Synergistic Neuroprotective Effects of a Natural Product Mixture against AD Hallmarks and Cognitive Decline in Caenorhabditis elegans and an SAMP8 Mice Model
Abstract
1. Introduction
2. Materials and Methods
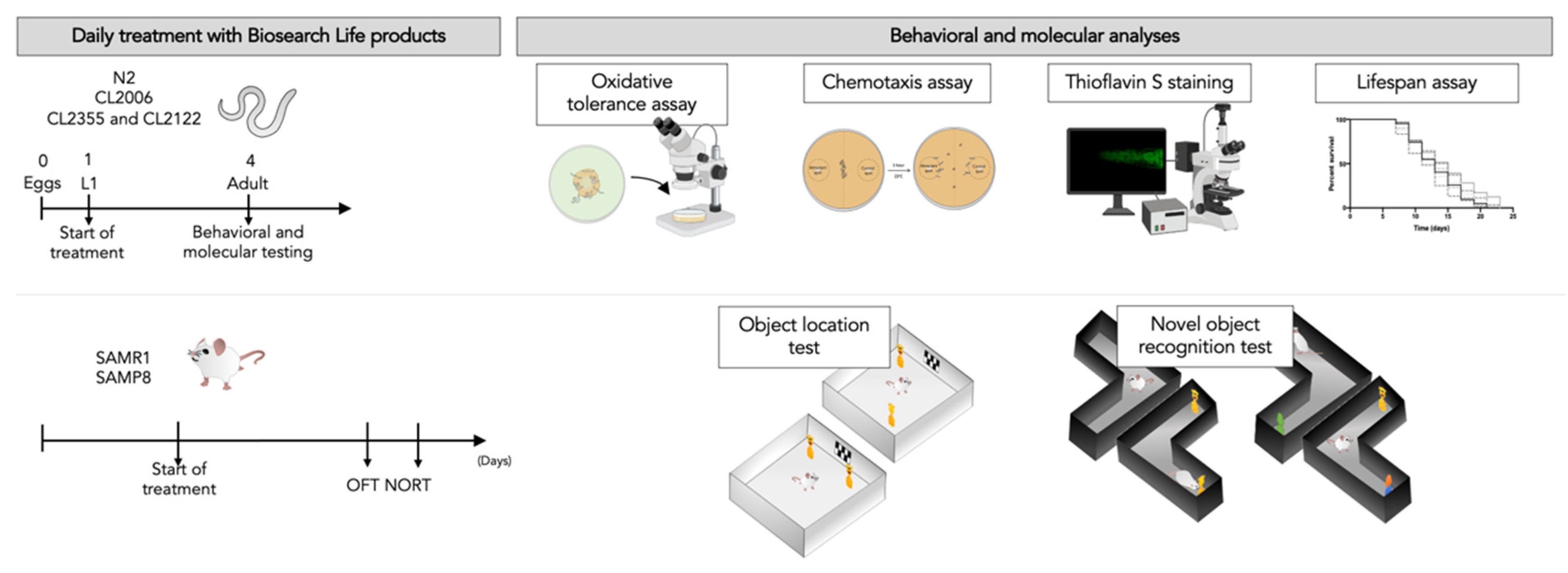
2.1. Study Design
2.2. Worm Strains, Maintenance, and General Methods
2.3. Mice and Maintenance
2.4. Compound Preparation and Treatments
2.5. C. elegans: Oxidative Tolerance Assay
2.6. C. elegans: Chemotaxis Assay
2.7. C. elegans: Thioflavin-S Staining Aß Aggregation
2.8. C. elegans: Lifespan Assay
2.9. SAMP8 Mice: Novel Object Recognition Test
2.10. SAMP8 Mice: Object Location Test
2.11. Statistics
3. Results
3.1. Natural Product Mixture Attenuates Oxidative Stress in C. elegans
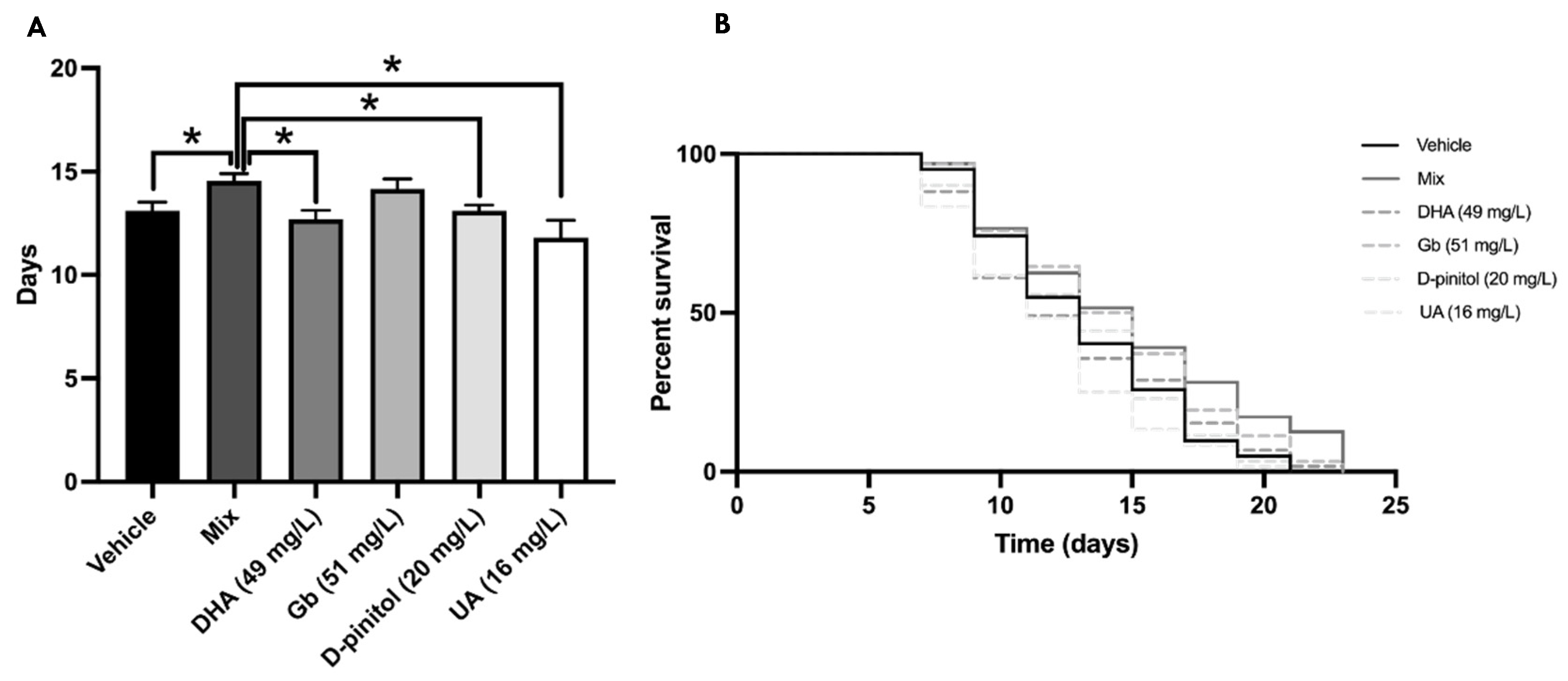
3.2. Mean Lifespan Extension by Natural Product Mixture in C. elegans
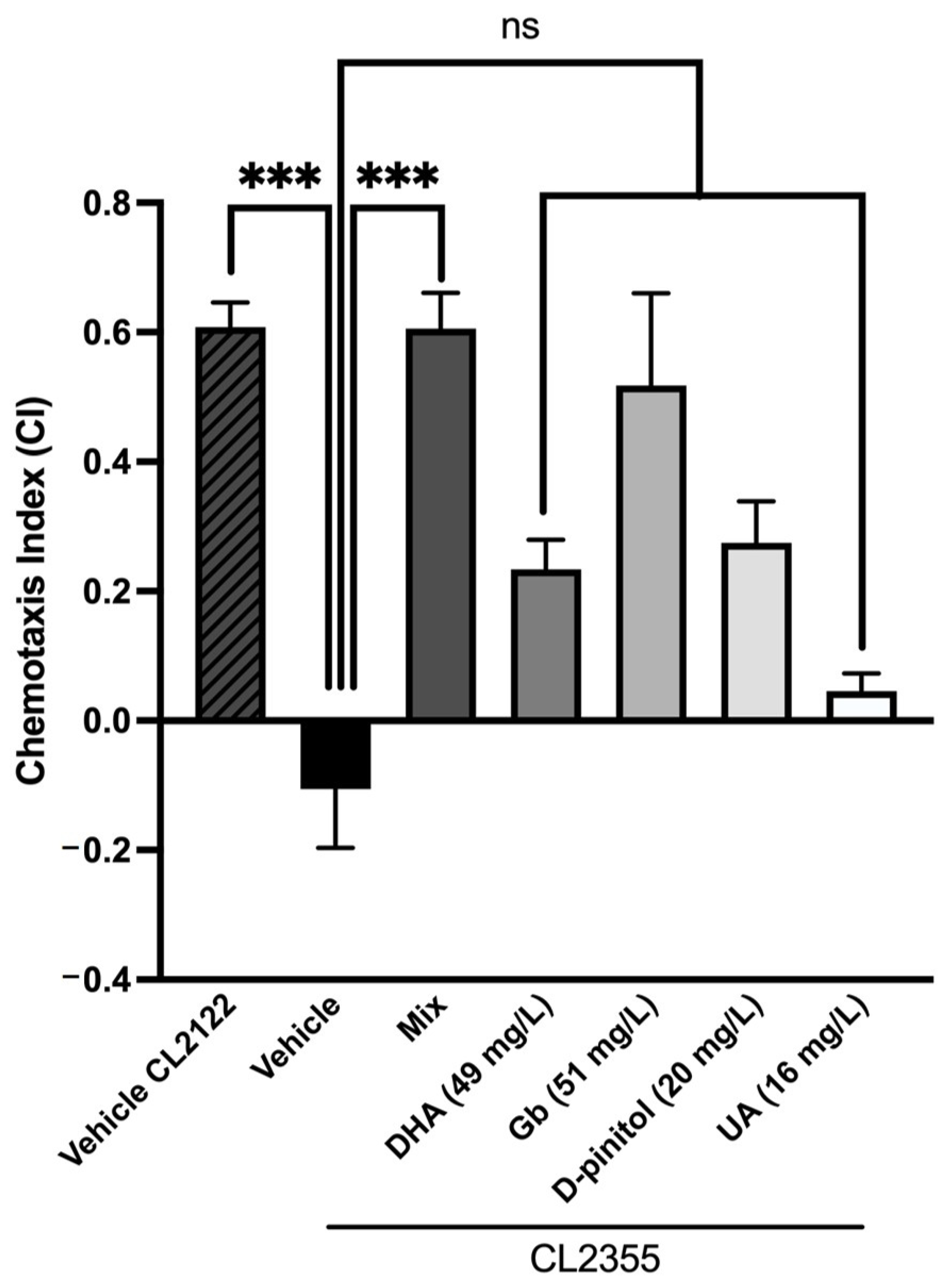
3.3. Natural Product Mixture Suppresses Neuronal Aβ Expression-Induced Defectd in Chemotaxis Behavior in Transgenic C. elegans (CL2355)
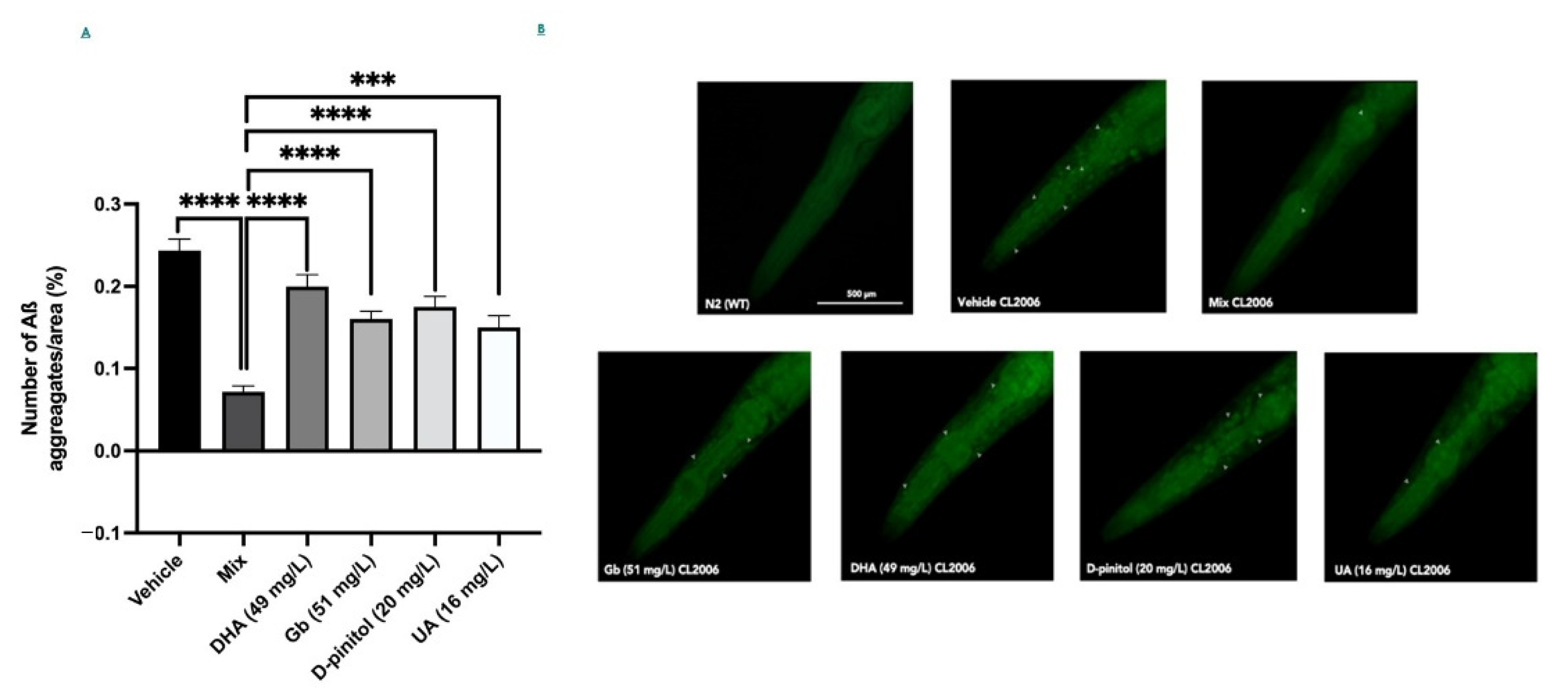
3.4. Natural Product Mixture Improves Amyloid-ß Burden in Transgenic C. elegans (CL2006)
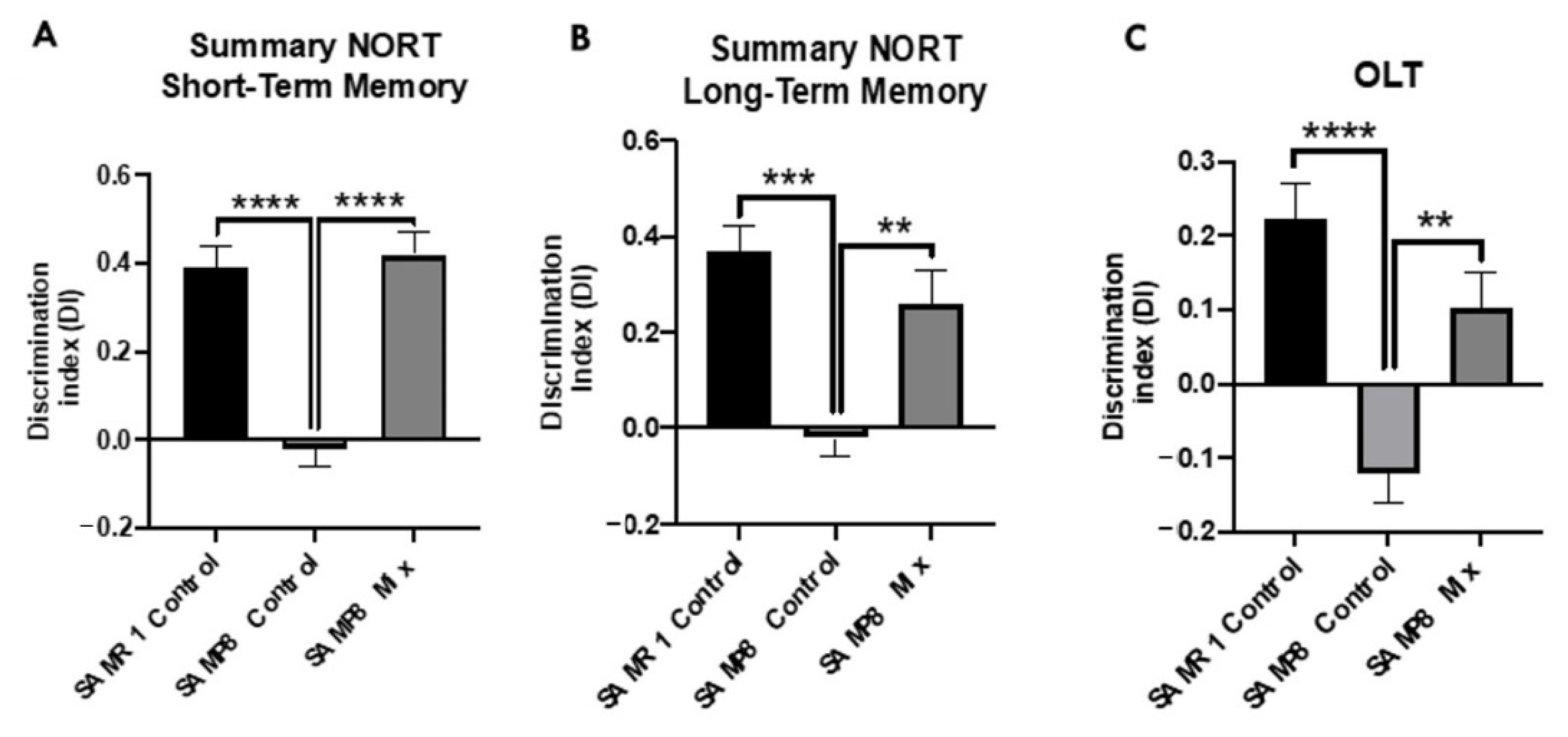
3.5. Product Mixture Improves Cognitive Decline Presented by the SAMP8 Mice Model
4. Discussion
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association 2018 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2018, 14, 367–429. [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Fan, L.; Mao, C.; Hu, X.; Zhang, S.; Yang, Z.; Hu, Z.; Sun, H.; Fan, Y.; Dong, Y.; Yang, J. New insights into the pathogenesis of Alzheimer’s disease. Front. Neurol. 2020, 10, 1312. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: Understanding the therapeutics strategies. Mol. Neurobiol. 2016, 53, 648–661. [Google Scholar] [CrossRef]
- Calkins, M.J.; Manczak, M.; Mao, P.; Shirendeb, U.; Reddy, P.H. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2011, 20, 4515–4529. [Google Scholar] [CrossRef]
- Manczak, M.; Anekonda, T.S.; Henson, E.; Park, B.S.; Quinn, J.; Reddy, P.H. Mitochondria are a direct site of Aβ accumulation in Alzheimer’s disease neurons: Implications for free radical generation and oxidative damage in disease progression. Hum. Mol. Genet. 2006, 15, 1437–1449. [Google Scholar] [CrossRef]
- Donev, R.; Kolev, M.; Millet, B.; Thome, J. Neuronal death in Alzheimer’s disease and therapeutic opportunities. J. Cell. Mol. Med. 2009, 13, 4329–4348. [Google Scholar] [CrossRef] [PubMed]
- Lanari, A.; Amenta, F.; Silvestrelli, G.; Tomassoni, D.; Parnetti, L. Neurotransmitter deficits in behavioural and psychological symptoms of Alzheimer’s disease. Mech. Ageing Dev. 2006, 127, 158–165. [Google Scholar] [CrossRef]
- Hajjar, I.; Hayek, S.S.; Goldstein, F.C.; Martin, G.; Jones, D.P.; Quyyumi, A. Oxidative stress predicts cognitive decline with aging in healthy adults: An observational study. J. Neuroinflammation 2018, 15, 17. [Google Scholar] [CrossRef]
- Head, E. Oxidative damage and cognitive dysfunction: Antioxidant treatments to promote healthy brain aging. Neurochem. Res. 2009, 34, 670–678. [Google Scholar] [CrossRef]
- Di Domenico, F.; Perluigi, M.; Butterfield, D.A.; Cornelius, C.; Calabrese, V. Oxidative damage in rat brain during aging: Interplay between energy and metabolic key target proteins. Neurochem. Res. 2010, 35, 2184–2192. [Google Scholar] [CrossRef]
- Kim, T.; Pae, C.; Yoon, S.; Jang, W.; Lee, N.J.; Kim, J.; Lee, S.; Lee, C.; Paik, I.; Lee, C. Decreased plasma antioxidants in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2006, 21, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Trushina, E.; McMurray, C.T. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience 2007, 145, 1233–1248. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. FDA Approves Controversial Alzheimer’s Drug despite Uncertainty over Effectiveness. BMJ 2021, 373, 1462. [Google Scholar] [CrossRef]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and inflammation in cognitive ageing and Alzheimer’s disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Canevelli, M.; Lucchini, F.; Quarata, F.; Bruno, G.; Cesari, M. Nutrition and dementia: Evidence for preventive approaches? Nutrients 2016, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Schelke, M.W.; Hackett, K.; Chen, J.L.; Shih, C.; Shum, J.; Montgomery, M.E.; Chiang, G.C.; Berkowitz, C.; Seifan, A.; Krikorian, R. Nutritional interventions for Alzheimer’s prevention: A clinical precision medicine approach. Ann. N. Y. Acad. Sci. 2016, 1367, 50. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Barbagallo, M. Nutritional prevention of cognitive decline and dementia. Acta Bio Med. Atenei Parm. 2018, 89, 276–290. [Google Scholar]
- Ge, W.; Ren, C.; Xing, L.; Guan, L.; Zhang, C.; Sun, X.; Wang, G.; Niu, H.; Qun, S. Ginkgo biloba extract improves cognitive function and increases neurogenesis by reducing Aβ pathology in 5× FAD mice. Am. J. Transl. Res. 2021, 13, 1471. [Google Scholar]
- Qin, Y.; Zhang, Y.; Tomic, I.; Hao, W.; Menger, M.D.; Liu, C.; Fassbender, K.; Liu, Y. Ginkgo biloba extract EGb 761 and its specific components elicit protective protein clearance through the autophagy-lysosomal pathway in tau-transgenic mice and cultured neurons. J. Alzheimer’s Dis. 2018, 65, 243–263. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Z.; Butko, P.; Christen, Y.; Lambert, M.P.; Klein, W.L.; Link, C.D.; Luo, Y. Amyloid-β-induced pathological behaviors are suppressed by Ginkgo biloba extract EGB 761 and ginkgolides in transgenic Caenorhabditis elegans. J. Neurosci. 2006, 26, 13102–13113. [Google Scholar] [CrossRef]
- Pitt, J.; Thorner, M.; Brautigan, D.; Larner, J.; Klein, W.L. Protection against the synaptic targeting and toxicity of Alzheimer’s-associated Aβ oligomers by insulin mimetic chiro-inositols. Faseb J. 2013, 27, 199–207. [Google Scholar] [CrossRef]
- Habtemariam, S. Antioxidant and anti-inflammatory mechanisms of neuroprotection by ursolic acid: Addressing brain injury, cerebral ischemia, cognition deficit, anxiety, and depression. Oxid. Med. Cell. Longev. 2019, 2019, 8512048. [Google Scholar] [CrossRef]
- Ramos-Hryb, A.B.; Pazini, F.L.; Kaster, M.P.; Rodrigues, A.L.S. Therapeutic potential of ursolic acid to manage neurodegenerative and psychiatric diseases. Cns Drugs 2017, 31, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Zhao, X.; Feng, J.; Song, F.; Pan, Y. Ursolic acid attenuates beta-amyloid-induced memory impairment in mice. Arq. Neuropsiquiatr. 2016, 74, 482–488. [Google Scholar] [CrossRef][Green Version]
- Patrick, R.P. Role of phosphatidylcholine-DHA in preventing APOE4-associated Alzheimer’s disease. Faseb J. 2019, 33, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ding, L.; Wen, M.; Che, H.; Huang, J.; Zhang, T.; Xue, C.; Mao, X.; Wang, Y. Mechanisms of DHA-enriched phospholipids in improving cognitive deficits in aged SAMP8 mice with high-fat diet. J. Nutr. Biochem. 2018, 59, 64–75. [Google Scholar] [CrossRef]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R.; Weiner, M. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Shin, S.J.; Hong, S.B.; Kim, S.; Nam, Y.; Kim, J.-J.; Lim, K.; Kim, J.-S.; Kim, J.; Jeon, S.G. Omega-3 Fatty Acid-Type Docosahexaenoic Acid Protects against Aβ-Mediated Mitochondrial Deficits and Pathomechanisms in Alzheimer’s Disease-Related Animal Model. Int. J. Mol. Sci. 2020, 21, 3879. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-H.; Youn, K.; Ho, C.-T.; Karwe, M.V.; Jeong, W.-S.; Jun, M. p-Coumaric acid and ursolic acid from corni fructus attenuated β-Amyloid25–35-induced toxicity through regulation of the NF-κB signaling pathway in PC12 cells. J. Agric. Food Chem. 2014, 62, 4911–4916. [Google Scholar] [CrossRef]
- Gohil, K.; Packer, L. Global gene expression analysis identifies cell and tissue specific actions of Ginkgo biloba extract, EGb 761. Cell. Mol. Biol. (Noisy-Le-Grand) 2002, 48, 625–631. [Google Scholar]
- Heo, H.-J.; Cho, H.-Y.; Hong, B.; Kim, H.-K.; Heo, T.-R.; Kim, E.-K.; Kim, S.-K.; Kim, C.-J.; Shin, D.-H. Ursolic acid of Origanum majorana L. reduces Abeta-induced oxidative injury. Mol. Cells 2002, 13, 5–11. [Google Scholar]
- Smith, J.V.; Luo, Y. Studies on molecular mechanisms of Ginkgo biloba extract. Appl. Microbiol. Biotechnol. 2004, 64, 465–472. [Google Scholar]
- Wei, T.; Ni, Y.; Hou, J.; Chen, C.; Zhao, B.; Xin, W. Hydrogen peroxide-induced oxidative damage and apoptosis in cerebellar granule cells: Protection by Ginkgo biloba extract. Pharm. Res. 2000, 41, 427–433. [Google Scholar] [CrossRef]
- Liu, H.; Ye, M.; Guo, H. An updated review of randomized clinical trials testing the improvement of cognitive function of Ginkgo biloba extract in healthy people and Alzheimer’s patients. Front. Pharm. 2020, 10, 1688. [Google Scholar] [CrossRef]
- Arellanes, I.C.; Choe, N.; Solomon, V.; He, X.; Kavin, B.; Martinez, A.E.; Kono, N.; Buennagel, D.P.; Hazra, N.; Kim, G. Brain delivery of supplemental docosahexaenoic acid (DHA): A randomized placebo-controlled clinical trial. EBioMedicine 2020, 59, 102883. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, A.M.; González-Ortiz, M.; Martínez-Abundis, E.; Acuña Ortega, N. Effect of ursolic acid on metabolic syndrome, insulin sensitivity, and inflammation. J. Med. Food 2017, 20, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Grossman, H.; Marzloff, G.; Luo, X.; LeRoith, D.; Sano, M.; Pasinetti, G. NIC5-15 as a treatment for Alzheimer’s: Safety, pharmacokinetics and clinical variables. Alzheimer’s Dement. 2009, 5, P259. [Google Scholar] [CrossRef]
- Skroza, D.; Mekinić, I.G.; Svilović, S.; Šimat, V.; Katalinić, V. Investigation of the potential synergistic effect of resveratrol with other phenolic compounds: A case of binary phenolic mixtures. J. Food Compos. Anal. 2015, 38, 13–18. [Google Scholar] [CrossRef]
- Wang, S.; Wang, D.; Liu, Z. Synergistic, additive and antagonistic effects of Potentilla fruticosa combined with EGb761 on antioxidant capacities and the possible mechanism. Ind. Crop. Prod. 2015, 67, 227–238. [Google Scholar] [CrossRef]
- Pemovska, T.; Bigenzahn, J.W.; Superti-Furga, G. Recent advances in combinatorial drug screening and synergy scoring. Curr. Opin. Pharm. 2018, 42, 102–110. [Google Scholar] [CrossRef]
- Wagner, H. Synergy research: Approaching a new generation of phytopharmaceuticals. Fitoterapia 2011, 82, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.; Vantipalli, M.C.; Lithgow, G.J. Using Caenorhabditis elegans as a model for aging and age—related diseases. Ann. N. Y. Acad. Sci. 2006, 1067, 120–128. [Google Scholar] [CrossRef]
- Link, C.D. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1995, 92, 9368–9372. [Google Scholar] [CrossRef]
- Link, C.D. Invertebrate models of Alzheimer’s disease. GenesBrain Behav. 2005, 4, 147–156. [Google Scholar] [CrossRef]
- Pallàs, M. Senescence-Accelerated Mice P8: A Tool to Study Brain Aging and Alzheimer’s Disease in a Mouse Model. Isrn Cell Biol. 2012, 2012, 917167. [Google Scholar] [CrossRef]
- Takeda, T. Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochem. Res. 2009, 34, 639–659. [Google Scholar] [CrossRef]
- Morley, J.E.; Farr, S.A.; Kumar, V.B.; Armbrecht, H.J. The SAMP8 Mouse: A Model to Develop Therapeutic Interventions for Alzheimers Disease. Curr. Pharm. Des. 2012, 18, 1123–1130. [Google Scholar] [CrossRef]
- Griñan-Ferré, C.; Palomera-Ávalos, V.; Puigoriol-Illamola, D.; Camins, A.; Porquet, D.; Plá, V.; Aguado, F.; Pallàs, M. Behaviour and cognitive changes correlated with hippocampal neuroinflammaging and neuronal markers in female SAMP8, a model of accelerated senescence. Exp. Gerontol. 2016, 80, 57–69. [Google Scholar] [CrossRef]
- Griñan-Ferré, C.; Pérez-Cáceres, D.; Gutiérrez-Zetina, S.M.; Camins, A.; Palomera-Avalos, V.; Ortuño-Sahagún, D.; Rodrigo, M.T.; Pallàs, M. Environmental enrichment improves behavior, cognition, and brain functional markers in young senescence-accelerated prone mice (SAMP8). Mol. Neurobiol. 2016, 53, 2435–2450. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Kumar, V.B.; Bernardo, A.E.; Farr, S.A.; Uezu, K.; Tumosa, N.; Flood, J.F. β-Amyloid precursor polypeptide in SAMP8 mice affects learning and memory. Peptides 2000, 21, 1761–1767. [Google Scholar] [CrossRef]
- Porquet, D.; Andrés-Benito, P.; Griñán-Ferré, C.; Camins, A.; Ferrer, I.; Canudas, A.M.; Del Valle, J.; Pallàs, M. Amyloid and tau pathology of familial Alzheimer’s disease APP/PS1 mouse model in a senescence phenotype background (SAMP8). Age (Omaha) 2015, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Poon, H.F. The senescence-accelerated prone mouse (SAMP8): A model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp. Gerontol. 2005, 40, 774–783. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Ennaceur, A.; Meliani, K. A new one-trial test for neurobiological studies of memory in rats. III. Spatial vs. non-spatial working memory. Behav. Brain Res. 1992, 51, 83–92. [Google Scholar] [CrossRef]
- Companys-Alemany, J.; Turcu, A.L.; Bellver-Sanchis, A.; Loza, M.I.; Brea, J.M.; Canudas, A.M.; Leiva, R.; Vázquez, S.; Pallàs, M.; Griñán-Ferré, C. A novel NMDA receptor antagonist protects against cognitive decline presented by senescent mice. Pharmaceutics 2020, 12, 284. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J. New approaches to symptomatic treatments for Alzheimer’s disease. Mol. Neurodegener. 2021, 16, 2. [Google Scholar]
- Knopman, D.S.; Jones, D.T.; Greicius, M.D. Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimer’s Dement. 2021, 17, 696–701. [Google Scholar] [CrossRef]
- Russo, P.; Frustaci, A.; Del Bufalo, A.; Fini, M.; Cesario, A. Multitarget drugs of plants origin acting on Alzheimer’s disease. Curr. Med. Chem. 2013, 20, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, M.; Li, X.; Yue, J.; Chen, J.; Yang, M.; Huang, X.; Zhu, L.; Hong, F.; Yang, S. Therapeutic effects of natural drugs on Alzheimer’s disease. Front. Pharm. 2019, 10, 1355. [Google Scholar] [CrossRef]
- Choi, R.C.Y.; Zhu, J.T.T.; Yung, A.W.Y.; Lee, P.S.C.; Xu, S.L.; Guo, A.J.Y.; Zhu, K.Y.; Dong, T.T.X.; Tsim, K.W.K. Synergistic action of flavonoids, baicalein, and daidzein in estrogenic and neuroprotective effects: A development of potential health products and therapeutic drugs against Alzheimer’s disease. Evid. Based Complement. Altern. Med. 2013, 2013, 635694. [Google Scholar] [CrossRef]
- Cheung, D.W.-S.; Koon, C.-M.; Wat, E.; Ko, C.-H.; Chan, J.Y.-W.; Yew, D.T.-W.; Leung, P.-C.; Chan, W.-Y.; Bik-San Lau, C.; Fung, K.-P. A herbal formula containing roots of Salvia miltiorrhiza (Danshen) and Pueraria lobata (Gegen) inhibits inflammatory mediators in LPS-stimulated RAW 264.7 macrophages through inhibition of nuclear factor κB (NFκB) pathway. J. Ethnopharmacol. 2013, 145, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Bayram, B.; Nikolai, S.; Huebbe, P.; Ozcelik, B.; Grimm, S.; Grune, T.; Frank, J.; Rimbach, G. Biomarkers of oxidative stress, antioxidant defence and inflammation are altered in the senescence-accelerated mouse prone 8. Age (Omaha) 2013, 35, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Youssef, P.; Chami, B.; Lim, J.; Middleton, T.; Sutherland, G.T.; Witting, P.K. Evidence supporting oxidative stress in a moderately affected area of the brain in Alzheimer’s disease. Sci. Rep. 2018, 8, 11553. [Google Scholar] [CrossRef]
- Honma, T.; Shinohara, N.; Ito, J.; Kijima, R.; Sugawara, S.; Arai, T.; Tsuduki, T.; Ikeda, I. High-fat diet intake accelerates aging, increases expression of Hsd11b1, and promotes lipid accumulation in liver of SAMP10 mouse. Biogerontology 2012, 13, 93–103. [Google Scholar] [CrossRef]
- Tsuduki, T.; Honma, T.; Nakagawa, K.; Ikeda, I.; Miyazawa, T. Long-term intake of fish oil increases oxidative stress and decreases lifespan in senescence-accelerated mice. Nutrition 2011, 27, 334–337. [Google Scholar] [CrossRef]
- Zhou, K.I.; Pincus, Z.; Slack, F.J. Longevity and stress in Caenorhabditis elegans. Aging (Albany Ny) 2011, 3, 733. [Google Scholar] [CrossRef]
- Van Raamsdonk, J.M.; Hekimi, S. Reactive oxygen species and aging in Caenorhabditis elegans: Causal or casual relationship? Antioxid. Redox Signal. 2010, 13, 1911–1953. [Google Scholar] [CrossRef]
- Nunomura, A.; Perry, G.; Aliev, G.; Hirai, K.; Takeda, A.; Balraj, E.K.; Jones, P.K.; Ghanbari, H.; Wataya, T.; Shimohama, S. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001, 60, 759–767. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Mormino, E.C.; Papp, K.V. Amyloid accumulation and cognitive decline in clinically normal older individuals: Implications for aging and early Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 64, S633–S646. [Google Scholar] [CrossRef]
- Näslund, J.; Haroutunian, V.; Mohs, R.; Davis, K.L.; Davies, P.; Greengard, P.; Buxbaum, J.D. Correlation between elevated levels of amyloid β-peptide in the brain and cognitive decline. JAMA 2000, 283, 1571–1577. [Google Scholar] [CrossRef]
- Kampkötter, A.; Pielarski, T.; Rohrig, R.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. The Ginkgo biloba extract EGb761 reduces stress sensitivity, ROS accumulation and expression of catalase and glutathione S-transferase 4 in Caenorhabditis elegans. Pharm. Res. 2007, 55, 139–147. [Google Scholar] [CrossRef]
- Sugawara, S.; Honma, T.; Ito, J.; Kijima, R.; Tsuduki, T. Fish oil changes the lifespan of Caenorhabditis elegans via lipid peroxidation. J. Clin. Biochem. Nutr. 2013, 52, 139–145. [Google Scholar] [CrossRef]
- Naß, J.; Abdelfatah, S.; Efferth, T. Ursolic acid enhances stress resistance, reduces ROS accumulation and prolongs life span in C. elegans serotonin-deficient mutants. Food Funct. 2021, 12, 2242–2256. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharm. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Fusco, D.; Colloca, G.; Monaco, M.R.L.; Cesari, M. Effects of antioxidant supplementation on the aging process. Clin. Interv. Aging 2007, 2, 377. [Google Scholar] [PubMed]
- Wu, Y.; Luo, Y.; Transgenic, C. elegans as a model in Alzheimer’s research. Curr. Alzheimer Res. 2005, 2, 37–45. [Google Scholar] [CrossRef]
- Hobert, O. Behavioral plasticity in C. elegans: Paradigms, circuits, genes. J. Neurobiol. 2003, 54, 203–223. [Google Scholar] [CrossRef] [PubMed]

| Strain | Genotype | Source |
|---|---|---|
| N2 (Bristol) | C. elegans wild-type | |
| CL2006 | dvIs2 (pCL12 (unc-54/human Abeta peptide 1–42 minigene) + rol-6(su1006)) | CGC |
| CL2355 | dvIs50 (pCL45 (snb-1::Abeta 1–42::3’ UTR(long) + mtl-2::GFP) I | CGC |
| CL2122 | dvIs15 ((pPD30.38) unc-54(vector) + (pCL26) mtl-2::GFP) | CGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griñán-Ferré, C.; Bellver-Sanchis, A.; Olivares-Martín, M.; Bañuelos-Hortigüela, O.; Pallàs, M. Synergistic Neuroprotective Effects of a Natural Product Mixture against AD Hallmarks and Cognitive Decline in Caenorhabditis elegans and an SAMP8 Mice Model. Nutrients 2021, 13, 2411. https://doi.org/10.3390/nu13072411
Griñán-Ferré C, Bellver-Sanchis A, Olivares-Martín M, Bañuelos-Hortigüela O, Pallàs M. Synergistic Neuroprotective Effects of a Natural Product Mixture against AD Hallmarks and Cognitive Decline in Caenorhabditis elegans and an SAMP8 Mice Model. Nutrients. 2021; 13(7):2411. https://doi.org/10.3390/nu13072411
Chicago/Turabian StyleGriñán-Ferré, Christian, Aina Bellver-Sanchis, Mónica Olivares-Martín, Oscar Bañuelos-Hortigüela, and Mercè Pallàs. 2021. "Synergistic Neuroprotective Effects of a Natural Product Mixture against AD Hallmarks and Cognitive Decline in Caenorhabditis elegans and an SAMP8 Mice Model" Nutrients 13, no. 7: 2411. https://doi.org/10.3390/nu13072411
APA StyleGriñán-Ferré, C., Bellver-Sanchis, A., Olivares-Martín, M., Bañuelos-Hortigüela, O., & Pallàs, M. (2021). Synergistic Neuroprotective Effects of a Natural Product Mixture against AD Hallmarks and Cognitive Decline in Caenorhabditis elegans and an SAMP8 Mice Model. Nutrients, 13(7), 2411. https://doi.org/10.3390/nu13072411








