Dietary Tryptophan Supplementation Alters Fat and Glucose Metabolism in a Low-Birthweight Piglet Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
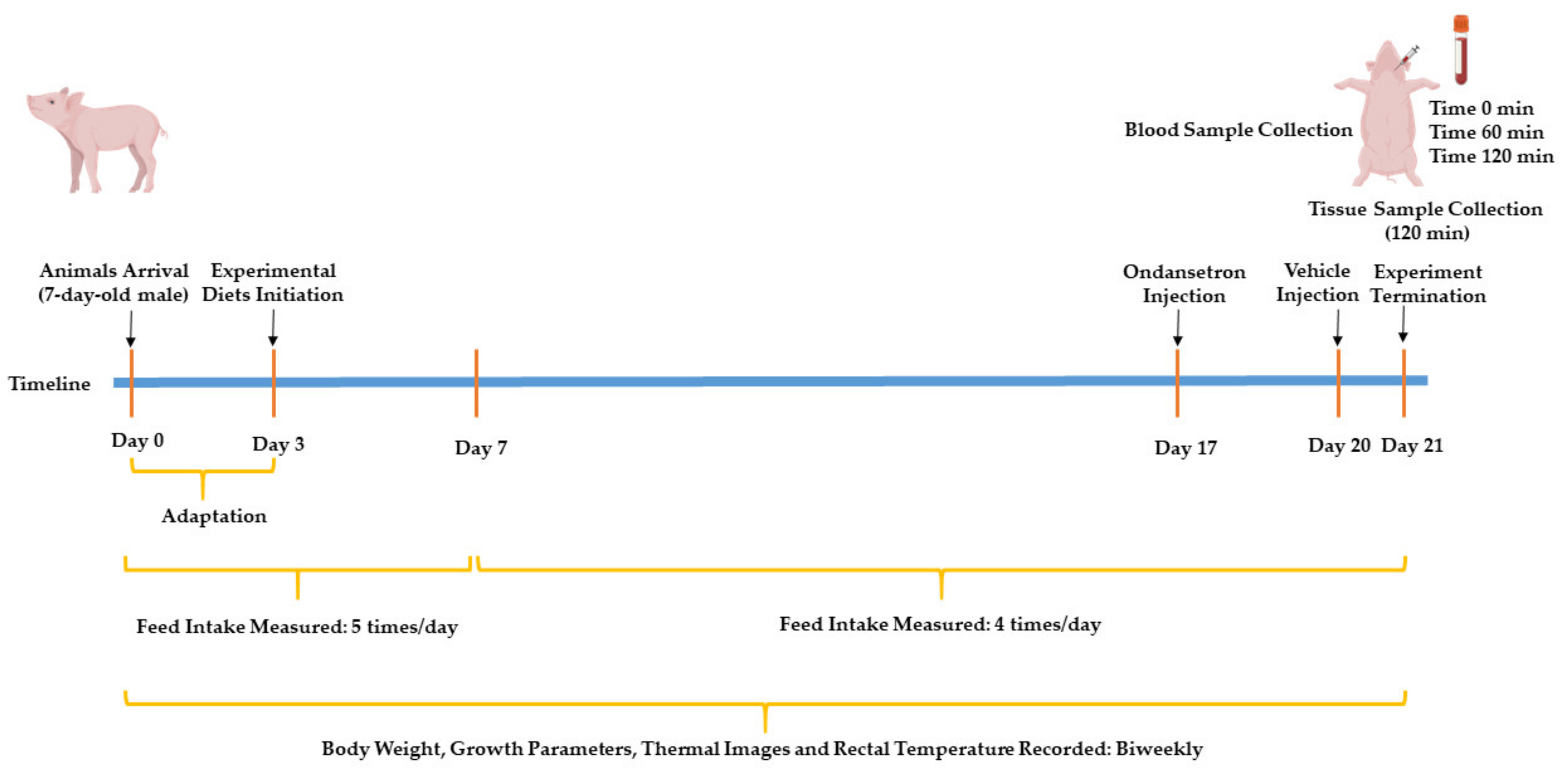
2.2. Experimental Design and Diets
2.2.1. Feed Intake and Growth Measurements
2.2.2. Thermal Images and Rectal Temperature
2.2.3. Blockade of 5-hydroxytryptamine Type 3 (5HT3) Receptors
2.2.4. Feed Samples Collection
2.2.5. Meal Test and Blood and Tissue Collection
2.3. Diet Proximate and Amino Acids Analysis
2.4. Thermal Radiation Analysis
2.5. Plasma Metabolites Analysis
2.6. H&E Staining and Gut Morphology Measurements
2.7. RNA Isolation and RT-qPCR
2.8. Immunoblot Analysis
2.9. Plasma Insulin
2.10. Statistical Analysis
3. Results
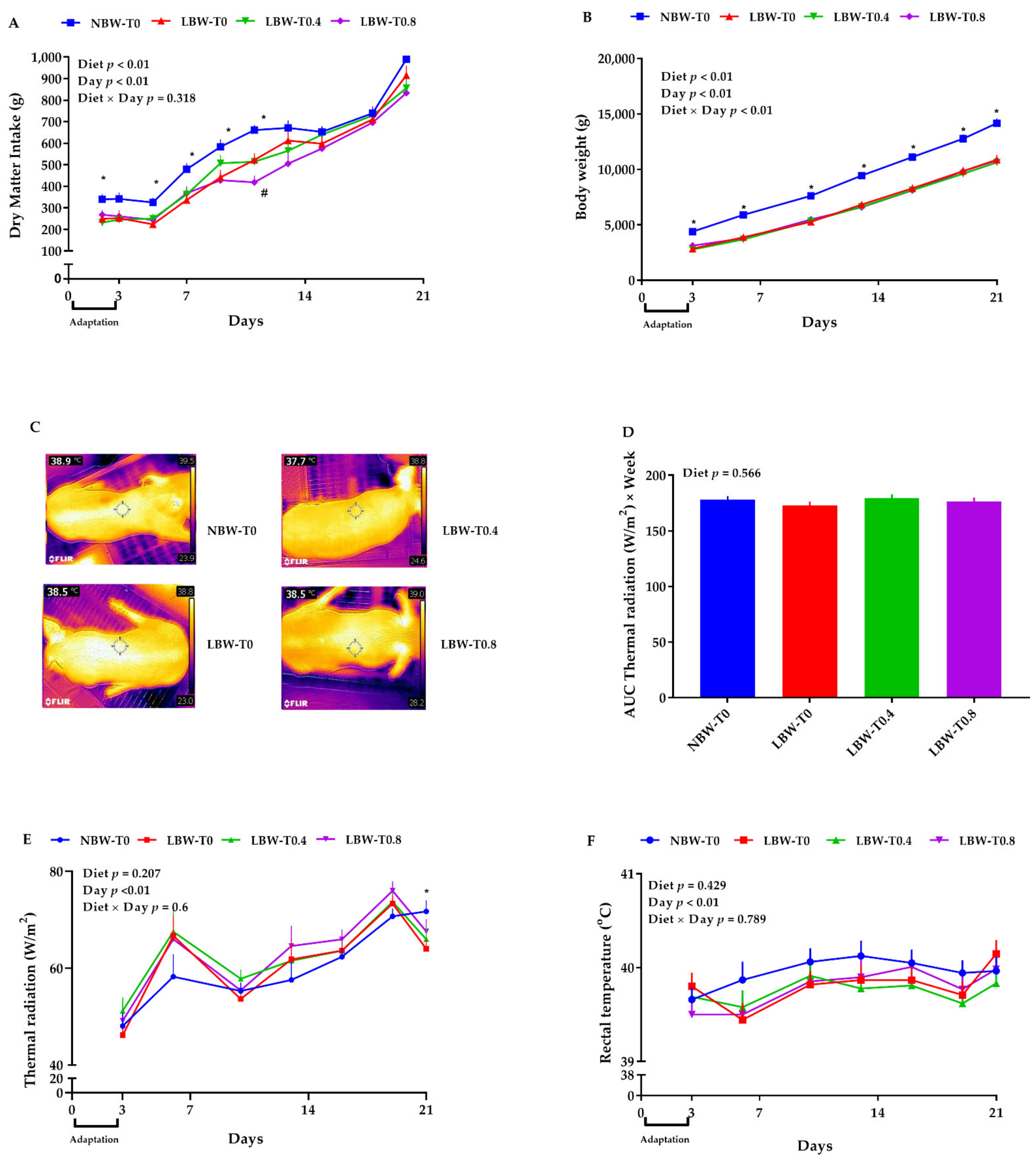
3.1. Feed Intake, Body Weight, and Growth Measurements
3.2. Thermal Radiation
3.3. Gut Histomorphology
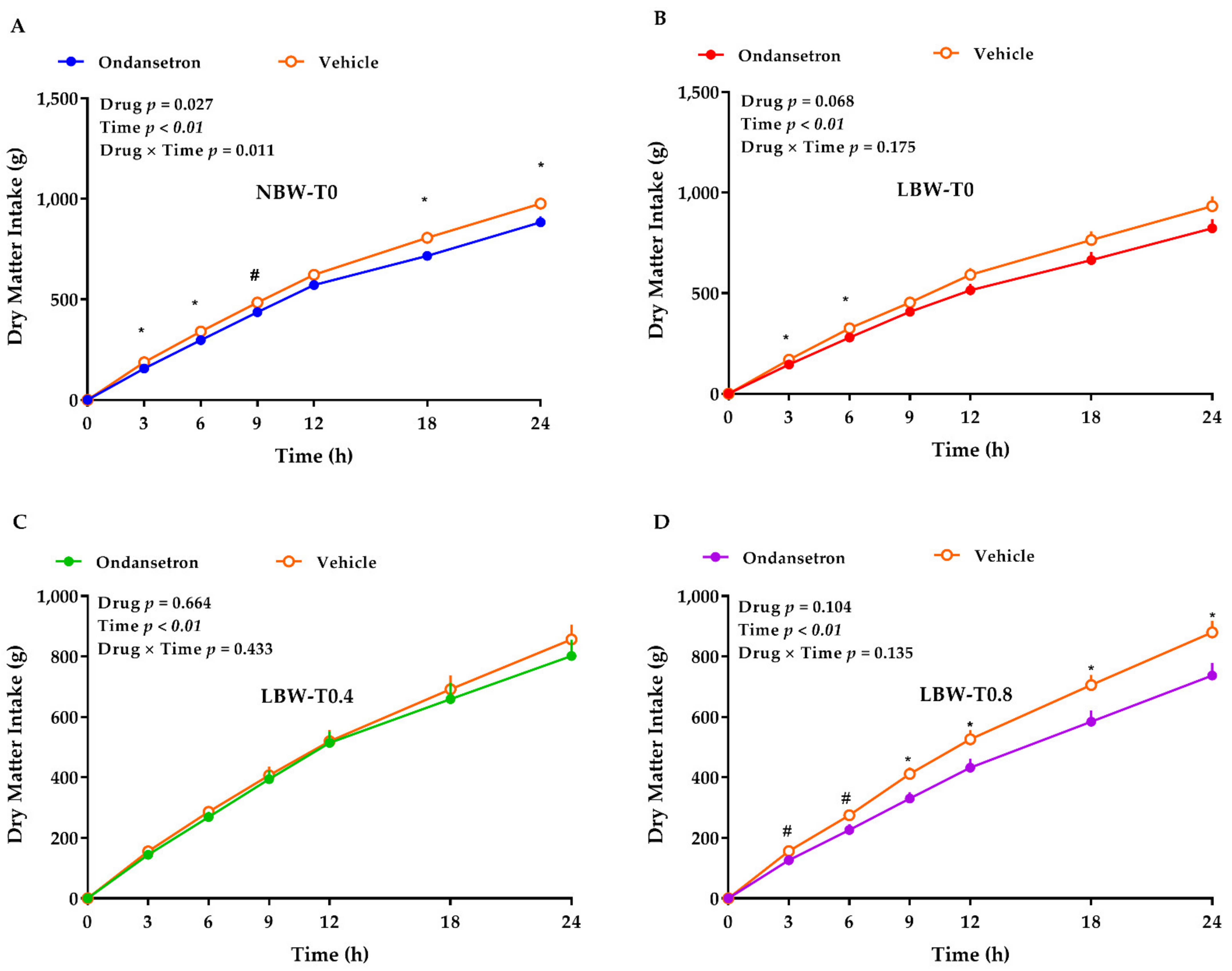
3.4. 5HT3 Receptors Blockade with Ondansetron
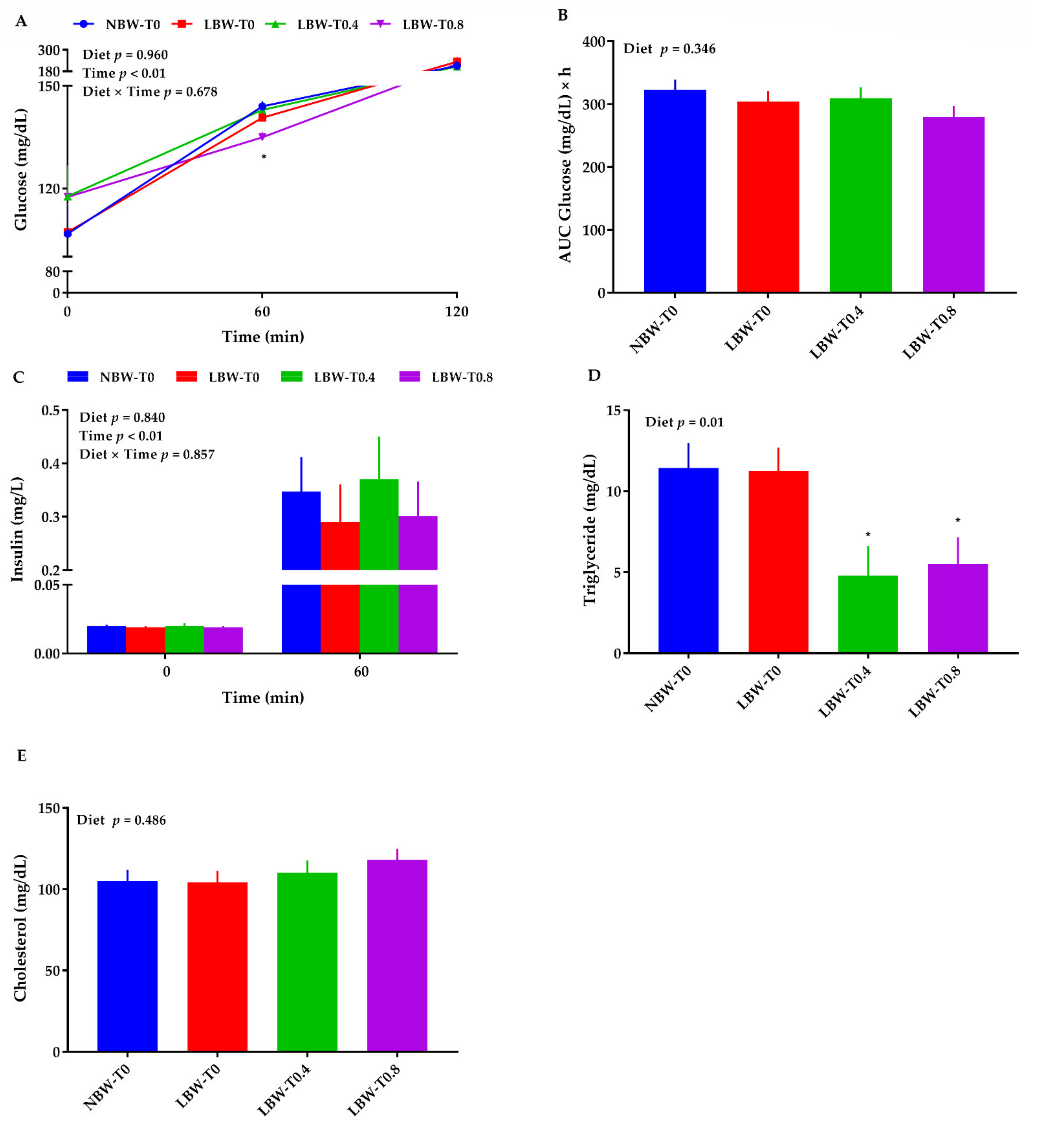
3.5. Blood Glucose, Triglycerides, Cholesterol, and Insulin
3.6. The mRNA Abundance of Key Molecules of Glucose and Lipid Metabolism in the Liver
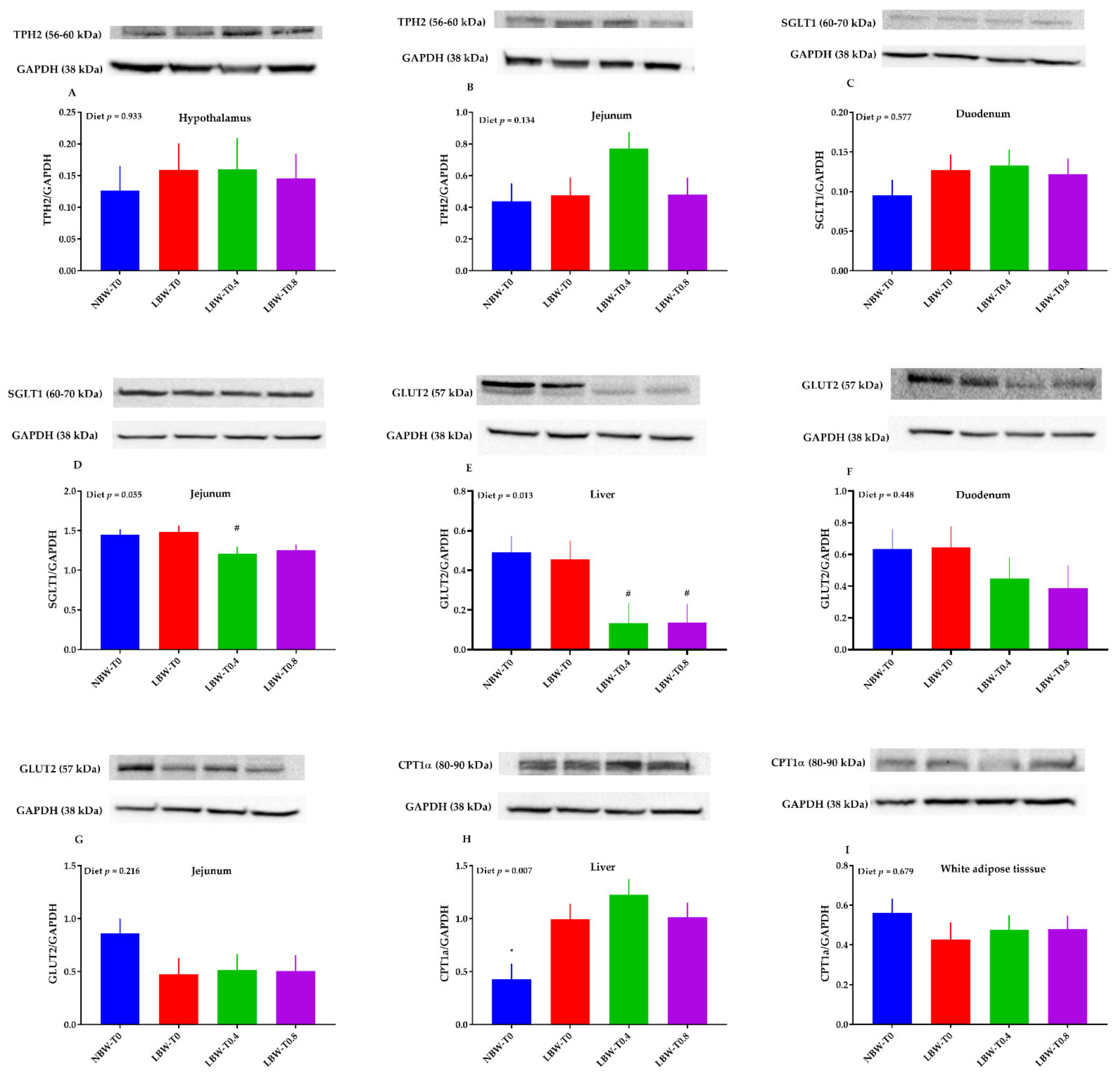
3.7. The Protein Abundance of Key Molecules of Glucose and Lipid Metabolism in the Hypothalamus, Liver, Jejunum, Duodenum, and White Adipose Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ananth, C.V.; Vintzileos, A.M. Distinguishing pathological from constitutional small for gestational age births in population-based studies. Early Hum. Dev. 2009, 85, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, I.; Gabbe, S.G. Intrauterine growth restriction. In Obstetrics: Normal and Problem Pregnancies, 3rd ed.; Gabbe, S.G., Niebyl, J.R., Simpson, J.L., Annas, G.J., Eds.; Churchill Livingstone: New York, NY, USA, 1996; Volume 3, pp. 863–886. [Google Scholar]
- Creasy, R.K.; Resnik, R. Intrauterine growth restriction. In Maternal-Fetal Medicine: Principles and Practice, 3rd ed.; Creasy, R.K., Resnik, R., Eds.; Saunders: Philadelphia, PA, USA, 1994; pp. 558–574. [Google Scholar]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.K.; Driscoll, A.K. Births: Final Data for 2019. National Vital Statistics Reports; National Center for Health Statistics: Hyattsville, MD, USA, 2021; Volume 70. [Google Scholar] [CrossRef]
- Ewing, A.C.; Ellington, S.R.; Shapiro-Mendoza, C.K.; Barfield, W.D.; Kourtis, A.P. Full-term small-for-gestational-age newborns in the US: Characteristics, trends, and morbidity. Matern. Child Health J. 2017, 21, 786–796. [Google Scholar] [CrossRef]
- Fontaine, M.A.; Diane, A.; Singh, V.P.; Mangat, R.; Krysa, J.A.; Nelson, R.; Willing, B.P.; Proctor, S.D. Low birth weight causes insulin resistance and aberrant intestinal lipid metabolism independent of microbiota abundance in Landrace–Large White pigs. FASEB J. 2019, 33, 9250–9262. [Google Scholar] [CrossRef] [PubMed]
- Giapros, V.; Vavva, E.; Siomou, E.; Kolios, G.; Tsabouri, S.; Cholevas, V.; Bairaktari, E.; Tzoufi, M.; Challa, A. Low-birth-weight, but not catch-up growth, correlates with insulin resistance and resistin level in SGA infants at 12 months. J. Matern. Fetal. Neonatal. Med. 2017, 30, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, W.; Wu, G.; Wang, J. Nutritional support for low birth weight infants: Insights from animal studies. Br. J. Nutr. 2017, 117, 1390–1402. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Y.; Tong, X.; Ye, H.; Li, S. Glucose and lipid metabolism in small-for-gestational-age infants at 72 hours of age. J. Clin. Endocrinol. Metab. 2007, 92, 681–684. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, S.; Yang, H.; Gong, F.; Wang, L.; Jiang, Y.; Yan, C.; Zhu, H.; Pan, H. Decreased insulin sensitivity and abnormal glucose metabolism start in preadolescence in low-birth-weight children—Meta-analysis and systematic review. Prim. Care Diabetes 2019, 13, 391–398. [Google Scholar] [CrossRef]
- Lane, R.H.; Flozak, A.S.; Ogata, E.S.; Bell, G.I.; Simmons, R.A. Altered hepatic gene expression of enzymes involved in energy metabolism in the growth-retarded fetal rat. Pediatr. Res. 1996, 39, 390–394. [Google Scholar] [CrossRef]
- Peterside, I.E.; Selak, M.A.; Simmons, R.A. Impaired oxidative phosphorylation in hepatic mitochondria in growth-retarded rats. Am. J. Physiol. Endocrinol. Metab. 2003, 285, 1258–1266. [Google Scholar] [CrossRef]
- Gomo, Z.A.R.; Nyatanga, K.; Chifamba, J.; Chinyanga, H.M.; Taderera, T.; Gwaunza, L.T.; Mushayamano, T.; Mahachi, C. Glucose tolerance study in low and normal birth weight young adults. Cent. Afr. J. Med. 2013, 59, 38–42. [Google Scholar]
- Ying, Z.; Ge, X.; Zhang, H.; Su, W.; Li, Y.; Zhou, L.; Zhang, L.; Wang, T. Effects of dietary methionine restriction on postnatal growth, insulin sensitivity, and glucose metabolism in intrauterine growth retardation pigs at 49 and 105 d of age. J. Anim. Sci. 2019, 97, 610–619. [Google Scholar] [CrossRef]
- Ying, Z.; Zhang, H.; Su, W.; Zhou, L.; Wang, F.; Li, Y.; Zhang, L.; Wang, T. Dietary methionine restriction alleviates hyperglycemia in pigs with intrauterine growth restriction by enhancing hepatic protein kinase B signaling and glycogen synthesis. J. Nutr. 2017, 147, 1892–1899. [Google Scholar] [CrossRef]
- Jones, J.N.; Gercel-Taylor, C.; Taylor, D.D. Altered cord serum lipid levels associated with small for gestational age infants. Obstet. Gynecol. 1999, 93, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, G.; Wang, X.; Wang, T.; Wu, G.; Li, D.; Wang, J. Intrauterine growth restriction alters the hepatic proteome in fetal pigs. J. Nutr. Biochem. 2013, 24, 954–959. [Google Scholar] [CrossRef]
- Cooper, J.E. The use of the pig as an animal model to study problems associated with low birthweight. Lab. Anim. 1975, 9, 329–336. [Google Scholar] [CrossRef]
- Ferenc, K.; Pietrzak, P.; Godlewski, M.M.; Piwowarski, J.; Kilianczyk, R.; Guilloteau, P.; Zabielski, R. Intrauterine growth retarded piglet as a model for humans–studies on the perinatal development of the gut structure and function. Reprod. Biol. 2014, 14, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Eiby, Y.A.; Wright, L.L.; Kalanjati, V.P.; Miller, S.M.; Bjorkman, S.T.; Keates, H.L.; Lumbers, E.R.; Colditz, P.B.; Lingwood, B.E. A pig model of the preterm neonate: Anthropometric and physiological characteristics. PLoS ONE 2013, 8, e68763. [Google Scholar] [CrossRef]
- Odle, J.; Lin, X.; Jacobi, S.K.; Kim, S.W.; Stahl, C.H. The suckling piglet as an agrimedical model for the study of pediatric nutrition and metabolism. Annu. Rev. Anim. Biosci. 2014, 2, 419–444. [Google Scholar] [CrossRef] [PubMed]
- Ardiansyah, S.; Shirakawa, H.; Inagawa, Y.; Koseki, T.; Komai, M. Regulation of blood pressure and glucose metabolism induced by L-tryptophan in stroke-prone spontaneously hypertensive rats. Nutr. Metab. 2011, 8, 1–7. [Google Scholar] [CrossRef]
- Ruan, Z.; Yang, Y.; Wen, Y.; Zhou, Y.; Fu, X.; Ding, S.; Liu, G.; Yao, K.; Wu, X.; Deng, Z.; et al. Metabolomic analysis of amino acid and fat metabolism in rats with l-tryptophan supplementation. Amino Acids 2014, 46, 2681–2691. [Google Scholar] [CrossRef] [PubMed]
- Akiba, Y.; Takahashi, K.; Horiguchi, M.; Ohtani, H.; Saitoh, S.; Ohkawara, H. L-tryptophan alleviates fatty liver and modifies hepatic microsomal mixed function oxidase in laying hens. Comp. Biochem. Physiol. A Physiol. 1992, 102, 769–774. [Google Scholar] [CrossRef]
- Rogers, S.R.; Pesti, G.M. The influence of dietary tryptophan on broiler chick growth and lipid metabolism as mediated by dietary protein levels. Poult. Sci. 1990, 69, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Inubushi, T.; Kamemura, N.; Oda, M.; Sakurai, J.; Nakaya, Y.; Harada, N.; Suenaga, M.; Matsunaga, Y.; Ishidoh, K.; Katunuma, N. L-tryptophan suppresses rise in blood glucose and preserves insulin secretion in type-2 diabetes mellitus rats. J. Nutr. Sci. Vitaminol. 2012, 58, 415–422. [Google Scholar] [CrossRef]
- Willemen, S.A.; Che, L.; Dewilde, S.; Van Hauwaert, M.L.; De Vos, M.; Huygelen, V.; Fransen, E.; Tambuyzer, B.R.; Casteleyn, C.; Van Cruchten, S.; et al. Enteric and serological distribution of serotonin and its precursor tryptophan in perinatal low and normal weight piglets. Animals 2014, 8, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.J.; Roberts, B.L.; Zhao, H.; Zhu, M.; Appleyard, S.M. Serotonin activates catecholamine neurons in the solitary tract nucleus by increasing spontaneous glutamate inputs. J. Neurosci. 2012, 32, 16530–16538. [Google Scholar] [CrossRef] [PubMed]
- Fetissov, S.O.; Meguid, M.M.; Chen, C.; Miyata, G. Synchronized release of dopamine and serotonin in the medial and lateral hypothalamus of rats. Neuroscience 2000, 101, 657–663. [Google Scholar] [CrossRef]
- Lam, D.D.; Garfield, A.S.; Marston, O.J.; Shaw, J.; Heisler, L.K. Brain serotonin system in the coordination of food intake and body weight. Pharmacol. Biochem. Behav. 2010, 97, 84–91. [Google Scholar] [CrossRef]
- Dépôt, M.; Caillé, G.; Mukherjee, J.; Katzman, M.A.; Cadieux, A.; Bradwejn, J. Acute and chronic role of 5-HT3 neuronal system on behavioral and neuroendocrine changes induced by intravenous cholecystokinin tetrapeptide administration in humans. Neuropsychopharmacology 1999, 20, 177–187. [Google Scholar] [CrossRef]
- McGlone, J. Guide for the Care and Use of Agricultural Animals in Research and Teaching, 3rd ed. Available online: https://www.aaalac.org/about/ag_guide_3rd_ed.pdf (accessed on 4 March 2019).
- Rafiee-Tari, N.; Fan, M.Z.; Archbold, T.; Arranz, E.; Corredig, M. Effect of milk protein composition and amount of β-casein on growth performance, gut hormones, and inflammatory cytokines in an in vivo piglet model. J. Dairy Sci. 2019, 102, 8604–8613. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhou, G.; Liao, Z.; Ahmad, H.; Liu, W.; Wang, T. Dietary L-arginine supplementation improves the intestinal development through increasing mucosal Akt and mammalian target of rapamycin signals in intra-uterine growth retarded piglets. Br. J. Nutr. 2012, 108, 1371–1381. [Google Scholar] [CrossRef]
- Zheng, P.; Song, Y.; Tian, Y.; Zhang, H.; Yu, B.; He, J.; Mao, X.; Yu, J.; Luo, Y.; Luo, J.; et al. Dietary arginine supplementation affects intestinal function by enhancing antioxidant capacity of a nitric oxide–independent pathway in low-birth-weight piglets. J. Nutr. 2018, 148, 1751–1759. [Google Scholar] [CrossRef]
- DeRouchey, J.M.; Goodband, R.D.; Tokach, M.D.; Nelssen, J.L.; Dritz, S.S. Nursery Swine Nutrient Recommendations and Feeding Management. In National Swine Nutrition Guide; Meisinger, D.J., Ed.; U.S. Pork Center of Excellence: Ames, IA, USA, 2010; pp. 65–79. [Google Scholar]
- Ebert, A.R.; Berman, A.S.; Harrell, R.J.; Kessler, A.M.; Cornelius, S.G.; Odle, J. Vegetable proteins enhance the growth of milk-fed piglets, despite lower apparent ileal digestibility. J. Nutr. 2005, 135, 2137–2143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Habibi, M.; Shili, C.; Sutton, J.; Goodarzi, P.; Maylem, E.R.; Spicer, L.; Pezeshki, A. Branched-chain amino acids partially recover the reduced growth of pigs fed with protein-restricted diets through both central and peripheral factors. Anim. Nutr. 2021. [Google Scholar] [CrossRef]
- Kim, S.W.; McPherson, R.L.; Wu, G. Dietary arginine supplementation enhances the growth of milk-fed young pigs. J. Nutr. 2004, 134, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.G.; Mueller, S.; Kreuzer, M.; Bigler, M.B.; Silacci, P.; Bee, G. Milk replacers supplemented with either L-arginine or L-carnitine potentially improve muscle maturation of early reared low birth weight piglets from hyperprolific sows. Animals 2018, 12, 43–53. [Google Scholar] [CrossRef]
- Jensen, G.M.; Grøndahl, M.L.; Nielsen, C.G.; Skadhauge, E.; Olsen, J.E.; Hansen, M.B. Effect of ondansetron on Salmonella typhimurium-induced net fluid accumulation in the pig jejunum in vivo. Comp. Biochem. Physiol. A Physiol. 1997, 118, 297–299. [Google Scholar] [CrossRef]
- Spring, S.; Premathilake, H.; Bradway, C.; Shili, C.; DeSilva, U.; Carter, S.; Pezeshki, A. Effect of very low-protein diets supplemented with branched-chain amino acids on energy balance, plasma metabolomics and fecal microbiome of pigs. Sci. Rep. 2020, 10, 15859. [Google Scholar] [CrossRef]
- Spring, S.; Premathilake, H.; DeSilva, U.; Shili, C.; Carter, S.; Pezeshki, A. Low protein-high carbohydrate diets alter energy balance, gut microbiota composition and blood metabolomics profile in young pigs. Sci. Rep. 2020, 10, 3318. [Google Scholar] [CrossRef]
- Shili, C.N.; Broomhead, J.N.; Spring, S.C.; Lanahan, M.B.; Pezeshki, A. A novel corn-expressed phytase improves daily weight gain, protein efficiency ratio and nutrients digestibility and alters fecal microbiota in pigs fed with very low protein diets. Animals 2020, 10, 1926. [Google Scholar] [CrossRef]
- AOAC. Official Method 982.30 E (a, b, c), chp, 45.3. 05 2006. Amino Acid profile without Tryptophan AAP-T; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Bakken, G.S. A heat transfer analysis of animals: Unifying concepts and the application of metabolism chamber data to field ecology. J. Theor. Biol. 1976, 60, 337–384. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Francisco, N.S.; Belloni, M.; Aguirre, G.M.; Caldara, F.R.; Nääs, I.A.; Garcia, R.G.; Almeida Paz, I.C.; Polycarpo, G.V. Infrared thermography applied to the evaluation of metabolic heat loss of chicks fed with different energy densities. Braz. J. Poult. Sci. 2011, 13, 113–118. [Google Scholar] [CrossRef]
- Digalakis, M.; Papamichail, M.; Glava, C.; Grammatoglou, X.; Sergentanis, T.N.; Papalois, A.; Bramis, J. Interposition of a reversed jejunal segment enhances intestinal adaptation in short bowel syndrome: An experimental study on pigs. J. Surg. Res. 2011, 171, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, A.; Muench, G.P.; Chelikani, P.K. Short communication: Expression of peptide YY, proglucagon, neuropeptide Y receptor Y2, and glucagon-like peptide-1 receptor in bovine peripheral tissues. J. Dairy Sci. 2012, 95, 5089–5094. [Google Scholar] [CrossRef]
- Shili, C.N.; Habibi, M.; Sutton, J.; Barnes, J.; Burch-Konda, J.; Pezeshki, A. Effect of a phytogenic water additive on growth performance, blood metabolites and gene expression of amino acid transporters in nursery pigs fed with low-protein/high-carbohydrate diets. Animals 2021, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, J.; Zhang, Y.; Liao, P.; Li, T.; Chen, L.; Yin, Y.; Wang, J.; Wu, G. Oral MSG administration alters hepatic expression of genes for lipid and nitrogen metabolism in suckling piglets. Amino Acids 2014, 46, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Duran-Montgé, P.; Theil, P.K.; Lauridsen, C.; Esteve-Garcia, E. Dietary fat source affects metabolism of fatty acids in pigs as evaluated by altered expression of lipogenic genes in liver and adipose tissues. Animal 2009, 3, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Wang, X.; Wang, Y.; Feng, J. Betaine affects muscle lipid metabolism via regulating the fatty acid uptake and oxidation in finishing pig. J. Anim. Sci. Biotechnol. 2017, 8, 72. [Google Scholar] [CrossRef]
- He, D.; Ma, J.; Long, K.; Wang, X.; Li, X.; Jiang, A.; Li, M. Differential expression of genes related to glucose metabolism in domesticated pigs and wild boar. Biosci. Biotechnol. Biochem. 2017, 81, 1478–1483. [Google Scholar] [CrossRef]
- Kang, P.; Liu, Y.; Zhu, H.; Zhang, J.; Shi, H.; Li, S.; Pi, D.; Leng, W.; Wang, X.; Wu, H.; et al. The effect of dietary asparagine supplementation on energy metabolism in liver of weaning pigs when challenged with lipopolysaccharide. Asian Australas J. Anim. Sci. 2018, 31, 548–555. [Google Scholar] [CrossRef]
- Xie, C.; Wang, Q.; Wang, J.; Tan, B.; Fan, Z.; Deng, Z.Y.; Wu, X.; Yin, Y. Developmental changes in hepatic glucose metabolism in a newborn piglet model: A comparative analysis for suckling period and early weaning period. Biochem. Biophys. Res. Commun. 2016, 470, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Liu, M.; Ren, W.; Duan, J.; Yang, G.; Zhao, Y.; Fang, R.; Chen, L.; Li, T.; Yin, Y. Effects of dietary supplementation with glutamate and aspartate on diquat-induced oxidative stress in piglets. PLoS ONE 2015, 10, e0122893. [Google Scholar] [CrossRef]
- Zuo, F.; Gu, Q.; Li, S.; Wei, H.; Peng, J. Effects of Different Methionine Sources on Methionine Metabolism in the IPEC-J2 Cells. Biomed. Res. Int. 2019, 2019, 5464906. [Google Scholar] [CrossRef]
- Pezeshki, A.; Chelikani, P.K. Effects of Roux-en-Y gastric bypass and ileal transposition surgeries on glucose and lipid metabolism in skeletal muscle and liver. Surg. Obes. Relat. Dis. 2014, 10, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, A.; Zapata, R.C.; Singh, A.; Yee, N.J.; Chelikani, P.K. Low protein diets produce divergent effects on energy balance. Sci. Rep. 2016, 6, 25145. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, A.; Fahim, A.; Chelikani, P.K. Dietary whey and casein differentially affect energy balance, gut hormones, glucose metabolism, and taste preference in diet-induced obese rats. J. Nutr. 2015, 145, 2236–2244. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Pogson, C.L. Tryptophan and the control of plasma glucose concentrations in the rat. Biochem. J. 1977, 168, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.S.; Fitzgerald, P.C.E.; Giesbertz, P.; Steinert, R.E.; Horowitz, M.; Feinle-Bisset, C. Effects of intragastric administration of tryptophan on the blood glucose response to a nutrient drink and energy intake, in lean and obese men. Nutrients 2018, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Ponter, A.A.; Sève, B.; Morgan, L.M. Intragastric tryptophan reduces glycemia after glucose, possibly via glucose-mediated insulinotropic polypeptide, in early-weaned piglets. J. Nutr. 1994, 124, 259–267. [Google Scholar] [CrossRef]
- Hedo, J.A.; Villanueva, M.L.; Marco, J. Elevation of plasma glucose and glucagon after tryptophan ingestion in man. Metabolism 1977, 26, 1131–1134. [Google Scholar] [CrossRef]
- Tsiolakis, D.; Marks, V. The differential effect of intragastric and intravenous tryptophan on plasma glucose, insulin, glucagon, GLI and GIP in the fasted rat. Horm. Metab. Res. 1984, 16, 226–229. [Google Scholar] [CrossRef]
- Lin, H.V.; Efanov, A.M.; Fang, X.; Beavers, L.S.; Wang, X.; Wang, J.; Gonzalez Valcarcel, I.C.; Ma, T. GPR142 controls tryptophan-induced insulin and incretin hormone secretion to improve glucose metabolism. PLoS ONE 2016, 11, e0157298. [Google Scholar] [CrossRef] [PubMed]
- Hajishafiee, M.; Elovaris, R.A.; Jones, K.L.; Heilbronn, L.K.; Horowitz, M.; Poppitt, S.D.; Feinle-Bisset, C. Effects of intragastric administration of L-tryptophan on the glycaemic response to a nutrient drink in men with type 2 diabetes—impacts on gastric emptying, glucoregulatory hormones and glucose absorption. Nutr. Diabetes 2021, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Liang, H.; Chisomo-Kasiya, H.; Mokrani, A.; Ge, X.; Ren, M.; Liu, B. Effects of dietary tryptophan levels on growth performance, whole body composition and gene expression levels related to glycometabolism for juvenile blunt snout bream, Megalobrama amblycephala. Aquac. Nutr. 2018, 24, 1474–1483. [Google Scholar] [CrossRef]
- Iynedjian, P.B. Molecular physiology of mammalian glucokinase. Cell. Mol. Life Sci. 2009, 66, 27–42. [Google Scholar] [CrossRef]
- Zelent, B.; Odili, S.; Buettger, C.; Shiota, C.; Grimsby, J.; Taub, R.; Magnuson, M.A.; Vanderkooi, J.M.; Matschinsky, F.M. Sugar binding to recombinant wild-type and mutant glucokinase monitored by kinetic measurement and tryptophan fluorescence. Biochem. J. 2008, 413, 269–280. [Google Scholar] [CrossRef]
- Ray, P.D.; Foster, D.O.; Lardy, H.A. Paths of carbon in gluconeogenesis and lipogenesis: IV. Inhibition by L-tryptophan of hepatic gluconeogenesis at the level of phosphoenolpyruvate formation. J. Biol. Chem. 1966, 241, 3904–3908. [Google Scholar] [CrossRef]
- Foster, D.O.; Ray, P.D.; Lardy, H.A. A paradoxical in vivo effect of L-tryptophan on the phosphoenolpyruvate carboxykinase of rat liver. Biochemistry 1966, 5, 563–569. [Google Scholar] [CrossRef]
- Rippe, D.F.; Berry, L.J. Effect of endotoxin on the activation of phosphoenolpyruvate carboxykinase by tryptophan. Infect. Immun. 1972, 6, 97–98. [Google Scholar] [CrossRef]
- Quinn, P.G.; Yeagley, D. Insulin regulation of PEPCK gene expression: A model for rapid and reversible modulation. Curr. Drug Targets-Immune Endocr. Metabol. Disord. 2005, 5, 423–437. [Google Scholar] [CrossRef]
- Karim, S.; Adams, D.H.; Lalor, P.F. Hepatic expression and cellular distribution of the glucose transporter family. World J. Gastroenterol. 2012, 18, 6771–6781. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Sounidaki, M.; Tsogka, K.; Antoniadis, N.; Antoniadi, G.; Liakopoulos, V.; Stefanidis, I. Indoleamine 2, 3-dioxygenase, by degrading L-tryptophan, enhances carnitine palmitoyltransferase I activity and fatty acid oxidation, and exerts fatty acid-dependent effects in human alloreactive CD4+ T-cells. Int. J. Mol. Med. 2016, 38, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Huang, F.F.; Qu, S. Melatonin: A potential intervention for hepatic steatosis. Lipids Health Dis. 2015, 14, 75. [Google Scholar] [CrossRef]
- Nishida, S.; Segawa, T.; Murai, I.; Nakagawa, S. Long-term melatonin administration reduces hyperinsulinemia and improves the altered fatty-acid compositions in type 2 diabetic rats via the restoration of Δ-5 desaturase activity. J. Pineal Res. 2002, 32, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Raka, F.; Adeli, K. The role of the gut microbiota in lipid and lipoprotein metabolism. J. Clin. Med. 2019, 8, 2227. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Kratz, M.; Damman, C.J.; Hullarg, M. Mechanisms linking the gut microbiome and glucose metabolism. J. Clin. Endocrinol. Metab. 2016, 101, 1445–1454. [Google Scholar] [CrossRef]
- Wenninger, J.; Meinitzer, A.; Holasek, S.; Schnedl, W.J.; Zelzer, S.; Mangge, H.; Herrmann, M.; Enko, D. Associations between tryptophan and iron metabolism observed in individuals with and without iron deficiency. Sci. Rep. 2019, 9, 14548. [Google Scholar] [CrossRef]
- Wessels, A.G.; Kluge, H.; Hirche, F.; Kiowski, A.; Bartelt, J.; Corrent, E.; Stangl, G.I. High leucine intake reduces the concentration of hypothalamic serotonin in piglets. J. Anim. Sci. 2016, 94, 26–29. [Google Scholar] [CrossRef]
- Van der Hoek, G.A.; Cooper, S.J. Ondansetron, a selective 5-HT3 receptor antagonist, reduces palatable food consumption in the nondeprived rat. Neuropharmacology 1994, 33, 805–811. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging roles for serotonin in regulating metabolism: New implications for an ancient molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Namkung, J.; Kim, H.; Park, S. Peripheral serotonin: A new player in systemic energy homeostasis. Mol. Cell. 2015, 38, 1023–1028. [Google Scholar] [CrossRef]
- Schoenichen, C.; Bode, C.; Duerschmied, D. Role of platelet serotonin in innate immune cell recruitment. Front. Biosci. 2019, 24, 514–526. [Google Scholar] [CrossRef]
- Christensen, R.D.; Henry, E.; Wiedmeier, S.E.; Stoddard, R.A.; Sola-Visner, M.C.; Lambert, D.K.; Kiehn, T.I.; Ainsworth, S. Thrombocytopenia among extremely low birth weight neonates: Data from a multihospital healthcare system. J. Perinatol. 2006, 26, 348–353. [Google Scholar] [CrossRef] [PubMed]



| Measurements | Diets 1 | SEM 2 | p-Value | |||
|---|---|---|---|---|---|---|
| NBW-T0 | LBW-T0 | LBW-T0.4 | LBW-T0.8 | |||
| Initial BW 3, g | 4393 * | 2853 | 2783 | 3123 | 145 | <0.01 |
| Final BW, g | 14,172 * | 10,877 | 10,634 | 10,818 | 335 | <0.01 |
| ADG 3, g/day | 543 * | 445 | 436 | 427 | 12 | <0.01 |
| ADMI 3, g/day | 554 * | 422 | 421 | 399 | 14 | <0.01 |
| ADPI 3, g/day | 119 * | 94 | 99 | 83 * | 2.7 | <0.01 |
| G:F 3, g/g | 1.00 | 1.05 | 0.97 | 1.07 | 0.01 | 0.26 |
| G:P 3, g/g | 4.65 | 4.77 | 4.30 | 5.14 | 0.10 | 0.02 |
| Heart girth, cm | 55.12 * | 50.00 | 50.42 | 51.37 | 0.53 | <0.01 |
| Wither height, cm | 34.75 * | 32.00 | 31.14 | 31.25 | 0.46 | <0.01 |
| Body length, cm | 54.00 * | 48.62 | 46.42 | 47.37 | 0.66 | <0.01 |
| Measurements | Diets 1 | SEM 2 | p-Value | |||
|---|---|---|---|---|---|---|
| NBW-T0 | LBW-T0 | LBW-T0.4 | LBW-T0.8 | |||
| Duodenum | ||||||
| Villi height, µm | 562 | 497 | 585 | 530 | 20 | 0.44 |
| Villi width, µm | 138 | 124 | 138 | 123 | 3 | 0.13 |
| Crypt depth, µm | 229 | 224 | 230 | 198 | 8 | 0.54 |
| Crypt width, µm | 64 | 55 | 60 | 55 | 2 | 0.12 |
| Muscle thickness, µm | 385 | 376 | 447 | 386 | 17 | 0.46 |
| Villi height:Crypt depth, µm:µm | 2.90 # | 2.03 | 2.57 | 2.69 | 0.13 | 0.13 |
| Jejunum | ||||||
| Villi height, µm | 619 | 595 | 589 | 594 | 28 | 0.98 |
| Villi width, µm | 121 | 106 | 118 | 125 | 3 | 0.23 |
| Crypt depth, µm | 200 | 211 | 221 | 256 # | 8 | 0.02 |
| Crypt width, µm | 48 | 49 | 53 | 55 | 1 | 0.24 |
| Muscle thickness, µm | 323 | 367 | 410 | 463 | 22 | 0.12 |
| Villi height:Crypt depth, µm:µm | 2.82 | 3.03 | 2.65 | 2.50 | 0.12 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goodarzi, P.; Habibi, M.; Roberts, K.; Sutton, J.; Shili, C.N.; Lin, D.; Pezeshki, A. Dietary Tryptophan Supplementation Alters Fat and Glucose Metabolism in a Low-Birthweight Piglet Model. Nutrients 2021, 13, 2561. https://doi.org/10.3390/nu13082561
Goodarzi P, Habibi M, Roberts K, Sutton J, Shili CN, Lin D, Pezeshki A. Dietary Tryptophan Supplementation Alters Fat and Glucose Metabolism in a Low-Birthweight Piglet Model. Nutrients. 2021; 13(8):2561. https://doi.org/10.3390/nu13082561
Chicago/Turabian StyleGoodarzi, Parniyan, Mohammad Habibi, Kennedy Roberts, Julia Sutton, Cedrick Ndhumba Shili, Dingbo Lin, and Adel Pezeshki. 2021. "Dietary Tryptophan Supplementation Alters Fat and Glucose Metabolism in a Low-Birthweight Piglet Model" Nutrients 13, no. 8: 2561. https://doi.org/10.3390/nu13082561
APA StyleGoodarzi, P., Habibi, M., Roberts, K., Sutton, J., Shili, C. N., Lin, D., & Pezeshki, A. (2021). Dietary Tryptophan Supplementation Alters Fat and Glucose Metabolism in a Low-Birthweight Piglet Model. Nutrients, 13(8), 2561. https://doi.org/10.3390/nu13082561









