Nutritional Status of Children with Cerebral Palsy in Gorkha, Nepal: Findings from the Nepal Cerebral Palsy Register
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Settings
2.2. Measures
2.3. Clinical Assessment
2.4. Anthropometric Measurements
2.5. Indicators Used to Define the Nutritional Status of Children
2.6. Statistical Analysis
3. Results
3.1. Overall Nutritional Status
3.2. Socio-Demographic Characteristics and Nutritional Status of Children with CP
3.3. Clinical Characteristics and Nutritional Status of Children with CP
3.3.1. Birthweight and Gestational Age
3.3.2. Timing of CP and Age of CP Diagnosis
3.3.3. Predominant Type, Topography, Motor Function Severity, Motor Speech Disorder and Functional Communication
3.3.4. Associated Impairments
3.4. Predictors of Underweight, Stuntin and Thinness Among Children with CP
3.4.1. Underweight
3.4.2. Stunting
3.4.3. Thinness
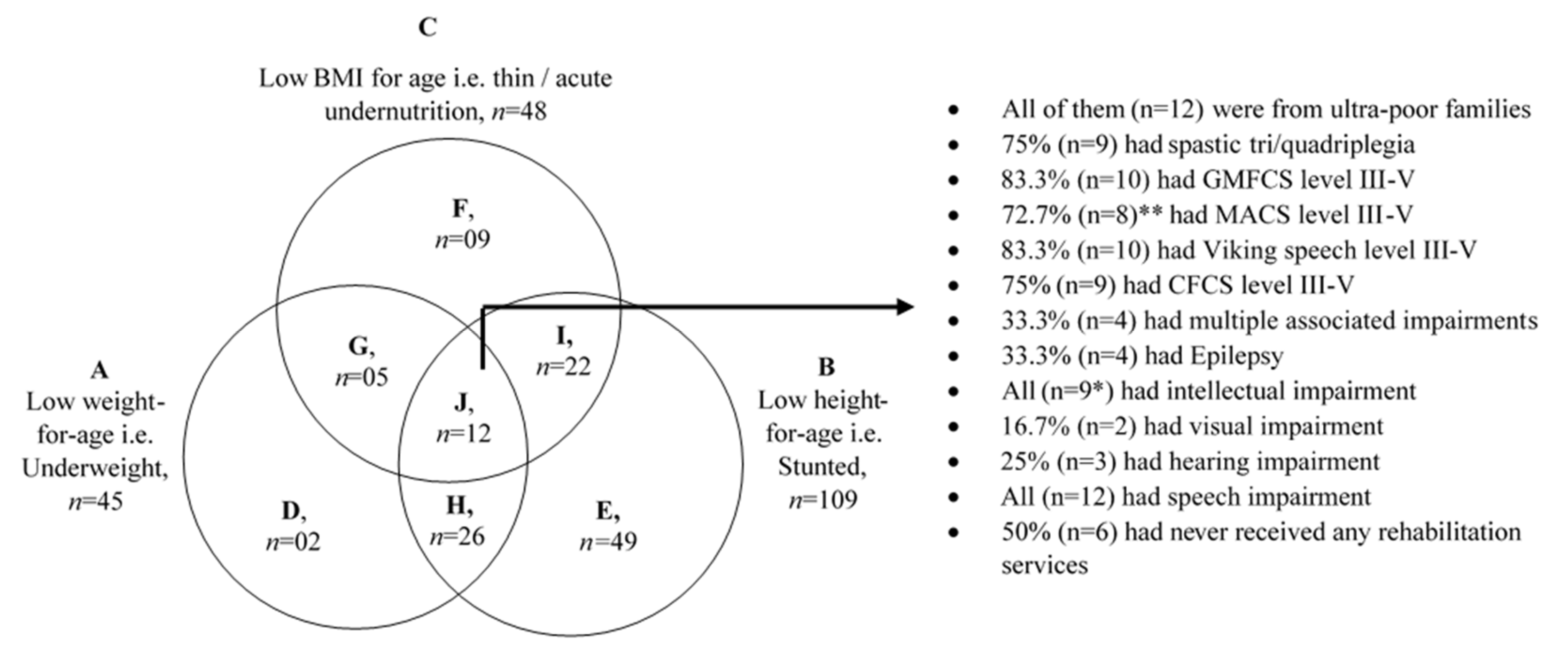
3.5. Children with Multiple Forms of Undernutrition
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. Suppl. 2007, 109, 8–14. [Google Scholar]
- Jahan, I.; Muhit, M.; Karim, T.; Smithers-Sheedy, H.; Novak, I.; Jones, C.; Badawi, N.; Khandaker, G. What makes children with cerebral palsy vulnerable to malnutrition? Findings from the Bangladesh cerebral palsy register (BCPR). Disabil. Rehabil. 2019, 41, 2247–2254. [Google Scholar] [CrossRef]
- Jahan, I.; Muhit, M.; Hardianto, D.; Karim, T.; Al Imam, M.H.; Das, M.C.; Smithers-Sheedy, H.; Badawi, N.; Khandaker, G. Nutritional status of children with cerebral palsy in remote Sumba Island of Indonesia: A community-based key informants study. Disabil. Rehabil. 2019, 43, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Karim, T.; Jahan, I.; Dossetor, R.; Giang, N.T.; Van Anh, N.T.; Dung, T.Q.; Chau, C.M.; Van Bang, N.; Badawi, N.; Khandaker, G.; et al. Nutritional status of children with cerebral palsy—Findings from prospective hospital-based surveillance in Vietnam indicate a need for action. Nutrients 2019, 11, 2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Power, R.; Muhit, M.; Heanoy, E.; Karim, T.; Badawi, N.; Akhter, R.; Khandaker, G. Health-related quality of life and mental health of adolescents with cerebral palsy in rural Bangladesh. PLoS ONE 2019, 14, e0217675. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; Karim, T.; Das, M.C.; Muhit, M.; Mcintyre, S.; Smithers-Sheedy, H.; Badawi, N.; Khandaker, G. Mortality in children with cerebral palsy in rural Bangladesh: A population-based surveillance study. Dev. Med. Child Neurol. 2019, 61, 1336–1343. [Google Scholar] [CrossRef]
- Aggarwal, S.; Chadha, R.; Pathak, R. Nutritional status and growth in children with cerebral palsy: A review. Int. J. Med. Sci. Public Health 2015, 4, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Aydin, K.; Akbas, Y.; Unay, B.; Arslan, M.; Cansu, A.; Sahin, S.; Dilber, C.; Gungor, O.; Aksoy, A.; Yuksel, D.; et al. A multicenter cross-sectional study to evaluate the clinical characteristics and nutritional status of children with cerebral palsy. Clin. Nutr. ESPEN 2018, 26, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kakooza-Mwesige, A.; Tumwine, J.K.; Eliasson, A.C.; Namusoke, H.K.; Forssberg, H. Malnutrition is common in Ugandan children with cerebral palsy, particularly those over the age of five and those who had neonatal complications. Acta Paediatr. 2015, 104, 1259–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Léonard, M.; Dain, E.; Pelc, K.; Dan, B.; De Laet, C. Nutritional status of neurologically impaired children: Impact on comorbidity. Arch. Pediatr. 2020, 27, 95–103. [Google Scholar] [CrossRef]
- Wang, F.; Cai, Q.; Shi, W.; Jiang, H.; Li, N.; Ma, D.; Wang, Q.; Luo, R.; Mu, D. A cross-sectional survey of growth and nutritional status in children with cerebral palsy in West China. Pediatr. Neurol. 2016, 58, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Karagiozoglou-Lampoudi, T.; Daskalou, E.; Vargiami, E.; Zafeiriou, D. Identification of feeding risk factors for impaired nutrition status in paediatric patients with cerebral palsy. Acta Paediatr. 2012, 101, 649–654. [Google Scholar] [CrossRef]
- Khandaker, G.; Muhit, M.; Karim, T.; Smithers-Sheedy, H.; Novak, I.; Jones, C.; Badawi, N. Epidemiology of cerebral palsy in Bangladesh: A population-based surveillance study. Dev. Med. Child Neurol. 2019, 61, 601–609. [Google Scholar] [CrossRef] [Green Version]
- Oskoui, M.; Coutinho, F.; Dykeman, J.; Jetté, N.; Pringsheim, T. An update on the prevalence of cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2013, 55, 509–519. [Google Scholar] [CrossRef]
- Herrera-Anaya, E.; Angarita-Fonseca, A.; Herrera-Galindo, V.M.; Martínez-Marín, R.D.; Rodríguez-Bayona, C.N. Association between gross motor function and nutritional status in children with cerebral palsy: A cross-sectional study from Colombia. Dev. Med. Child Neurol. 2016, 58, 936–941. [Google Scholar] [CrossRef] [Green Version]
- Banskota, B.; Shrestha, S.; Rajbhandari, T.; Banskota, A.; Spiegel, D. A snapshot of 1001 children presenting with cerebral palsy to a children’s disability hospital. J. Nepal. Health Res. Counc. 2015, 13, 31–37. [Google Scholar] [PubMed]
- Thapa, R. Retrospective descriptive study of cerebral palsy in Nepal. J. Autism Dev. Disord. 2016, 46, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- LeBrun, D.G.; Banskota, B.; Banskota, A.K.; Rajbhandari, T.; Baldwin, K.D.; Spiegel, D.A. Socioeconomic status influences functional severity of untreated cerebral palsy in Nepal: A prospective analysis and systematic review. Clin. Orthop. Relat. Res. 2019, 477, 10. [Google Scholar] [CrossRef] [PubMed]
- Local Level Authorities, Nepal. 2017. Available online: http://rapnepal.com/sites/default/files/report-publication/local_govt_of_75_districts23mar2017.pdf (accessed on 18 July 2021).
- Central Bureau of Statistics. National Population and Housing Census 2011 (National Report). 2012. Available online: https://unstats.un.org/unsd/demographic-social/census/documents/Nepal/Nepal-Census-2011-Vol1.pdf (accessed on 18 July 2021).
- Mackey, S.; Murthy, G.V.; Muhit, M.A.; Islam, J.J.; Foster, A. Validation of the key informant method to identify children with disabilities: Methods and results from a pilot study in Bangladesh. J. Trop. Pediatr. 2012, 58, 269–274. [Google Scholar] [CrossRef]
- Cans, C. Surveillance of cerebral palsy in Europe: A collaboration of cerebral palsy surveys and registers. Dev. Med. Child Neurol. 2000, 42, 816–824. [Google Scholar] [CrossRef]
- ACPR. Report of the Australian Cerebral Palsy Register, Birth Years 1995–2012. Australia, 12 December 2018. Available online: https://www.ausacpdm.org.au/resources/australian-cerebral-palsy-register/ (accessed on 18 July 2021).
- Rosenbaum, P.L.; Palisano, R.J.; Bartlett, D.J.; Galuppi, B.E.; Russell, D.J. Development of the gross motor function classification system for cerebral palsy. Dev. Med. Child Neurol. 2008, 50, 249–253. [Google Scholar] [CrossRef]
- Eliasson, A.C.; Krumlinde-Sundholm, L.; Rösblad, B.; Beckung, E.; Arner, M.; Öhrvall, A.M.; Rosenbaum, P. The Manual Ability Classification System (MACS) for children with cerebral palsy: Scale development and evidence of validity and reliability. Dev. Med. Child. Neurol. 2006, 48, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Pennington, L.; Virella, D.; Mjøen, T.; da Graça Andrada, M.; Murray, J.; Colver, A.; Himmelmann, K.; Rackauskaite, G.; Greitane, A.; Prasauskiene, A.; et al. Development of The Viking Speech Scale to classify the speech of children with cerebral palsy. Res. Dev. Disabil. 2013, 34, 3202–3210. [Google Scholar] [CrossRef]
- Hidecker, M.J.; Paneth, N.; Rosenbaum, P.L.; Kent, R.D.; Lillie, J.; Eulenberg, J.B.; Chester, J.R.K.E.; Johnson, B.; Michalsen, L.; Evatt, M.; et al. Developing and validating the Communication Function Classification System for individuals with cerebral palsy. Dev. Med. Child Neurol. 2011, 53, 704–710. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Expert Committee on Physical Status: The Use and Interpretation of Anthropometry. Switzerland, 1995. Available online: https://apps.who.int/iris/bitstream/handle/10665/37003/WHO_TRS_854.pdf;jsessionid=08FC457AB2A52609E61DE1F499FAF4BE?sequence=1 (accessed on 18 July 2021).
- Stevenson, R.D. Use of segmental measures to estimate stature in children with cerebral palsy. Arch. Pediatr. Adolesc. Med. 1995, 149, 658–662. [Google Scholar] [CrossRef]
- Ministry of Health, Nepal; New ERA; ICF. Nepal Demographic and Health Survey 2016. Ministry of Health, Nepal: Kathmandu, Nepal, 2017. Available online: https://www.dhsprogram.com/pubs/pdf/fr336/fr336.pdf (accessed on 18 July 2021).
- Polack, S.; Adams, M.; O’banion, D.; Baltussen, M.; Asante, S.; Kerac, M.; Gladstone, M.; Zuurmond, M. Children with cerebral palsy in Ghana: Malnutrition, feeding challenges, and caregiver quality of life. Dev. Med. Child Neurol. 2018, 60, 914–921. [Google Scholar] [CrossRef]
- Ruiz Brunner, M.D.; Cieri, M.E.; Rodriguez Marco, M.P.; Schroeder, A.S.; Cuestas, E. Nutritional status of children with cerebral palsy attending rehabilitation centers. Dev. Med. Child Neurol. 2020, 62, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Dahlseng, M.O.; Finbråten, A.K.; Júlíusson, P.B.; Skranes, J.; Andersen, G.; Vik, T. Feeding problems, growth and nutritional status in children with cerebral palsy. Acta Paediatr. 2012, 101, 92–98. [Google Scholar] [CrossRef]
- Benfer, K.A.; Weir, K.A.; Bell, K.L.; Ware, R.S.; Davies, P.S.; Boyd, R.N. Oropharyngeal dysphagia and gross motor skills in children with cerebral palsy. Pediatrics 2013, 131, e1553–e1562. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.H.; Chung, C.Y.; Lee, K.M.; Cho, B.C.; Moon, S.J.; Kim, J.; Park, M.S. Differences in body composition according to gross motor function in children with cerebral palsy. Arch. Phys. Med. Rehabil. 2017, 98, 2295–2300. [Google Scholar] [CrossRef]
- Walker, J.L.; Bell, K.L.; Boyd, R.N.; Davies, P.S. Energy requirements in preschool-age children with cerebral palsy. Am. J. Clin. Nutr. 2012, 96, 1309–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpato, E.; Staiano, A.; Molteni, M.; Terrone, G.; Mazzocchi, A.; Agostoni, C. Nutritional assessment and intervention in children with cerebral palsy: A practical approach. Int. J. Food Sci. Nutr. 2017, 68, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Mlinda, S.J.; Leyna, G.H.; Massawe, A. The effect of a practical nutrition education programme on feeding skills of caregivers of children with cerebral palsy at Muhimbili National Hospital, in Tanzania. Child Care Health Dev. 2018, 44, 452–461. [Google Scholar] [CrossRef]
- Donkor, C.M.; Lee, J.; Lelijveld, N.; Adams, M.; Baltussen, M.M.; Nyante, G.G.; Kerac, M.; Polack, S.; Zuurmond, M. Improving nutritional status of children with Cerebral palsy: A qualitative study of caregiver experiences and community-based training in Ghana. Food Sci. Nutr. 2019, 7, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banks, L.M.; Walsham, M.; Neupane, S.; Neupane, S.; Pradhananga, Y.; Maharjan, M.; Blanchet, K.; Kuper, H. Access to social protection among people with disabilities: Mixed methods research from Tanahun, Nepal. Eur. J. Dev. Res. 2019, 31, 929–956. [Google Scholar] [CrossRef] [Green Version]
| Indicator | Weight-for-Age z Score (WAZ) | Height-for-Age z Score (HAZ) | BMI-for-Age z Score (BAZ) | Weight-for-Height-z Score (WHZ) | MUAC-for-Age-z Score (MUACZ) |
|---|---|---|---|---|---|
| n | 87 1 | 170 2 | 164 3 | 26 2,4 | 28 2,4 |
| Mean (SD) | −2.2 (1.9) | −2.9 (2.6) | −0.5 (4.1) | −0.5 (1.6) | −0.9 (1.4) |
| Median (IQR) | −2.1 (−3.8, −0.9) | −2.8 (−4.5, −1.4) | −1.1 (−2.5, 0.6) | −0.1 (−1.7, 0.5) | −0.6 (−2.2, 0.1) |
| Overnutrition, n (%) (z score: >+2 SD) | 4 (4.6) | 6 (3.5) | 13 (7.9) | 1 (3.8) | 0 (0.0) |
| Normal, n (%) (z score: −2 SD to +2 SD) | 38 (43.7) | 55 (32.4) | 103 (62.8) | 21 (80.8) | 21 (75.0) |
| Moderate undernutrition, n (%) (z score: >−3 SD to <−2.0 SD) | 11 (12.6) | 30 (17.6) | 19 (11.6) | 2 (7.7) | 3 (10.7) |
| Severe undernutrition, n (%) (z score: ≤−3.0 SD) | 34 (39.1) | 79 (46.5) | 29 (17.7) | 2 (7.7) | 4 (14.3) |
| Characteristics | Total n = 182 | Weight for Age z Score (WAZ), n = 83 1 | p Value 4 | Height for Age z Score (HAZ), n = 164 2 | p Value 4 | BMI for Age z Score (BAZ), n = 151 3 | p Value 4 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal, n = 38 | Underweight, n = 45 | Normal, n = 55 | Stunted, n = 109 | Normal, n = 103 | Thin, n = 48 | |||||
| Age group (in years), n = 181 6 | ||||||||||
| 0–4 | 26 (14.4) | 15 (40.5) | 9 (20.4) | 0.08 5 | 9 (16.4) | 12 (11.1) | 0.51 | 17 (16.7) | 5 (10.4) | 0.51 |
| 5–9 | 63 (34.8) | 22 (59.5) | 34 (77.3) | 21 (38.2) | 37 (34.3) | 36 (35.3) | 16 (33.3) | |||
| 10–14 | 54 (29.8) | 0 | 1 (2.3) 2 | 13 (23.6) | 37 (34.3) | 31 (30.4) | 14 (29.2) | |||
| ≥15 | 38 (21.0) | - | - | 12 (21.8) | 22 (20.4) | 18 (17.6) | 13 (27.1) | |||
| Sex, n = 182 | ||||||||||
| Male | 114 (62.6) | 28 (73.7) | 23 (51.1) | 0.03 | 41 (74.5) | 62 (56.9) | 0.03 | 65 (63.1) | 29 (60.4) | 0.75 |
| Female | 68 (37.4) | 10 (26.3) | 22 (48.9) | 14 (25.5) | 47 (43.1) | 38 (36.9) | 19 (39.6) | |||
| Mother’s educational level (completed years of formal schooling) 6, n = 170 | ||||||||||
| 0–4 | 113 (66.5) | 14 (42.4) | 28 (63.6) | 0.06 | 28 (56.0) | 77 (74.8) | 0.05 | 64 (66.7) | 33 (68.8) | 0.44 |
| 5–9 | 32 (18.8) | 7 (21.2) | 10 (22.7) | 11 (22.0) | 15 (14.6) | 15 (15.6) | 10 (20.8) | |||
| ≥10 | 25 (14.7) | 12 (36.4) | 6 (13.6) | 11 (22.0) | 11 (10.7) | 17 (17.7) | 5 (10.4) | |||
| Father’s educational level (completed years of formal schooling)6, n = 167 | ||||||||||
| 0–4 | 77 (46.1) | 13 (37.1) | 21 (47.7) | 0.58 | 25 (50.0) | 46 (46.0) | 0. 87 | 45 (47.9) | 19 (41.3) | 0.62 |
| 5–9 | 52 (31.1) | 11 (31.4) | 13 (29.5) | 16 (32.0) | 33 (33.0) | 29 (30.9) | 18 (39.1) | |||
| ≥10 | 38 (22.8) | 11 (31.4) | 10 (22.7) | 9 (18.0) | 21 (21.0) | 20 (21.3) | 9 (19.6) | |||
| Mother’s occupation at birth of child with CP 6, n = 167 | ||||||||||
| Agricultural work | 81 (48.5) | 13 (37.1) | 20 (47.6) | 0.70 5 | 20 (40.0) | 51 (51.5) | 0.29 5 | 42 (43.8) | 25 (56.8) | 0.46 5 |
| Business | 11 (6.6) | 3 (8.6) | 4 (9.5) | 2 (4.0) | 7 (7.1) | 6 (6.3) | 2 (4.5) | |||
| No IGA | 66 (39.5) | 16 (45.7) | 14 (33.3) | 26 (52.0) | 35 (35.4) | 41 (42.7) | 16 (36.4) | |||
| Other IGA | 9 (5.4) | 3 (8.6) | 4 (9.5) | 2 (4.0) | 6 (6.1) | 7 (7.3) | 1 (2.3) | |||
| Father’s occupation at birth of child with CP 6, n = 171 | ||||||||||
| Agricultural work | 59 (34.5) | 10 (27.8) | 15 (34.9) | 0.38 5 | 17 (33.3) | 36 (35.3) | 0.48 5 | 33 (34.0) | 12 (26.1) | 0.62 5 |
| Business | 11 (6.4) | 3 (8.3) | 2 (4.7) | 1 (2.0) | 8 (7.8) | 5 (5.2) | 2 (4.3) | |||
| No IGA | 8 (4.7) | 0 (0.0) | 3 (7.0) | 3 (5.9) | 4 (3.9) | 5 (5.2) | 1 (2.2) | |||
| Other IGA | 93 (54.4) | 23 (63.9) | 23 (53.5) | 30 (58.8) | 54 (52.9) | 54 (55.7) | 31 (67.4) | |||
| Monthly family income, NRs (USD) 6,7, n = 181 | ||||||||||
| Median (IQR) | 10,000 (7000, 20,000) (82 (57, 164)) | 17,000 (9750, 20,500) (140 (80.0 168)) | 12,000 (7000, 20,000) (98 (57, 164)) | 0.14 | 12,000 (8000, 20,000)(98 (66, 164)) | 10,500 (7000, 20,000) (86 (57, 164)) | 0.88 | 12,000 (7000, 20,000) (98 (57, 164) | 13,500 (8250, 20,000) (111 (68, 164)) | 0.55 |
| 1000–14,999 (10–149.9) | 103 (56.9) | 13 (34.2) | 26 (59.1) | 0.03 5 | 29 (52.7) | 62 (57.4) | 0.09 | 54 (52.9) | 24 (50.0) | 0.93 |
| 15,000–29,999 (150–299.9) | 61 (33.7) | 21 (55.3) | 12 (27.3) | 24 (43.6) | 33 (30.6) | 37 (36.3) | 19 (39.6) | |||
| ≥30,000 (≥300) | 17 (9.4) | 4 (10.5) | 6 (13.6) | 2 (3.6) | 13 (12.0) | 11 (10.8) | 5 (10.4) | |||
| Source of drinking water 6, n = 177 | ||||||||||
| Improved 8 | 163 (92.6) | 31 (86.1) | 40 (90.9) | 0.72 5 | 48 (90.6) | 99 (92.5) | 0.76 5 | 91 (91.9) | 43 (89.6) | 0.76 5 |
| Unimproved | 13 (7.4) | 5 (13.9) | 4 (9.1) | 5 (9.4) | 8 (7.5) | 8 (8.1) | 5 (10.4) | |||
| Type of latrin used 6 | ||||||||||
| Sanitary 9 | 169 (97.1) | 34 (94.4) | 40 (97.6) | 0.60 5 | 48 (94.2) | 103 (98.1) | 0.33 5 | 95 (96.9) | 44 (95.7) | 0.65 5 |
| Non-sanitary | 5 (2.9) | 2 (5.6) | 1 (2.4) | 3 (5.8) | 2 (1.9) | 3 (3.1) | 2 (4.3) | |||
| Attendance at mainstream and/or special school 6, n = 174 | ||||||||||
| No | 83 (47.7) | 7 (18.4) | 20 (47.6) | 0.01 | 14 (26.4) | 63 (60.0) | <0.001 | 37 (37.4) | 28 (59.6) | 0.02 |
| Yes | 53 (30.5) | 13 (34.2) | 6 (14.3) | 28 (52.8) | 22 (21.0) | 39 (39.4) | 9 (19.1) | |||
| N/A (aged <6 year) | 38 (21.8) | 18 (47.4) | 16 (38.1) | 11 (20.8) | 20 (19.0) | 23 (23.2) | 10 (21.3) | |||
| Risk Factors and Clinical Characteristics | Total, n = 182 | Weight for Age z Score (WAZ) 1, n = 83 | p Value 4 | Height for Age z Score (HAZ) 2, n = 164 | p Value 4 | BMI for Age z Score (BAZ) 3, n = 151 | p Value 4 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal, n = 38 | Underweight, n = 45 | Normal, n = 55 | Stunted, n = 109 | Normal, n = 103 | Thin, n = 48 | |||||
| Birthweight (BW) among known 6, n = 78 | ||||||||||
| Normal BW | 57 (73.1) | 19 (79.2) | 19 (57.6) | 0.09 | 22 (100.0) | 29 (60.4) | 0.001 | 35 (74.5) | 14 (66.7) | 0.51 |
| Low BW | 21 (26.9) | 5 (20.8) | 14 (42.4) | 0 (0.0) | 19 (39.6) | 12 (25.5) | 7 (33.3) | |||
| Gestational age 6, n = 170 | ||||||||||
| Pre-term | 18 (10.6) | 4 (12.1) | 8 (18.6) | 0.44 | 3 (6.1) | 15 (14.6) | 0.13 | 14 (14.7) | 3 (6.5) | 0.16 |
| Term | 152 (89.4) | 29 (87.9) | 35 (81.4) | 46 (93.9) | 88 (85.4) | 81 (85.3) | 43 (93.5) | |||
| Epilepsy, n = 182 | ||||||||||
| No | 132 (72.5) | 24 (63.2) | 33 (73.3) | 0.32 | 41 (74.5) | 75 (68.8) | 0.45 | 78 (75.7) | 31 (64.6) | 0.15 |
| Yes | 50 (27.5) | 14 (36.8) | 12 (26.7) | 14 (25.5) | 34 (31.2) | 25 (24.3) | 17 (35.4) | |||
| Intellectual 6, n = 132 | ||||||||||
| No | 47 (35.6) | 14 (50.0) | 9 (26.5) | 0.06 | 21 (51.2) | 23 (28.7) | 0.01 | 35 (46.1) | 9 (27.3) | 0.07 |
| Yes | 85 (64.4) | 14 (50.0) | 25 (73.5) | 20 (48.8) | 57 (71.3) | 41 (53.9) | 24 (72.7) | |||
| Visual 6, n = 179 | ||||||||||
| No | 161 (89.9) | 32 (84.2) | 37 (84.1) | 1.00 | 49 (92.5) | 95 (88.0) | 0.38 | 91 (90.1) | 41 (87.2) | 0.60 |
| Yes | 18 (10.1) | 6 (15.8) | 7 (15.9) | 4 (7.5) | 13 (12.0) | 10 (9.9) | 6 (12.8) | |||
| Hearing 6, n = 179 | ||||||||||
| No | 143 (79.9) | 29 (76.3) | 33 (73.3) | 0.76 | 42 (79.2) | 86 (78.9) | 0.96 | 83 (81.4) | 36 (78.3) | 0.66 |
| Yes | 36 (20.1) | 9 (23.7) | 12 (26.7) | 11 (20.8) | 23 (21.1) | 19 (18.6) | 10 (21.7) | |||
| Speech 6, n = 181 | ||||||||||
| No | 36 (19.9) | 10 (26.3) | 6 (13.3) | 0.13 | 16 (29.6) | 18 (16.5) | 0.05 | 26 (25.5) | 6 (12.5) | 0.07 |
| Yes | 145 (80.1) | 28 (73.7) | 39 (86.7) | 38 (70.4) | 91 (83.5) | 76 (74.5) | 42 (87.5) | |||
| Number of associated impairments, n = 182 | ||||||||||
| None | 23 (12.6) | 6 (15.8) | 5 (11.1) | 0.80 | 12 (21.8) | 9 (8.3) | 0.03 | 17 (16.5) | 5 (10.4) | 0.28 |
| ≤2 | 113 (62.1) | 20 (52.6) | 26 (57.8) | 33 (60.0) | 68 (62.4) | 65 (63.1) | 28 (58.3) | |||
| Multiple | 46 (25.3) | 12 (31.6) | 14 (31.1) | 10 (18.2) | 32 (29.4) | 21 (20.4) | 15 (31.3) | |||
| Timing of CP 6, n = 180 | ||||||||||
| Pre & Peri | 159 (88.3) | 31 (81.6) | 42 (93.3) | 0.17 5 | 45 (83.3) | 99 (91.7) | 0.12 | 88 (88.1) | 43 (89.6) | 0.78 |
| Postnatal | 21 (11.7) | 7 (18.4) | 3 (6.7) | 9 (16.7) | 9 (8.3) | 12 (11.9) | 5 (10.4) | |||
| Swallowing difficulties 6, n = 179 | ||||||||||
| No | 117 (65.4) | 24 (63.2) | 24 (54.5) | 0.43 | 40 (74.1) | 67 (61.5) | 0.11 | 69 (67.6) | 29 (61.7) | 0.48 |
| Yes | 62 (34.6) | 14 (36.8) | 20 (45.5) | 14 (25.9) | 42 (38.5) | 33 (32.4) | 18 (38.3) | |||
| Predominant type of CP, n = 182 | ||||||||||
| Spastic | 141 (77.5) | 24 (63.2) | 39 (86.7) | 0.025 | 39 (70.9) | 89 (81.7) | <0.0015 | 74 (71.8) | 40 (83.3) | 0.42 5 |
| Dyskinesia | 7 (3.8) | 2 (5.3) | 0 (0.0) | 2 (3.6) | 4 (3.7) | 4 (3.9) | 2 (4.2) | |||
| Ataxia | 16 (8.8) | 7 (18.4) | 1 (2.2) | 12 (21.8) | 2 (1.8) | 11 (10.7) | 3 (6.3) | |||
| Hypotonia | 18 (9.9) | 5 (13.2) | 5 (11.1) | 2 (3.6) | 14 (12.8) | 14 (13.6) | 3 (6.3) | |||
| Topography of CP, n = 141 | ||||||||||
| Mono/Hemiplegia | 54 (38.3) | 10 (41.7) | 11 (28.2) | 0.545 | 24 (61.5) | 26 (29.2) | 0.0025 | 31 (41.9) | 12 (30.0) | 0.18 5 |
| Diplegia | 16 (11.3) | 3 (12.5) | 5 (12.8) | 4 (10.3) | 10 (11.2) | 10 (13.5) | 3 (7.5) | |||
| Tri/Quadriplegia | 71 (50.4) | 11 (45.8) | 23 (59.0) | 11 (28.2) | 53 (59.6) | 33 (44.6) | 25 (62.5) | |||
| CP diagnosis age (in months) 6, n = 168 | ||||||||||
| ≤30 | 78 (46.4) | 19 (52.8) | 18 (46.2) | 0.85 | 21 (42.9) | 48 (46.2) | 0.02 | 39 (41.5) | 23 (50.0) | 0.54 |
| 31–60 | 38 (22.6) | 8 (22.2) | 10 (25.6) | 6 (12.2) | 30 (28.8) | 23 (24.5) | 8 (17.4) | |||
| ≥61 | 52 (31.0) | 9 (25.0) | 11 (28.2) | 22 (44.9) | 26 (25.0) | 32 (34.0) | 15 (32.6) | |||
| GMFCS level, n = 182 | ||||||||||
| I–II | 82 (45.1) | 22 (57.9) | 13 (28.9) | 0.01 | 37 (67.3) | 37 (33.9) | <0.001 | 54 (52.4) | 15 (31.3) | 0.01 |
| III–V | 100 (54.9) | 16 (42.1) | 32 (71.1) | 18 (32.7) | 72 (66.1) | 49 (47.6) | 33 (68.8) | |||
| MACS level, n = 177 | ||||||||||
| I–II | 88 (49.7) | 17 (47.2) | 16 (37.2) | 0.14 | 35 (66.0) | 45 (42.5) | 0.002 | 58 (58.6) | 17 (35.4) | 0.02 |
| III–V | 72 (40.7) | 10 (27.8) | 21 (48.8) | 12 (22.6) | 55 (51.9) | 32 (32.3) | 26 (54.2) | |||
| Not applicable 7 | 17 (9.6) | 9 (25.0) | 6 (14.0) | 6 (11.3) | 6 (5.7) | 9 (9.1) | 5 (10.4) | |||
| Viking speech level 6, n = 180 | ||||||||||
| I–II | 61 (33.9) | 12 (31.6) | 11 (24.4) | 0.25 | 25 (46.3) | 32 (29.6) | 0.02 | 40 (39.6) | 14 (29.2) | 0.46 |
| III–IV | 102 (56.7) | 17 (44.7) | 28 (62.2) | 23 (42.6) | 70 (64.8) | 52 (51.5) | 29 (60.4) | |||
| Not applicable | 17 (9.4) | 9 (23.7) | 6 (13.3) | 6 (11.1) | 6 (5.6) | 9 (8.9) | 5 (10.4) | |||
| CFCS level 6, n = 180 | ||||||||||
| I–II | 82 (45.6) | 20 (52.6) | 15 (33.3) | 0.08 | 32 (59.3) | 43 (39.8) | 0.02 | 55 (54.5) | 17 (35.4) | 0.03 |
| III–V | 98 (54.4) | 18 (47.4) | 30 (66.7) | 22 (40.7) | 65 (60.2) | 46 (45.5) | 31 (64.6) | |||
| Predictors 1 | Underweight (WAZ < −2 SD), n = 45 | Stunting (HAZ < −2 SD), n = 109 | Thin (BAZ < −2 SD), n = 48 | |||
|---|---|---|---|---|---|---|
| aOR [95% CI] | p Value | aOR [95% CI] | p Value | aOR [95% CI] | p Value | |
| Sex | ||||||
| Male | Ref | Ref | - | |||
| Female | 2.1 (0.5, 9.2) | 0.30 | 1.8 (0.6, 5.6) | 0.28 | ||
| Attendance to mainstream school | ||||||
| Yes | Ref | Ref | Ref | |||
| No | 3.8 (0.9, 16.8) | 0.08 | 4.0 (1.2, 13.2) | 0.02 | 2.2 (0.7, 6.3) | 0.15 |
| GMFCS level | ||||||
| I–II | Ref | Ref | Ref | |||
| III–V | 2.9 (0.6, 13.0) | 0.16 | 8.2 (1.6, 40.8) | 0.01 | 1.6 (0.6, 4.1) | 0.31 |
| MACS level | ||||||
| I–II | - | Ref | - | |||
| III–V | 0.1 (0.0, 0.9) | 0.04 | ||||
| CFCS level | ||||||
| I–II | - | Ref | Ref | |||
| III–V | 1.7 (0.4, 7.1) | 0.44 | 1.3 (0.5, 3.2) | 0.58 | ||
| Viking speech level | ||||||
| I–II | - | Ref | - | |||
| III–V | 1.2 (0.3, 4.3) | 0.77 | ||||
| Intellectual impairment | ||||||
| No | - | Ref | - | |||
| Yes | 2.5 (0.8, 8.2) | 0.11 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahan, I.; Muhit, M.; Al Imam, M.H.; Ghose, R.; Chhetri, A.B.; Badawi, N.; Khandaker, G. Nutritional Status of Children with Cerebral Palsy in Gorkha, Nepal: Findings from the Nepal Cerebral Palsy Register. Nutrients 2021, 13, 2537. https://doi.org/10.3390/nu13082537
Jahan I, Muhit M, Al Imam MH, Ghose R, Chhetri AB, Badawi N, Khandaker G. Nutritional Status of Children with Cerebral Palsy in Gorkha, Nepal: Findings from the Nepal Cerebral Palsy Register. Nutrients. 2021; 13(8):2537. https://doi.org/10.3390/nu13082537
Chicago/Turabian StyleJahan, Israt, Mohammad Muhit, Mahmudul Hassan Al Imam, Ratul Ghose, Amir Banjara Chhetri, Nadia Badawi, and Gulam Khandaker. 2021. "Nutritional Status of Children with Cerebral Palsy in Gorkha, Nepal: Findings from the Nepal Cerebral Palsy Register" Nutrients 13, no. 8: 2537. https://doi.org/10.3390/nu13082537
APA StyleJahan, I., Muhit, M., Al Imam, M. H., Ghose, R., Chhetri, A. B., Badawi, N., & Khandaker, G. (2021). Nutritional Status of Children with Cerebral Palsy in Gorkha, Nepal: Findings from the Nepal Cerebral Palsy Register. Nutrients, 13(8), 2537. https://doi.org/10.3390/nu13082537





