: The Good, the Bad, and the Ugly. Annu. Rev. Physiol. 2003, 65, 45–79. [Google Scholar] [CrossRef]
- Verdecchia, P.; Schillaci, G.; Borgioni, C.; Ciucci, A.; Gattobigio, R.; Zampi, I.; Reboldi, G.; Porcellati, C. Prognostic Significance of Serial Changes in Left Ventricular Mass in Essential Hypertension. Circulation 1998, 97, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, L.J.; Parrinello, G.; Amato, P.; Licata, G. Trends of Congestive Heart Failure Epidemiology: Contrast with Clinical Trial Results. Cardiologia 1999, 44, 801–808. [Google Scholar]
- Lohse, M.J.; Engelhardt, S.; Eschenhagen, T. What Is the Role of Beta-Adrenergic Signaling in Heart Failure? Circ. Res. 2003, 93, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Lee, S.J.; Jobe, S.M.; Markham, B.E.; Kitsis, R.N. cis-Acting Sequences That Mediate Induction of Beta-Myosin Heavy Chain Gene Expression During Left Ventricular Hypertrophy Due to Aortic Constriction. Circulation 1997, 96, 3943–3953. [Google Scholar] [CrossRef] [PubMed]
- Gusterson, R.J.; Jazrawi, E.; Adcock, I.M.; Latchman, D.S. The Transcriptional Co-Activators CREB-Binding Protein (CBP) and p300 Play a Critical Role in Cardiac Hypertrophy That Is Dependent on Their Histone Acetyltransferase Activity. J. Biol. Chem. 2003, 278, 6838–6847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanazume, T.; Hasegawa, K.; Morimoto, T.; Kawamura, T.; Wada, H.; Matsumori, A.; Kawase, Y.; Hirai, M.; Kita, T. Cardiac p300 Is Involved in Myocyte Growth with Decompensated Heart Failure. Mol. Cell. Biol. 2003, 23, 3593–3606. [Google Scholar] [CrossRef] [Green Version]
- Slepak, T.I.; Webster, K.A.; Zang, J.; Prentice, H.; O’Dowd, A.; Hicks, M.N.; Bishopric, N.H. Control of Cardiac-Specific Transcription by p300 Through Myocyte Enhancer factor-2D. J. Biol. Chem. 2001, 276, 7575–7585. [Google Scholar] [CrossRef] [Green Version]
- Morin, S.; Paradis, P.; Aries, A.; Nemer, M. Serum Response Factor-GATA Ternary Complex Required for Nuclear Signaling by a G-Protein-Coupled Receptor. Mol. Cell. Biol. 2001, 21, 1036–1044. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, S.; Kawamura, T.; Morimoto, T.; Ono, K.; Wada, H.; Kawase, Y.; Matsumori, A.; Nishio, R.; Kita, T.; Hasegawa, K. Histone Acetyltransferase Activity of p300 Is Required for the Promotion of Left Ventricular Remodeling After Myocardial Infarction in Adult Mice In Vivo. Circulation 2006, 113, 679–690. [Google Scholar] [CrossRef]
- Takaya, T.; Kawamura, T.; Morimoto, T.; Ono, K.; Kita, T.; Shimatsu, A.; Hasegawa, K. Identification of p300-Targeted Acetylated Residues in GATA4 During Hypertrophic Responses in Cardiac Myocytes. J. Biol. Chem. 2008, 283, 9828–9835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunagawa, Y.; Morimoto, T.; Takaya, T.; Kaichi, S.; Wada, H.; Kawamura, T.; Fujita, M.; Shimatsu, A.; Kita, T.; Hasegawa, K. Cyclin-Dependent kinase-9 Is a Component of the p300/GATA4 Complex Required for Phenylephrine-Induced Hypertrophy in Cardiomyocytes. J. Biol. Chem. 2010, 285, 9556–9568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanazume, T.; Morimoto, T.; Wada, H.; Kawamura, T.; Hasegawa, K. Biological Role of p300 in Cardiac Myocytes. Mol. Cell. Biochem. 2003, 248, 115–119. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Fang, H.; Shen, J.; Jin, Y.; Zhao, Y.; Wang, R.; Fu, Y.; Tian, Y.; Yu, H.; Zhang, J. Curcumin Alleviates LPS-Induced Oxidative Stress, Inflammation and Apoptosis in Bovine Mammary Epithelial Cells via the NFE2L2 Signaling Pathway. Toxins 2021, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.C.; Mittal, S. Antitumor Activity of Curcumin in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 9435. [Google Scholar] [CrossRef] [PubMed]
- Bulku, E.; Stohs, S.J.; Cicero, L.; Brooks, T.; Halley, H.; Ray, S.D. Curcumin exposure modulates multiple pro-apoptotic and anti-apoptotic signaling pathways to antagonize acetaminophen-induced toxicity. Curr. Neurovasc. Res. 2012, 9, 58–71. [Google Scholar] [CrossRef]
- Balasubramanyam, K.; Varier, R.A.; Altaf, M.; Swaminathan, V.; Siddappa, N.B.; Ranga, U.; Kundu, T.K. Curcumin, a Novel p300/CREB-Binding Protein-Specific Inhibitor of Acetyltransferase, Represses the Acetylation of Histone/Nonhistone Proteins and Histone Acetyltransferase-Dependent Chromatin Transcription. J. Biol. Chem. 2004, 279, 51163–51171. [Google Scholar] [CrossRef] [Green Version]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunagawa, Y.; Miyazaki, Y.; Funamoto, M.; Shimizu, K.; Shimizu, S.; Nurmila, S.; Katanasaka, Y.; Ito, M.; Ogawa, T.; Ozawa-Umeta, H.; et al. A novel amorphous preparation improved curcumin bioavailability in healthy volunteers: A single-dose, double-blind, two-way crossover study. J. Funct. Foods 2021, 81, 104443. [Google Scholar] [CrossRef]
- Morimoto, T.; Sunagawa, Y.; Kawamura, T.; Takaya, T.; Wada, H.; Nagasawa, A.; Komeda, M.; Fujita, M.; Shimatsu, A.; Kita, T.; et al. The Dietary Compound Curcumin Inhibits p300 Histone Acetyltransferase Activity and Prevents Heart Failure in Rats. J. Clin. Investig. 2008, 118, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Sunagawa, Y.; Fujita, M.; Hasegawa, K. Novel Heart Failure Therapy Targeting Transcriptional Pathway in Cardiomyocytes by a Natural Compound, Curcumin. Circ. J. 2010, 74, 1059–1066. [Google Scholar] [CrossRef] [Green Version]
- Sunagawa, Y.; Morimoto, T.; Wada, H.; Takaya, T.; Katanasaka, Y.; Kawamura, T.; Yanagi, S.; Marui, A.; Sakata, R.; Shimatsu, A.; et al. A Natural p300-Specific Histone Acetyltransferase Inhibitor, Curcumin, in Addition to Angiotensin-Converting Enzyme Inhibitor, Exerts Beneficial Effects on Left Ventricular Systolic Function After Myocardial Infarction in Rats. Circ. J. 2011, 75, 2151–2159. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, T.; Funamoto, M.; Sunagawa, Y.; Katanasaka, Y.; Miyazaki, Y.; Hasegawa, K. Noble Heart Failure Therapy Using Food Compositions. Yakugaku Zasshi 2018, 138, 1263–1269. [Google Scholar] [CrossRef] [Green Version]
- Sari, N.; Katanasaka, Y.; Honda, H.; Miyazaki, Y.; Sunagawa, Y.; Funamoto, M.; Shimizu, K.; Shimizu, S.; Wada, H.; Hasegawa, K.; et al. Cacao Bean Polyphenols Inhibit Cardiac Hypertrophy and Systolic Dysfunction in Pressure Overload-Induced Heart Failure Model Mice. Planta Med. 2020, 86, 1304–1312. [Google Scholar] [CrossRef]
- Sunagawa, Y.; Sono, S.; Katanasaka, Y.; Funamoto, M.; Hirano, S.; Miyazaki, Y.; Hojo, Y.; Suzuki, H.; Morimoto, E.; Marui, A.; et al. Optimal Dose-Setting Study of Curcumin for Improvement of Left Ventricular Systolic Function After Myocardial Infarction in Rats. J. Pharmacol. Sci. 2014, 126, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Sunagawa, Y.; Funamoto, M.; Sono, S.; Shimizu, K.; Shimizu, S.; Genpei, M.; Miyazaki, Y.; Katanasaka, Y.; Morimoto, E.; Ueno, M.; et al. Curcumin and Its Demethoxy Derivatives Possess p300 HAT Inhibitory Activity and Suppress Hypertrophic Responses in Cardiomyocytes. J. Pharmacol. Sci. 2018, 136, 212–217. [Google Scholar] [CrossRef]
- Shimizu, K.; Sunagawa, Y.; Funamoto, M.; Wakabayashi, H.; Genpei, M.; Miyazaki, Y.; Katanasaka, Y.; Sari, N.; Shimizu, S.; Katayama, A.; et al. The Synthetic Curcumin Analogue GO-Y030 Effectively Suppresses the Development of Pressure Overload-Induced Heart Failure in Mice. Sci. Rep. 2020, 10, 7172. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, W.E.; Li, M.; Ren, H.; Chen, C.; Wang, J.; Yang, J.; Zeng, C. Curcumin Exerts Its Anti-Hypertensive Effect by Down-Regulating the AT1 Receptor in Vascular Smooth Muscle Cells. Sci. Rep. 2016, 6, 25579. [Google Scholar] [CrossRef]
- Nakmareong, S.; Kukongviriyapan, U.; Pakdeechote, P.; Donpunha, W.; Kukongviriyapan, V.; Kongyingyoes, B.; Sompamit, K.; Phisalaphong, C. Antioxidant and Vascular Protective Effects of Curcumin and Tetrahydrocurcumin in Rats with L-NAME-Induced Hypertension. Naunyn. Schmiedebergs. Arch. Pharmacol. 2011, 383, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Muta, K.; Obata, Y.; Oka, S.; Abe, S.; Minami, K.; Kitamura, M.; Endo, D.; Koji, T.; Nishino, T. Curcumin Ameliorates Nephrosclerosis via Suppression of Histone Acetylation Independent of Hypertension. Nephrol. Dial. Transplant. 2016, 31, 1615–1623. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.Q.; Taylor, D.A.; Fleming, W.W. Sustained hypertension in Dahl rats. Negative correlation of agonist response to blood pressure. Hypertension 1995, 25, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, F.M.; Andersen, C.B.; Leyssac, P.P.; Holstein-Rathlou, N.H. Dynamic autoregulation and renal injury in Dahl rats. Hypertension 1997, 30, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Rimbaud, S.; Ruiz, M.; Piquereau, J.; Mateo, P.; Fortin, D.; Veksler, V.; Garnier, A.; Ventura-Clapier, R. Resveratrol improves survival, hemodynamics and energetics in a rat model of hypertension leading to heart failure. PLoS ONE 2011, 6, e26391. [Google Scholar] [CrossRef] [Green Version]
- Vaněčková, I.; Vokurková, M.; Rauchová, H.; Dobešová, Z.; Pecháňová, O.; Kuneš, J.; Vorlíček, J.; Zicha, J. Chronic antioxidant therapy lowers blood pressure in adult but not in young Dahl salt hypertensive rats: The role of sympathetic nervous system. Acta Physiol. 2013, 208, 340–349. [Google Scholar] [CrossRef]
- Wei, J.Q.; Shehadeh, L.A.; Mitrani, J.M.; Pessanha, M.; Slepak, T.I.; Webster, K.A.; Bishopric, N.H. Quantitative Control of Adaptive Cardiac Hypertrophy by Acetyltransferase p300. Circulation 2008, 118, 934–946. [Google Scholar] [CrossRef] [Green Version]
- Kuno, A.; Hori, Y.S.; Hosoda, R.; Tanno, M.; Miura, T.; Shimamoto, K.; Horio, Y. Resveratrol Improves Cardiomyopathy in Dystrophin-Deficient Mice Through SIRT1 Protein-Mediated Modulation of p300 Protein. J. Biol. Chem. 2013, 288, 5963–5972. [Google Scholar] [CrossRef] [Green Version]
- Poizat, C.; Puri, P.L.; Bai, Y.; Kedes, L. Phosphorylation-Dependent Degradation of p300 by Doxorubicin-Activated p38 Mitogen-Activated Protein Kinase in Cardiac Cells. Mol. Cell. Biol. 2005, 25, 2673–2687. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.E.; Han, C.; Zhao, R.; Wani, G.; Zhu, Q.; Gong, L.; Battu, A.; Racoma, I.; Sharma, N.; Wani, A.A. p38 MAPK- and Akt-Mediated p300 Phosphorylation Regulates Its Degradation to Facilitate Nucleotide Excision Repair. Nucleic Acids Res. 2013, 41, 1722–1733. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.L.; McKinsey, T.A.; Chang, S.; Antos, C.L.; Hill, J.A.; Olson, E.N. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 2002, 110, 479–488. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, C.M.; Zhu, W.; Wang, Q.; Jia, C.; Kee, H.J.; Li, L.; Hannenhalli, S.; Epstein, J.A. Hopx and Hdac2 Interact to Modulate Gata4 Acetylation and Embryonic Cardiac Myocyte Proliferation. Dev. Cell 2010, 19, 450–459. [Google Scholar] [CrossRef] [Green Version]
- Yamamura, S.; Izumiya, Y.; Araki, S.; Nakamura, T.; Kimura, Y.; Hanatani, S.; Yamada, T.; Ishida, T.; Yamamoto, M.; Onoue, Y.; et al. Cardiomyocyte Sirt (Sirtuin) 7 Ameliorates Stress-Induced Cardiac Hypertrophy by Interacting with and Deacetylating GATA4. Hypertension 2020, 75, 98–108. [Google Scholar] [CrossRef]
- Zhou, M.S.; Jaimes, E.A.; Raij, L. Atorvastatin Prevents End-Organ Injury in Salt-Sensitive Hypertension: Role of eNOS and Oxidant Stress. Hypertension 2004, 44, 186–190. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, H.; Raij, L. Nitric Oxide Synthase Activity and Renal Injury in Genetic Hypertension. Hypertension 1998, 31, 266–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kai, H.; Mori, T.; Tokuda, K.; Takayama, N.; Tahara, N.; Takemiya, K.; Kudo, H.; Sugi, Y.; Fukui, D.; Yasukawa, H.; et al. Pressure Overload-Induced Transient Oxidative Stress Mediates Perivascular Inflammation and Cardiac Fibrosis Through Angiotensin II. Hypertens. Res. 2006, 29, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of Curcumin, a Component of Golden Spice, and Its Miraculous Biological Activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ahn, Y.; Hong, M.H.; Joo, S.Y.; Kim, K.H.; Sohn, I.S.; Park, H.W.; Hong, Y.J.; Kim, J.H.; Kim, W.; et al. Curcumin Attenuates Inflammatory Responses of TNF-alpha-Stimulated Human Endothelial Cells. J. Cardiovasc. Pharmacol. 2007, 50, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Mito, S.; Watanabe, K.; Harima, M.; Thandavarayan, R.A.; Veeraveedu, P.T.; Sukumaran, V.; Suzuki, K.; Kodama, M.; Aizawa, Y. Curcumin Ameliorates Cardiac Inflammation in Rats with Autoimmune Myocarditis. Biol. Pharm. Bull. 2011, 34, 974–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative Preparation of Curcumin for Improved Oral Bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
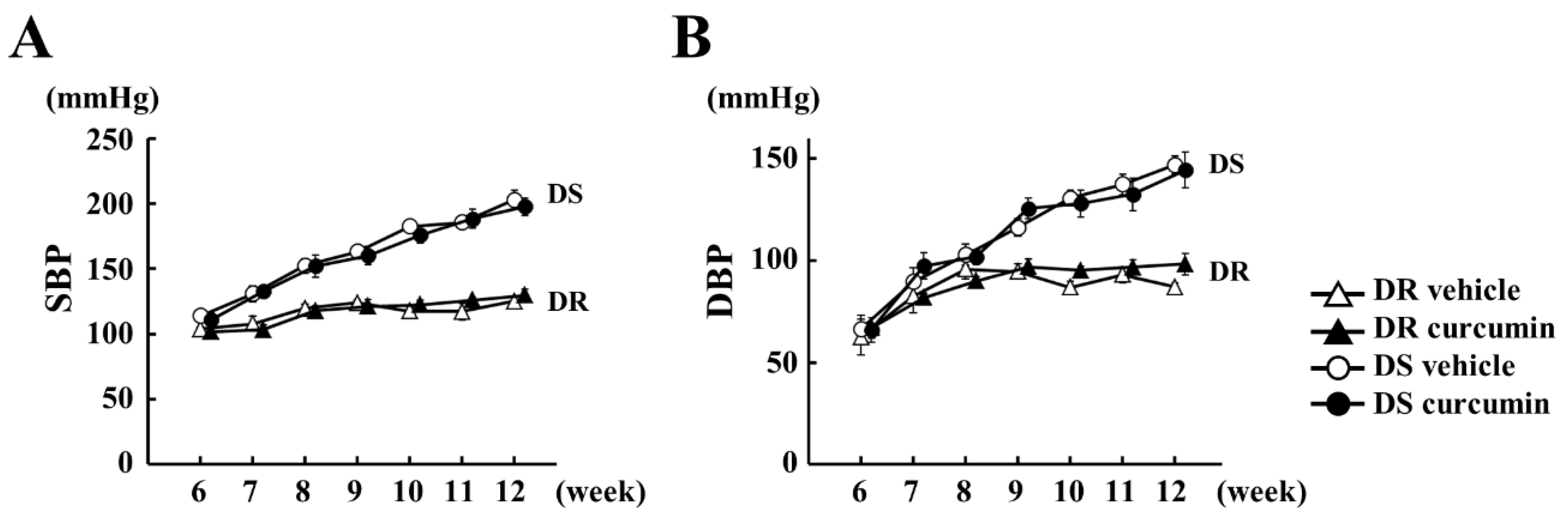
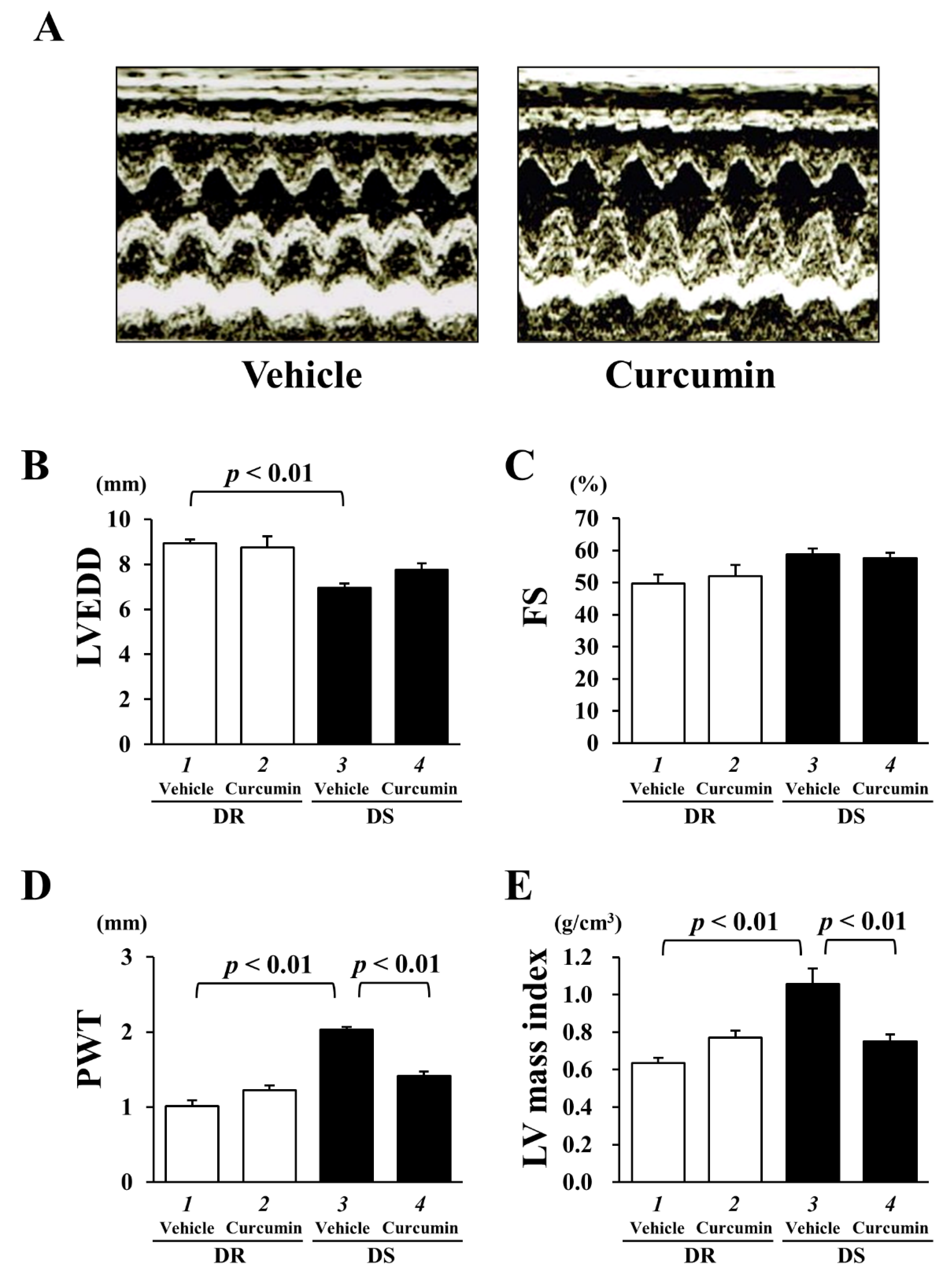
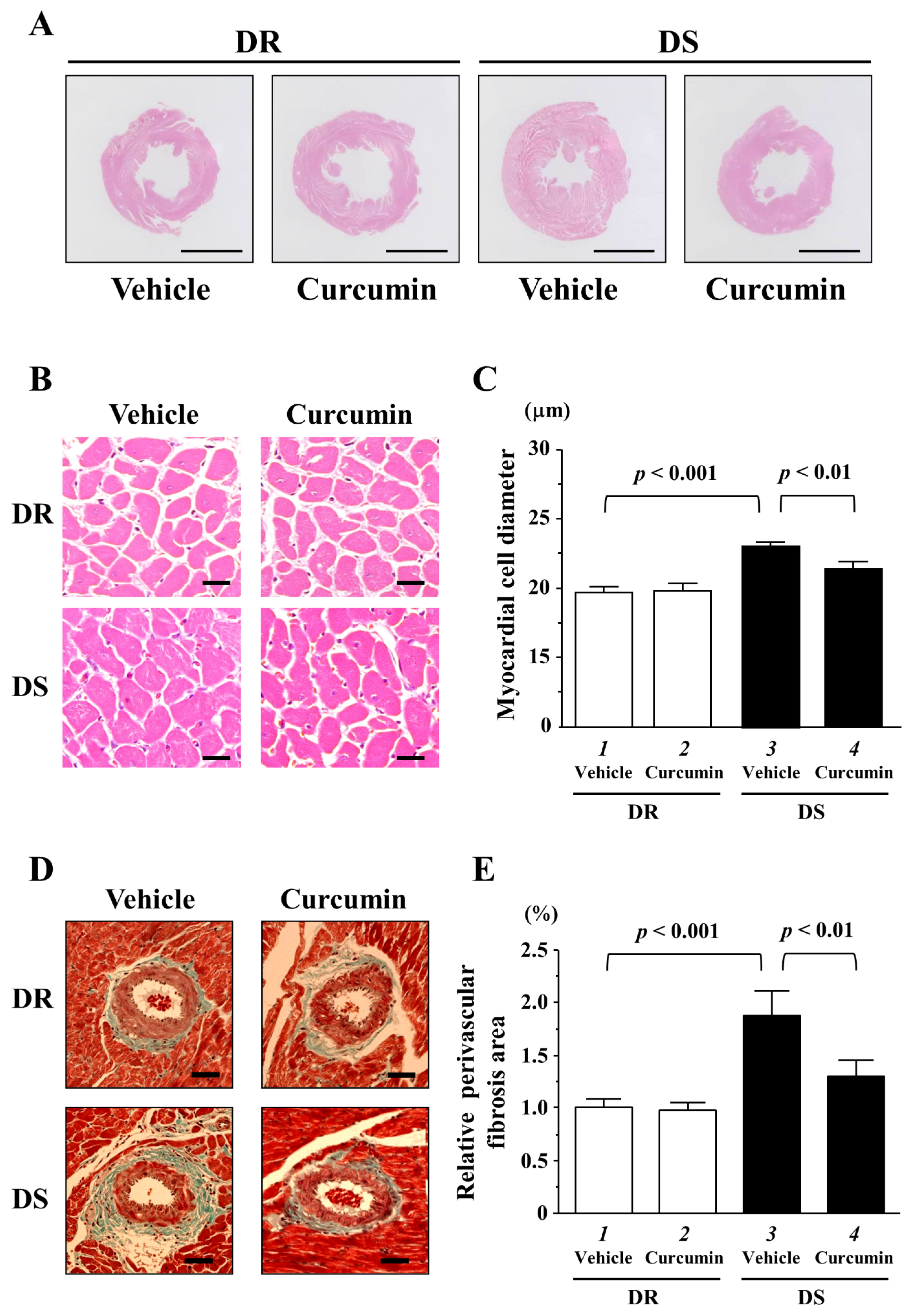
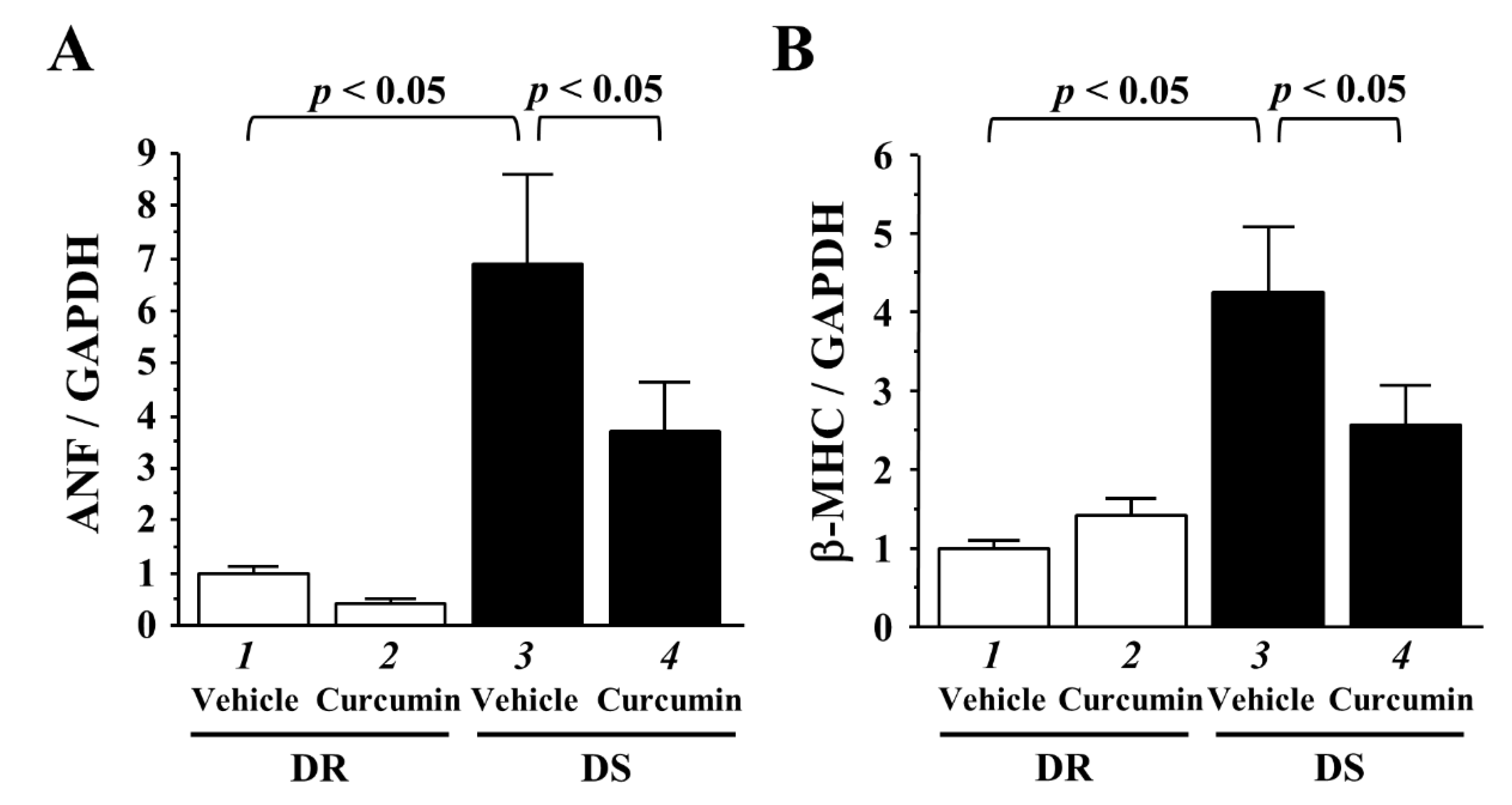
| n | SBP mmHg | DBP mmHg | HR/Min | LVEDD mm | PWT mm | FS % | |
|---|---|---|---|---|---|---|---|
| DR vehicle | 5 | 104 ± 1 | 62 ± 9 | 439 ± 8 | 7.46 ± 0.29 | 0.80 ± 0.06 | 57.6 ± 4.1 |
| DR curcumin | 5 | 102 ± 3 | 67 ± 5 | 426 ± 20 | 7.53 ± 0.20 | 0.88 ± 0.09 | 57.7 ± 2.3 |
| DS vehicle | 8 | 111 ± 3 | 67 ± 6 | 416 ± 22 | 6.86 ± 0.25 | 0.95 ± 0.04 | 61.1 ± 1.8 |
| DS curcumin | 8 | 111 ± 4 | 67 ± 6 | 391 ± 11 | 6.92 ± 0.15 | 1.00 ± 0.06 | 60.9 ± 1.7 |
| BW g | HW g | LVW mg | HW/BW mg/g | LVW/BW mg/g | SBP mmHg | DBP mmHg | HR /min | |
|---|---|---|---|---|---|---|---|---|
| DR vehicle | 376 ± 10 | 1.09 ± 0.03 | 809 ± 29 | 2.91 ± 0.08 | 2.16 ± 0.10 | 125 ± 3 | 89 ± 3 | 390 ± 16 |
| DR curcumin | 378 ± 8 | 1.11 ± 0.03 | 817 ± 17 | 2.93 ± 0.04 | 2.16 ± 0.06 | 129 ± 5 | 96 ± 6 | 378 ± 13 |
| DS vehicle | 319 ± 13 * | 1.24 ± 0.03 * | 965 ± 39 * | 3.91 ± 0.10 * | 3.05 ± 0.11 * | 203 ± 7 * | 147 ± 4 * | 389 ± 10 |
| DS curcumin | 311 ± 6 * | 1.12 ± 0.03 # | 873 ± 28 # | 3.62 ± 0.10 # | 2.68 ± 0.11 # | 198 ± 6 * | 139 ± 9 * | 398 ± 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunagawa, Y.; Funamoto, M.; Shimizu, K.; Shimizu, S.; Sari, N.; Katanasaka, Y.; Miyazaki, Y.; Kakeya, H.; Hasegawa, K.; Morimoto, T. Curcumin, an Inhibitor of p300-HAT Activity, Suppresses the Development of Hypertension-Induced Left Ventricular Hypertrophy with Preserved Ejection Fraction in Dahl Rats. Nutrients 2021, 13, 2608. https://doi.org/10.3390/nu13082608
Sunagawa Y, Funamoto M, Shimizu K, Shimizu S, Sari N, Katanasaka Y, Miyazaki Y, Kakeya H, Hasegawa K, Morimoto T. Curcumin, an Inhibitor of p300-HAT Activity, Suppresses the Development of Hypertension-Induced Left Ventricular Hypertrophy with Preserved Ejection Fraction in Dahl Rats. Nutrients. 2021; 13(8):2608. https://doi.org/10.3390/nu13082608
Chicago/Turabian StyleSunagawa, Yoichi, Masafumi Funamoto, Kana Shimizu, Satoshi Shimizu, Nurmila Sari, Yasufumi Katanasaka, Yusuke Miyazaki, Hideaki Kakeya, Koji Hasegawa, and Tatsuya Morimoto. 2021. "Curcumin, an Inhibitor of p300-HAT Activity, Suppresses the Development of Hypertension-Induced Left Ventricular Hypertrophy with Preserved Ejection Fraction in Dahl Rats" Nutrients 13, no. 8: 2608. https://doi.org/10.3390/nu13082608
APA StyleSunagawa, Y., Funamoto, M., Shimizu, K., Shimizu, S., Sari, N., Katanasaka, Y., Miyazaki, Y., Kakeya, H., Hasegawa, K., & Morimoto, T. (2021). Curcumin, an Inhibitor of p300-HAT Activity, Suppresses the Development of Hypertension-Induced Left Ventricular Hypertrophy with Preserved Ejection Fraction in Dahl Rats. Nutrients, 13(8), 2608. https://doi.org/10.3390/nu13082608






