Selenium Modulates the Allergic Response to Whey Protein in a Mouse Model for Cow’s Milk Allergy
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
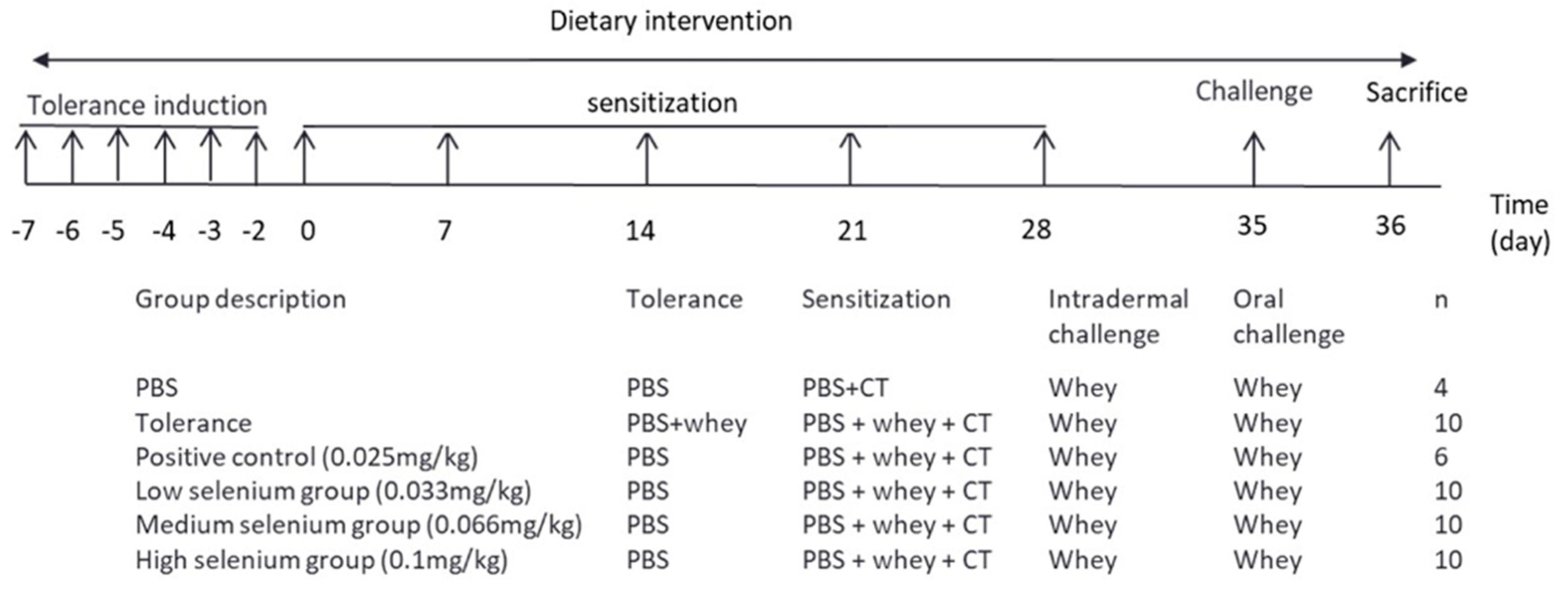
2.2. Experimental Design
2.3. Measurement of Allergen-Specific Immunoglobulins and Mouse Mast Cell Proteases-1 (mMCP-1) in Serum
2.4. Flow Cytometric Analysis of Immune Cells
2.5. Cytokine Measurement after Ex Vivo Antigen-Specific Stimulation of Splenocytes
2.6. Statistical Analysis
3. Results
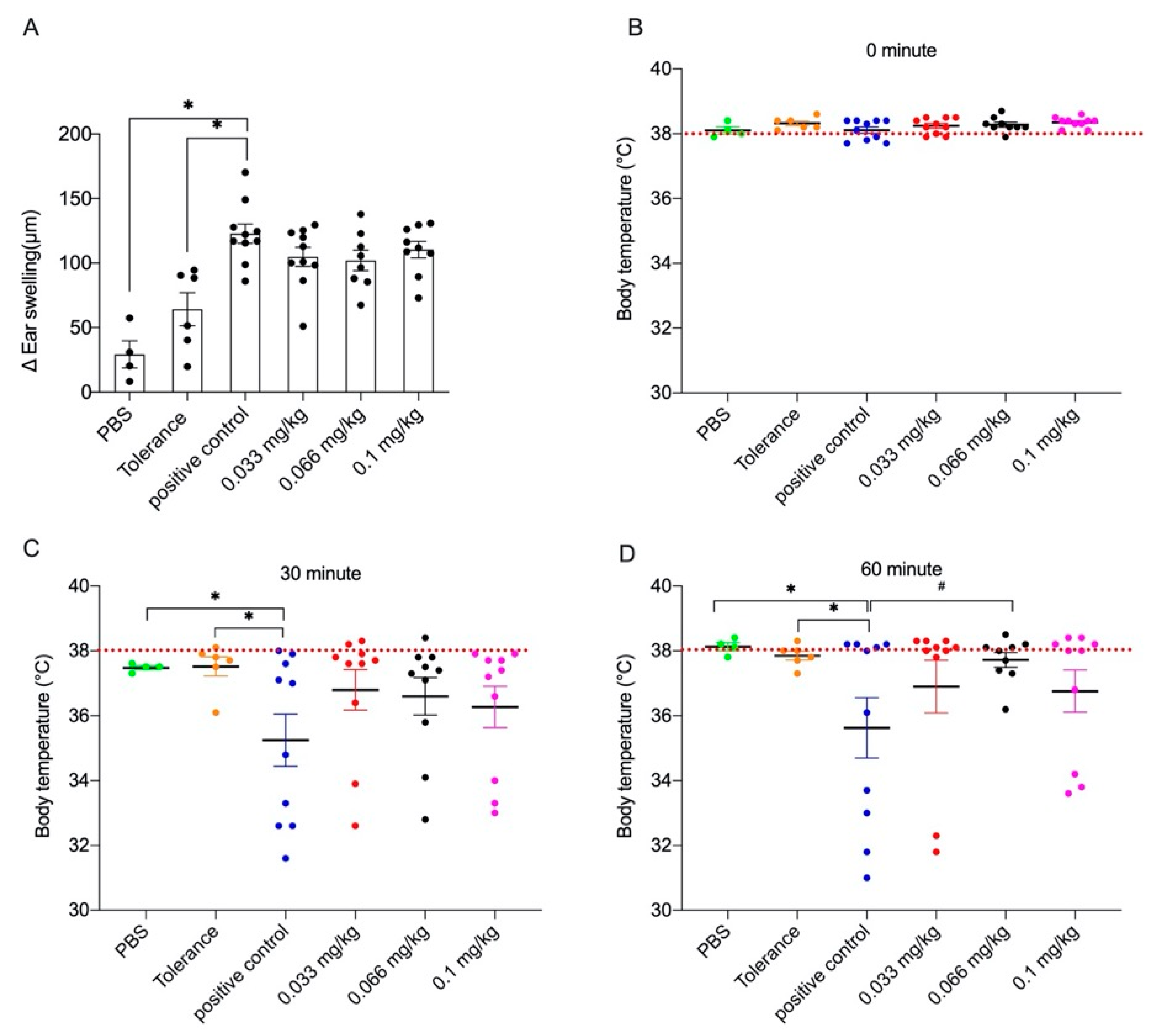
3.1. Acute Allergic Skin Response and Body Temperature after Challenge
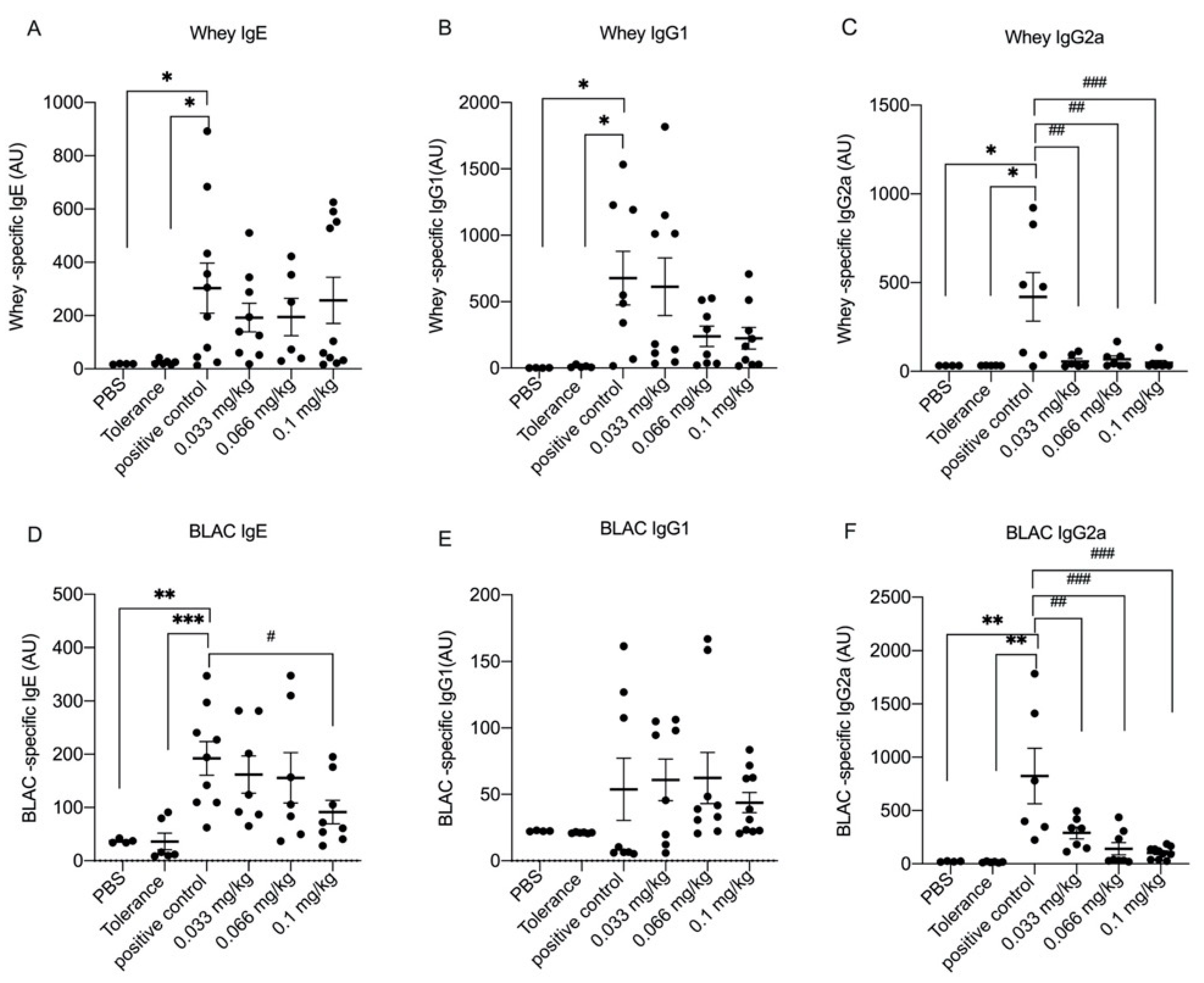
3.2. Dietary Se Supplementation Downregulates Whey- and BLAC-Specific Immunoglobulin Levels
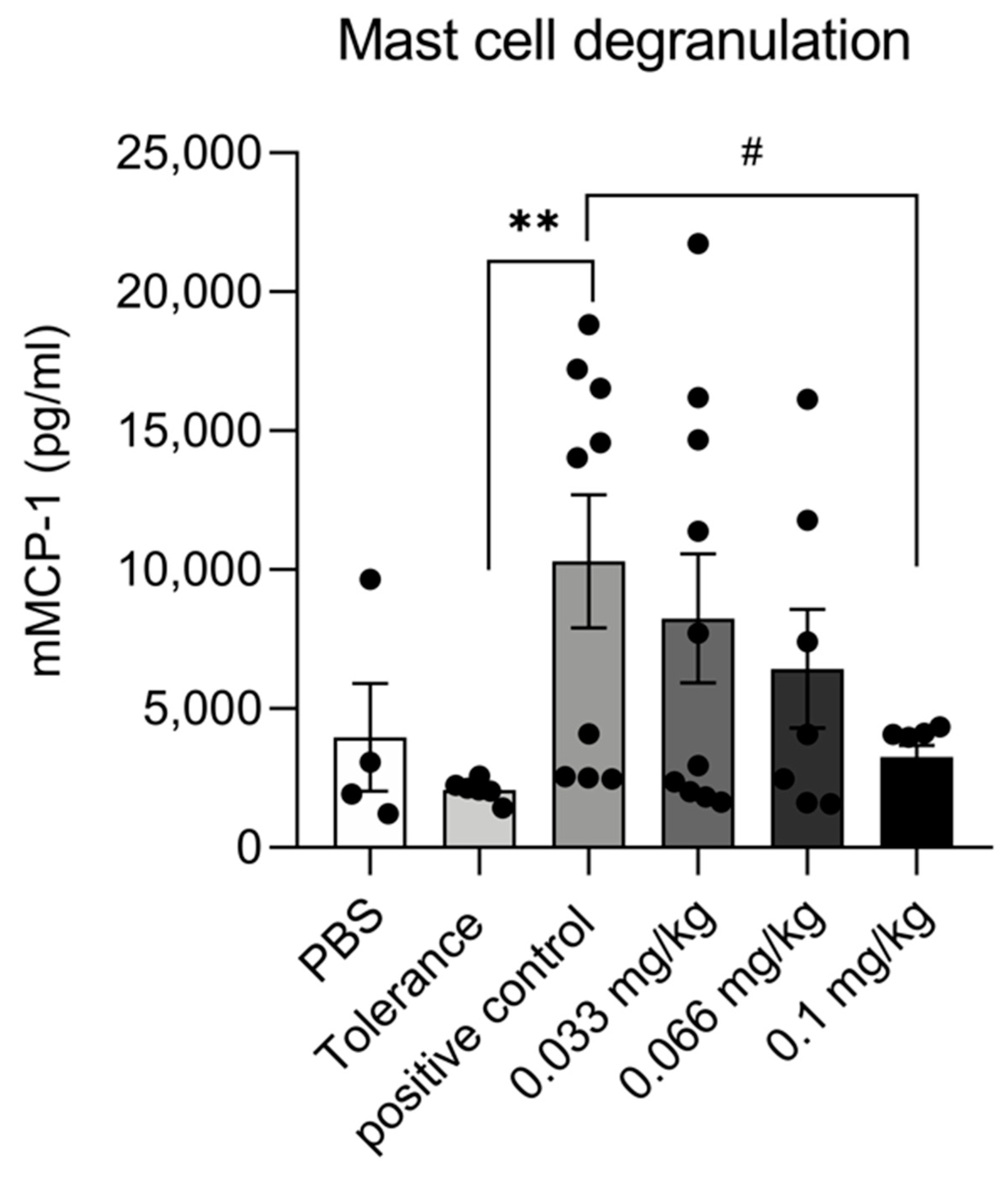
3.3. Dietary Se Supplementation Reduces mMCP-1 Production
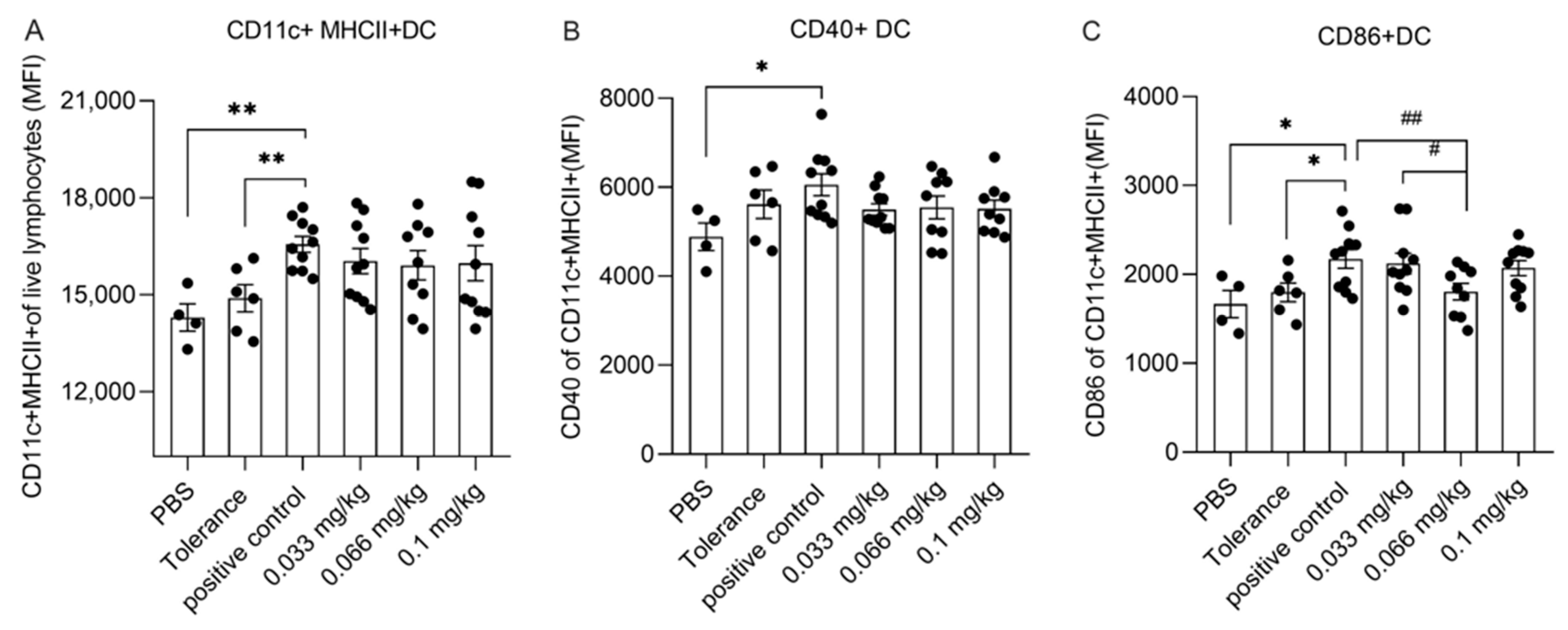
3.4. Dietary Se Supplementation Affects Dendritic Cell Activation
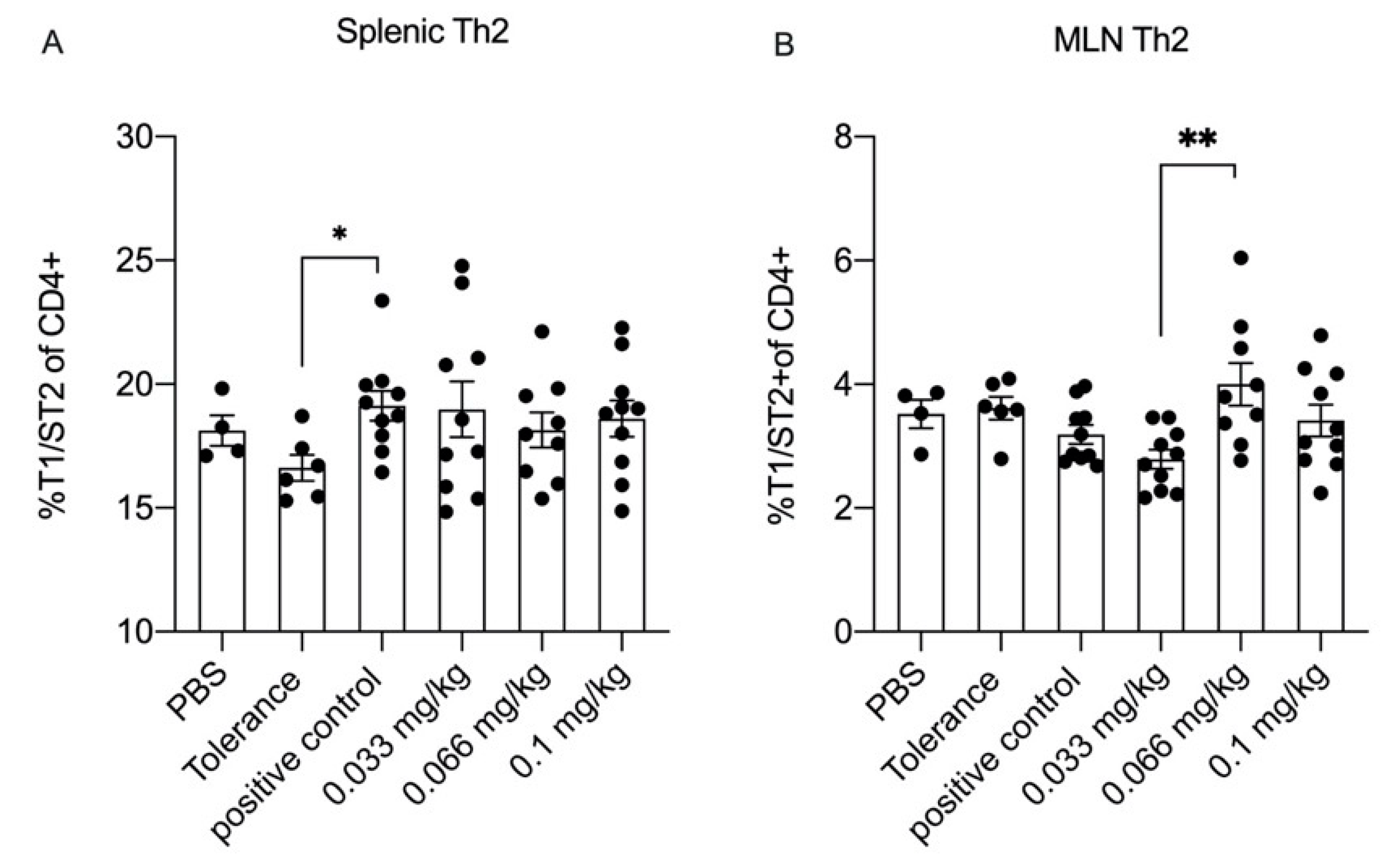
3.5. Dietary Se Only Modestly Affects T Cell Differentiation
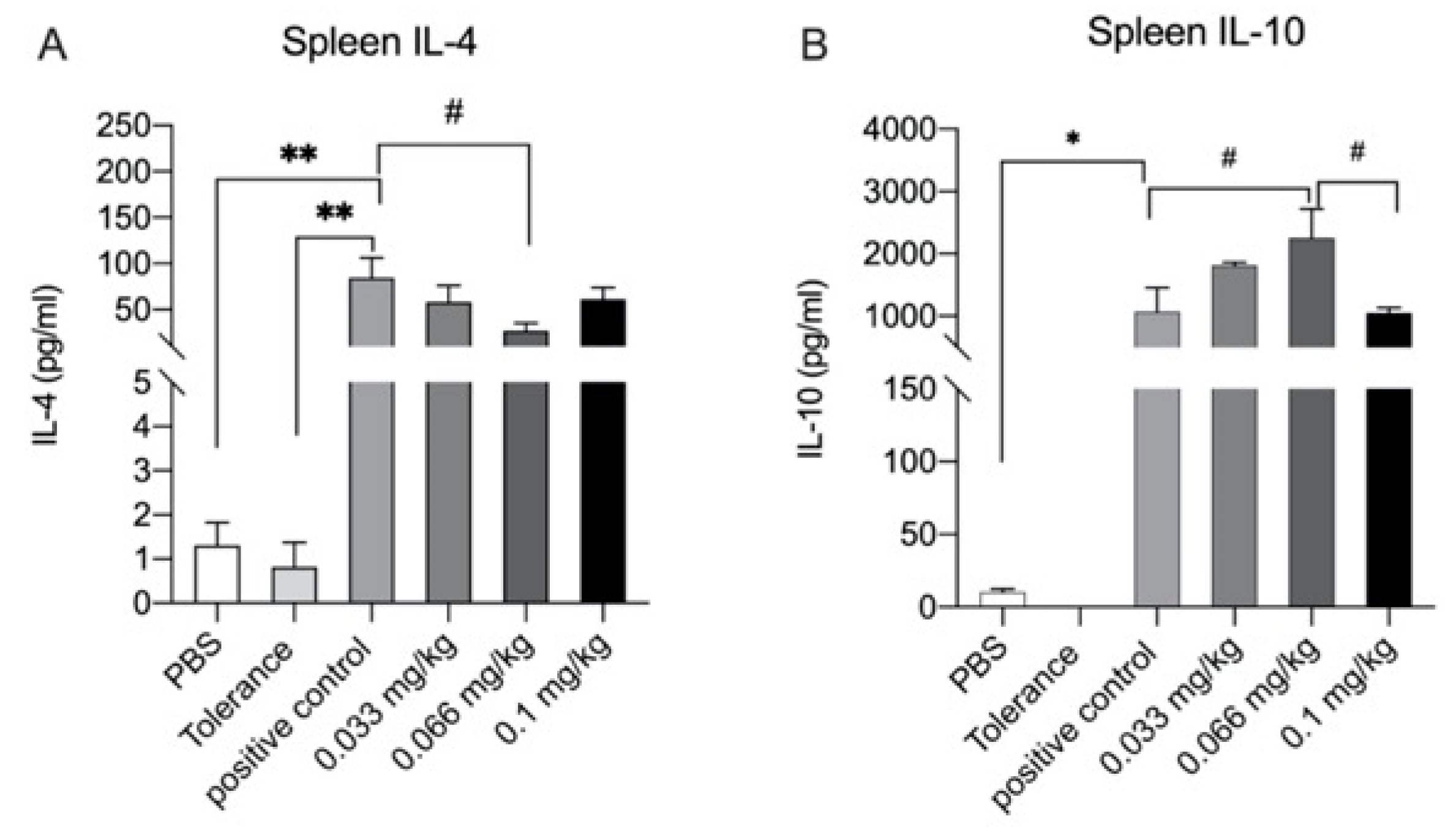
3.6. Cytokine Production after Ex Vivo Stimulation with Whey
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Auria, E.; Salvatore, S.; Pozzi, E.; Mantegazza, C.; Sartorio, M.U.A.; Pensabene, L.; Baldassarre, M.E.; Agosti, M.; Vandenplas, Y.; Zuccotti, G. Cow’s milk allergy: Immunomodulation by dietary intervention. Nutrients 2019, 11, 1399. [Google Scholar] [CrossRef]
- Ludman, S.; Shah, N.; Fox, A.T. Managing cows’ milk allergy in children. BMJ 2013, 347, f5424. [Google Scholar] [CrossRef] [PubMed]
- Mousan, G.; Kamat, D. Cow’s Milk Protein Allergy. Clin. Pediatr. 2016, 55, 1054–1063. [Google Scholar] [CrossRef]
- Santos, A.; Dias, A.; Pinheiro, J.A. Predictive factors for the persistence of cow’s milk allergy. Pediatr. Allergy Immunol. 2010, 21, 1127–1134. [Google Scholar] [CrossRef]
- Savage, J.; Johns, C.B. Food allergy: Epidemiology and natural history. Immunol. Allergy Clinics. 2015, 35, 45–59. [Google Scholar] [CrossRef]
- Stoffel, N.U.; Uyoga, M.A.; Mutuku, F.M.; Frost, J.N.; Mwasi, E.; Paganini, D.; van der Klis, F.R.M.; Malhotra, I.J.; LaBeaud, A.D.; Ricci, C.; et al. Iron deficiency anemia at time of vaccination predicts decreased vaccine response and iron supplementation at time of vaccination increases humoral vaccine response: A birth cohort study and a randomized trial follow-up study in Kenyan infants. Front. Immunol. 2020, 11, 1313. [Google Scholar] [CrossRef]
- Jones, S.M.; Burks, A.W. Food Allergy. N. Engl. J. Med. 2017, 377, 1168–1176. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Virkud, Y.V.; Wang, J.; Shreffler, W.G. Enhancing the Safety and Efficacy of Food Allergy Immunotherapy: A Review of Adjunctive Therapies. Clin. Rev. Allergy Immunol. 2018, 55, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ho, H.E.; Bunyavanich, S. The gut microbiome in food allergy. Ann. Allergy Asthma Immunol. 2019, 122, 7. [Google Scholar] [CrossRef] [PubMed]
- Heine, R.G. Food allergy prevention and treatment by targeted nutrition. Ann. Nutr. Metab. 2018, 72, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, W.C.; Kelley, D.S.; Taylor, P.C. The effects of dietary selenium on the immune system in healthy men. Biol. Trace Elem. Res. 2006, 81, 5. [Google Scholar] [CrossRef]
- Kamer, B.; Wasowicz, W.; Pyziak, K.; Kamer-Bartosinska, A.; Gromadzinska, J.; Pasowska, R. Role of selenium and zinc in the pathogenesis of food allergy in infants and young children. Arch. Med. Sci. 2012, 8, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Weijing Sun, Z.; Wang, Q.; Deng, G.; Guo, Y.; Guo, M. Selenium suppresses inflammation by inducing microRNA-146a in Staphylococcus aureus-infected mouse mastitis model. Oncotarget 2017, 8, 110949. [Google Scholar]
- Guo, Y.; Pan, D.; Li, H.; Sun, Y.; Zeng, X.; Yan, B. Antioxidant and immunomodulatory activity of selenium exopolysaccharide produced by Lactococcus lactis subsp. lactis. Food Chem. 2013, 138, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Broome, C.S.; McArdle, F.; Kyle, J.A.; Andrews, F.; Lowe, N.M.; Hart, C.A.; Arthur, J.R.; Jackson, M.J. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am. J. Clin. Nutr. 2004, 80, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, C.; Pan, T.; Yao, H.; Li, S. Selenium accelerates chicken dendritic cells differentiation and affects selenoproteins expression. Dev. Comp. Immunol. 2017, 77, 30–37. [Google Scholar] [CrossRef]
- Hoffmann, F.W.; Hashimoto, A.C.; Shafer, L.A.; Dow, S.; Berry, M.J.; Hoffmann, P.R. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J. Nutr. 2010, 140, 1155–1161. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Al-Quraishy, S.; Dkhil, M.A.; Wunderlich, F.; Sies, H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv. Nutr. 2015, 6, 73–82. [Google Scholar] [CrossRef]
- Xue, H.; Wang, W.; Li, Y.; Shan, Z.; Li, Y.; Teng, X.; Gao, Y.; Fan, C.; Teng, W. Selenium upregulates CD4(+)CD25(+) regulatory T cells in iodine-induced autoimmune thyroiditis model of NOD.H-2(h4) mice. Endocr. J. 2010, 57, 595–601. [Google Scholar] [CrossRef]
- Soni, C.; Sinha, I.; Fasnacht, M.J.; Olsen, N.J.; Rahman, Z.S.M.; Sinha, R. Selenium supplementation suppresses immunological and serological features of lupus in B6.Sle1b mice. Autoimmunity 2019, 52, 57–68. [Google Scholar] [CrossRef]
- Lv, J.; Ai, P.; Lei, S.; Zhou, F.; Chen, S.; Zhang, Y. Selenium levels and skin diseases: Systematic review and meta-analysis. J. Trace Elem. Med. Biol. 2020, 62, 126548. [Google Scholar] [PubMed]
- Misso, N.L.; Powers, K.A.; Gillon, R.L.; Stewart, G.A.; Thompson, P.J. Reduced platelet glutathione peroxidase activity and serum selenium concentration in atopic asthmatic patients. Clin. Exp. Allergy 1996, 26, 838–847. [Google Scholar] [CrossRef]
- Spallholz, J.E.; Boylan, L.M.; Larsen, H.S. Advances in understanding selenium’s role in the immune system. Ann. N. Y. Acad. Sci. 1990, 587, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Chan, Q.; Afton, S.E.; Caruso, J.A. Selenium speciation profiles in selenite-enriched soybean (Glycine Max) by HPLC-ICPMS and ESI-ITMS. Metallomics 2010, 2, 147–153. [Google Scholar] [CrossRef]
- Zhang, Y.; Cartland, S.P.; Henriquez, R.; Patel, S.; Gammelgaard, B.; Flouda, K.; Hawkins, C.L.; Rayner, B.S. Selenomethionine supplementation reduces lesion burden, improves vessel function and modulates the inflammatory response within the setting of atherosclerosis. Redox Biol. 2020, 29, 101409. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Deguchi, T.; Sakazaki, F.; Ogino, H.; Okuno, T.; Ueno, H. Supplementary seleno-L-methionine suppresses active cutaneous anaphylaxis reaction. Biol. Pharm. Bull. 2013, 36, 1969–1974. [Google Scholar] [CrossRef]
- Bhatia, P.; Aureli, F.; D’Amato, M.; Prakash, R.; Cameotra, S.S.; Nagaraja, T.P.; Cubadda, F. Selenium bioaccessibility and speciation in biofortified Pleurotus mushrooms grown on selenium-rich agricultural residues. Food Chem. 2013, 140, 225–230. [Google Scholar] [CrossRef]
- Kerperien, J.; Veening-Griffioen, D.; Oja, A.; Wehkamp, T.; Jeurink, P.V.; Garssen, J.; Knippels, L.M.J.; Willemsen, L.E.M. Dietary vitamin D supplementation is ineffective in preventing murine cow’s milk allergy, Irrespective of the presence of nondigestible oligosaccharides. Int. Arch. Allergy Immunol. 2020, 181, 908–918. [Google Scholar] [CrossRef] [PubMed]
- van Sadelhoff, J.H.J.; Hogenkamp, A.; Wiertsema, S.P.; Harthoorn, L.F.; Loonstra, R.; Hartog, A.; Garssen, J. A free amino acid-based diet partially prevents symptoms of cow’s milk allergy in mice after oral sensitization with whey. Immun. Inflamm. Disease 2020, 8, 93–105. [Google Scholar]
- Liu, L.L.; Yao, H.; Zhang, X.L.; Zhang, H.L.; Chao, P.L.; Tong, M.L.; Liu, G.L.; Lin, L.R.; Fan, L.; Zhang, Z.Y.; et al. Characteristics of patients suffering from cow milk allergy. Int. Immunopharmacol. 2012, 14, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Ren, Z.; Huang, Y.; Song, Y.; Lin, D.; Li, J.; Ma, Y.; Wu, X.; Qiu, F.; Xiao, Q. Selenizing Hericium erinaceus polysaccharides induces dendritic cells maturation through MAPK and NF-κB signaling pathways. Int. J. Biol. Macromol. 2017, 97, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991, 9, 271–296. [Google Scholar] [CrossRef]
- Dieli, F. Dendritic cells and the handling of antigen. Clin. Exp. Immunol. 2003, 134, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Frischmeyer-Guerrerio, P.A.; Guerrerio, A.L.; Chichester, K.L.; Bieneman, A.P.; Hamilton, R.A.; Wood, R.A.; Schroeder, J.T. Dendritic cell and T cell responses in children with food allergy. Clin. Exp. Allergy 2011, 41, 61–71. [Google Scholar] [CrossRef][Green Version]
- Arakawa, T.; Sugiyama, T.; Matsuura, H.; Okuno, T.; Ogino, H.; Sakazaki, F.; Ueno, H. Effects of supplementary seleno-L-methionine on atopic dermatitis-like skin lesions in mice. Biol. Pharm. Bull. 2018, 41, 1456–1462. [Google Scholar] [CrossRef]
- Safaralizadeh, R.; Nourizadeh, M.; Zare, A.; Kardar, G.A.; Pourpak, Z. Influence of selenium on mast cell mediator release. Biol. Trace Elem. Res. 2013, 154, 299–303. [Google Scholar] [CrossRef]
- Ho, I.C.; Miaw, S.C. Regulation of IL-4 Expression in Immunity and Diseases. Adv. Exp. Med. Biol. 2016, 941, 31–77. [Google Scholar] [PubMed]
- MacNeil, A.J.; Yang, Y.J.; Lin, T.-J. MAPK kinase 3 specifically regulates Fcεri-Mediated IL-4 production by mast cells. J. Immunol. 2011, 187, 3374–3382. [Google Scholar] [CrossRef]
- Toyoshima, S.; Wakamatsu, E.; Ishida, Y.; Obata, Y.; Kurashima, Y.; Kiyono, H.; Abe, R. The spleen is the site where mast cells are induced in the development of food allergy. Int. Immunol. 2017, 29, 6. [Google Scholar] [CrossRef]
- McLeod, J.J.A.; Baker, B.; Ryan, J.J. Mast cell production and response to IL-4 and IL-13. Cytokine 2015, 75, 57–61. [Google Scholar] [CrossRef]
- Bailey, D.P.; Kashyap, M.; Andew Bouton, L.; Murray, P.J.; Ryan, J.J. Interleukin-10 induces apoptosis in developing mast cells and macrophages. J. Leukoc. Biol. 2006, 80, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Jutel, M.; Akdis, M.; Blaser, K.; Akdis, C. Mechanisms of allergen specific immunotherapy—T-cell tolerance and more. Allergy 2006, 61, 796–807. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, A.R.; Kim, D.K.; Kim, H.W.; Park, Y.H.; Jang, G.H.; Kim, B.; Park, Y.M.; You, J.S.; Kim, H.S.; et al. Interleukin-10-producing CD5+ B cells inhibit mast cells during immunoglobulin E-mediated allergic responses. Sci. Signal. 2015, 8, ra28. [Google Scholar] [CrossRef]
- Nakajima-Adachi, H.; Kikuchi, A.; Fujimura, Y.; Shibahara, K.; Makino, T.; Goseki-Sone, M.; Kihara-Fujioka, M.; Nochi, T.; Kurashima, Y.; Igarashi, O.; et al. Peyer’s patches and mesenteric lymph nodes cooperatively promote enteropathy in a mouse model of food allergy. PLoS ONE. 2014, 9, e107492. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, M.; O’mahony, L.; Akdis, M. Role of regulatory cells in oral tolerance. Allergy Asthma Immunol. Res. 2017, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.X.; Chang, B.; Zhu, J.F.; Yang, F.L.; Li, Y.; Jiang, X.F.; Wang, D.N.; Lu, C.L.; Sun, X. Sodium selenite ameliorates dextran sulfate sodium-induced chronic colitis in mice by decreasing Th1, Th17, and γδT and increasing CD4(+)CD25(+) regulatory T-cell responses. World J. Gastroenterol. 2017, 23, 3850–3863. [Google Scholar] [CrossRef]
- Peng, X.; Cui, H.-m.; Deng, J.; Zuo, Z.; Cui, W. Low dietary selenium induce increased apoptotic thymic cells and alter peripheral blood T cell subsets in chicken. Biol. Trace Elem. Res. 2011, 142, 167–173. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Thijssen, S.; Chen, H.; Garssen, J.; Knippels, L.M.J.; Hogenkamp, A. Selenium Modulates the Allergic Response to Whey Protein in a Mouse Model for Cow’s Milk Allergy. Nutrients 2021, 13, 2479. https://doi.org/10.3390/nu13082479
Zhao X, Thijssen S, Chen H, Garssen J, Knippels LMJ, Hogenkamp A. Selenium Modulates the Allergic Response to Whey Protein in a Mouse Model for Cow’s Milk Allergy. Nutrients. 2021; 13(8):2479. https://doi.org/10.3390/nu13082479
Chicago/Turabian StyleZhao, Xiaoli, Suzan Thijssen, Hongbing Chen, Johan Garssen, Leon M. J. Knippels, and Astrid Hogenkamp. 2021. "Selenium Modulates the Allergic Response to Whey Protein in a Mouse Model for Cow’s Milk Allergy" Nutrients 13, no. 8: 2479. https://doi.org/10.3390/nu13082479
APA StyleZhao, X., Thijssen, S., Chen, H., Garssen, J., Knippels, L. M. J., & Hogenkamp, A. (2021). Selenium Modulates the Allergic Response to Whey Protein in a Mouse Model for Cow’s Milk Allergy. Nutrients, 13(8), 2479. https://doi.org/10.3390/nu13082479





