Cytotoxic, Genotoxic and Senolytic Potential of Native and Micellar Curcumin
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Lines and Culture Conditions
2.3. MTT Assay
2.4. Apoptosis and Necrosis
2.5. Senescence
2.6. Determination of Senolytic Activity
2.7. Comet Assays (Single Cell Gel Electrophoresis, SCGE)
2.8. ROS
2.9. Statistics
3. Results
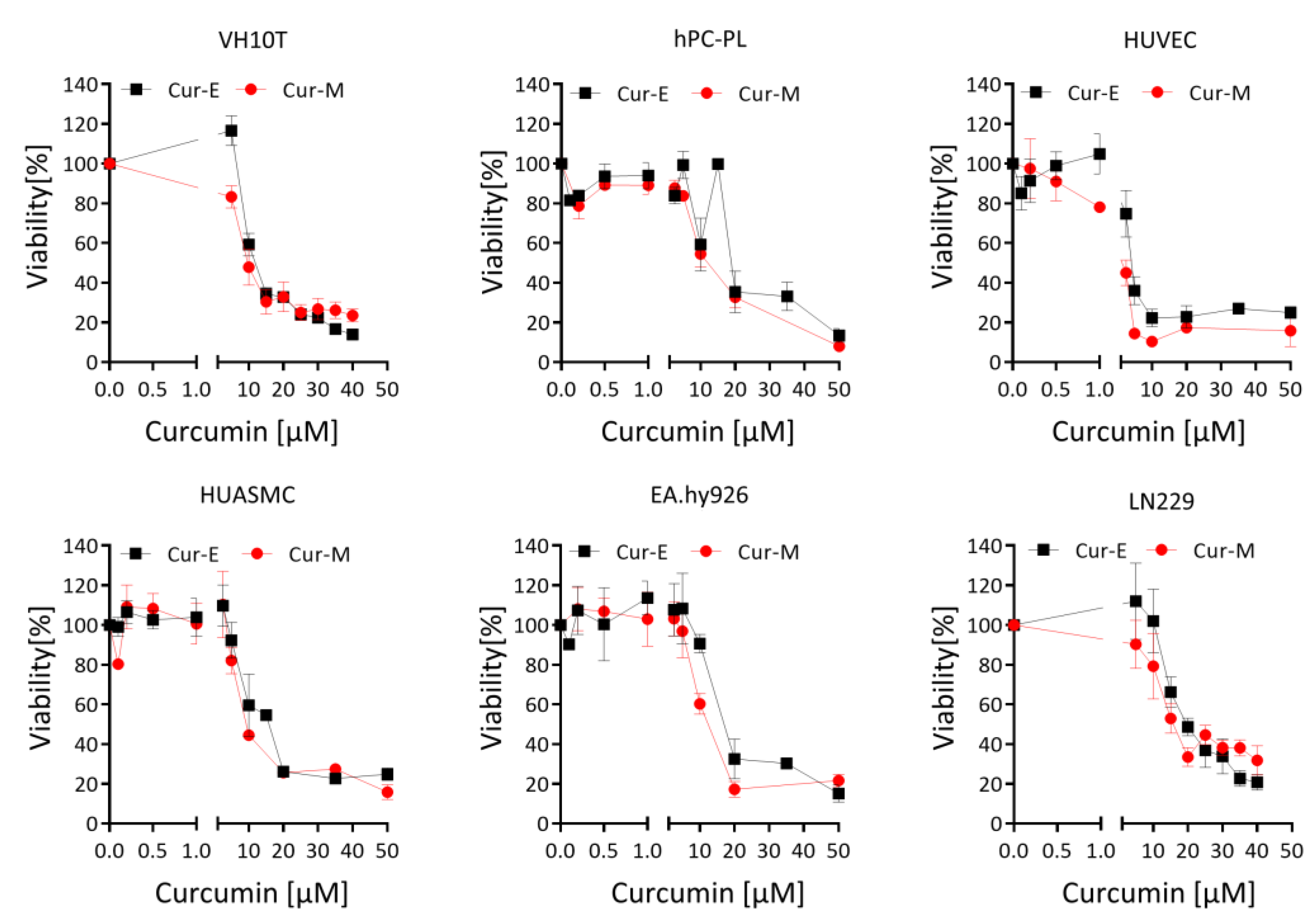
3.1. Effect of Cur-E and Cur-M on the Viability of Cells
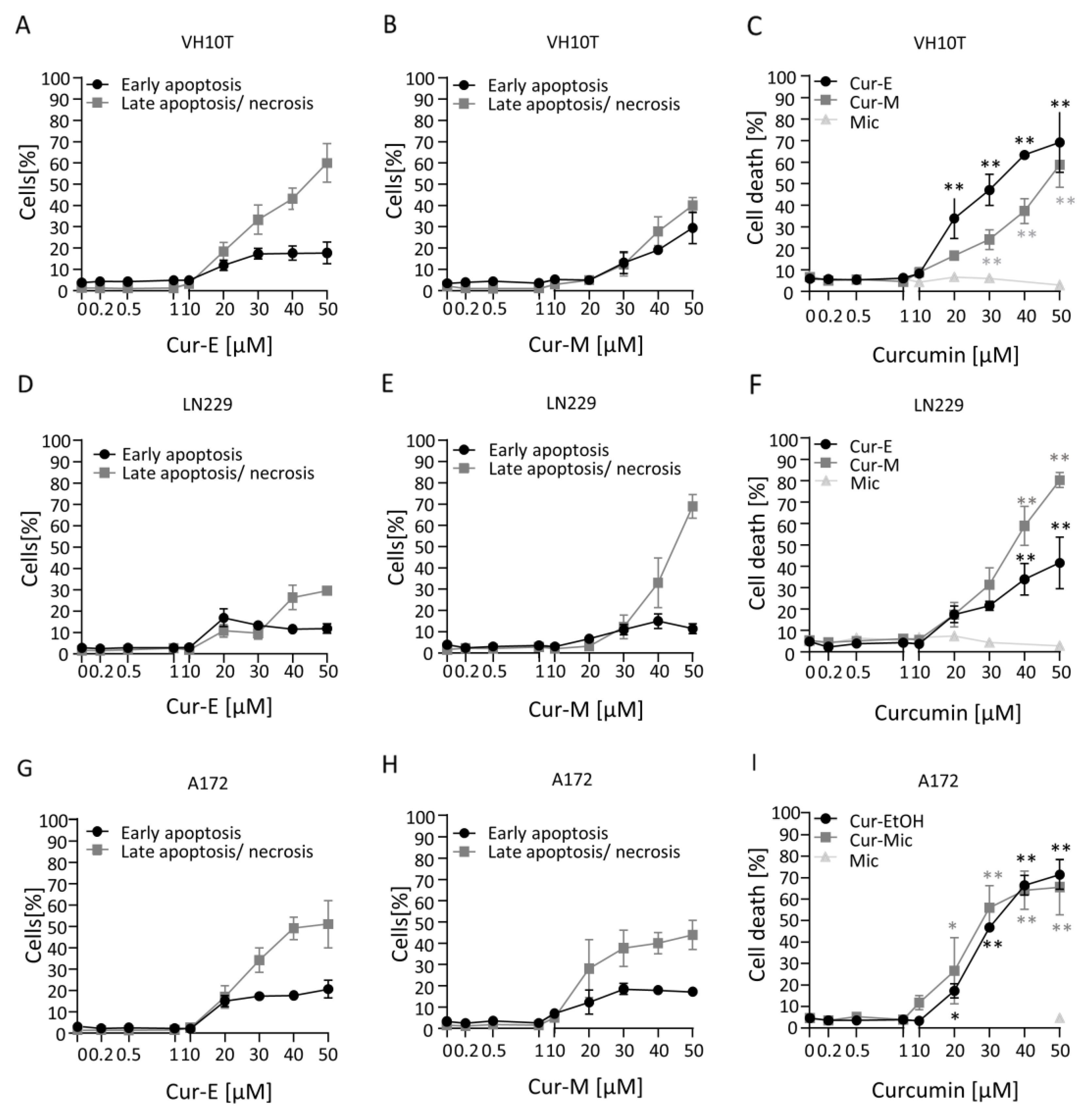
3.2. Cur-E and Cur-M Induce Cell Death by Apoptosis
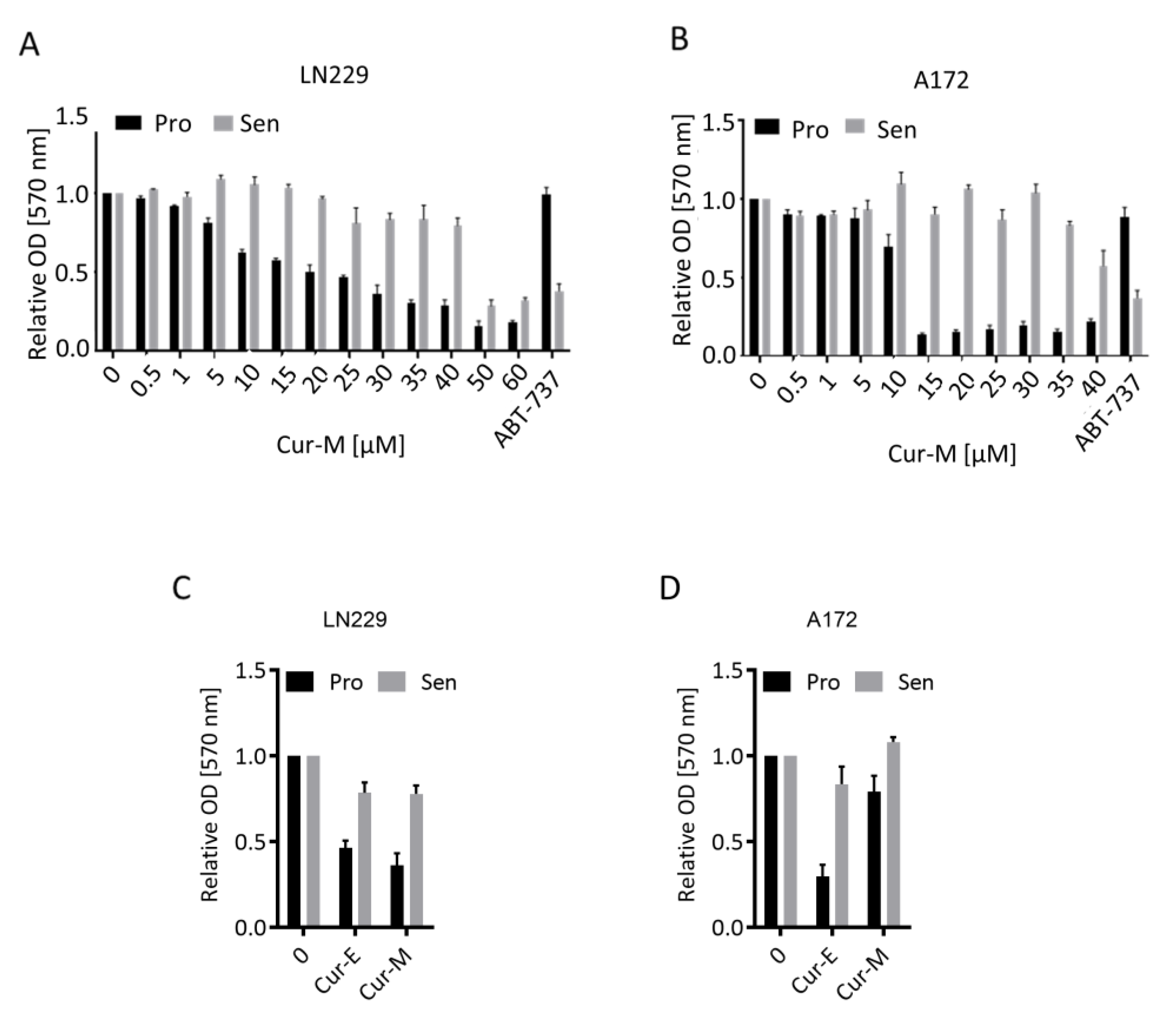
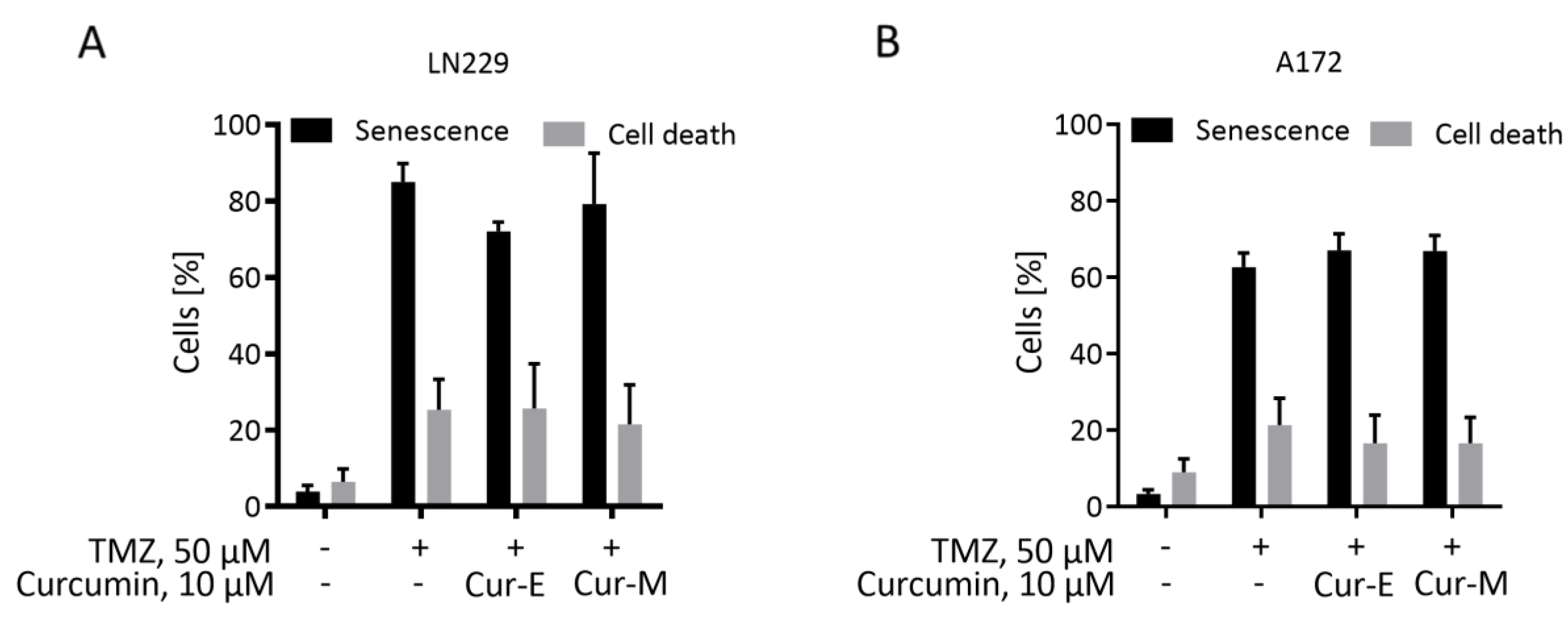
3.3. Cur-E and Cur-M Have No Senolytic Activity
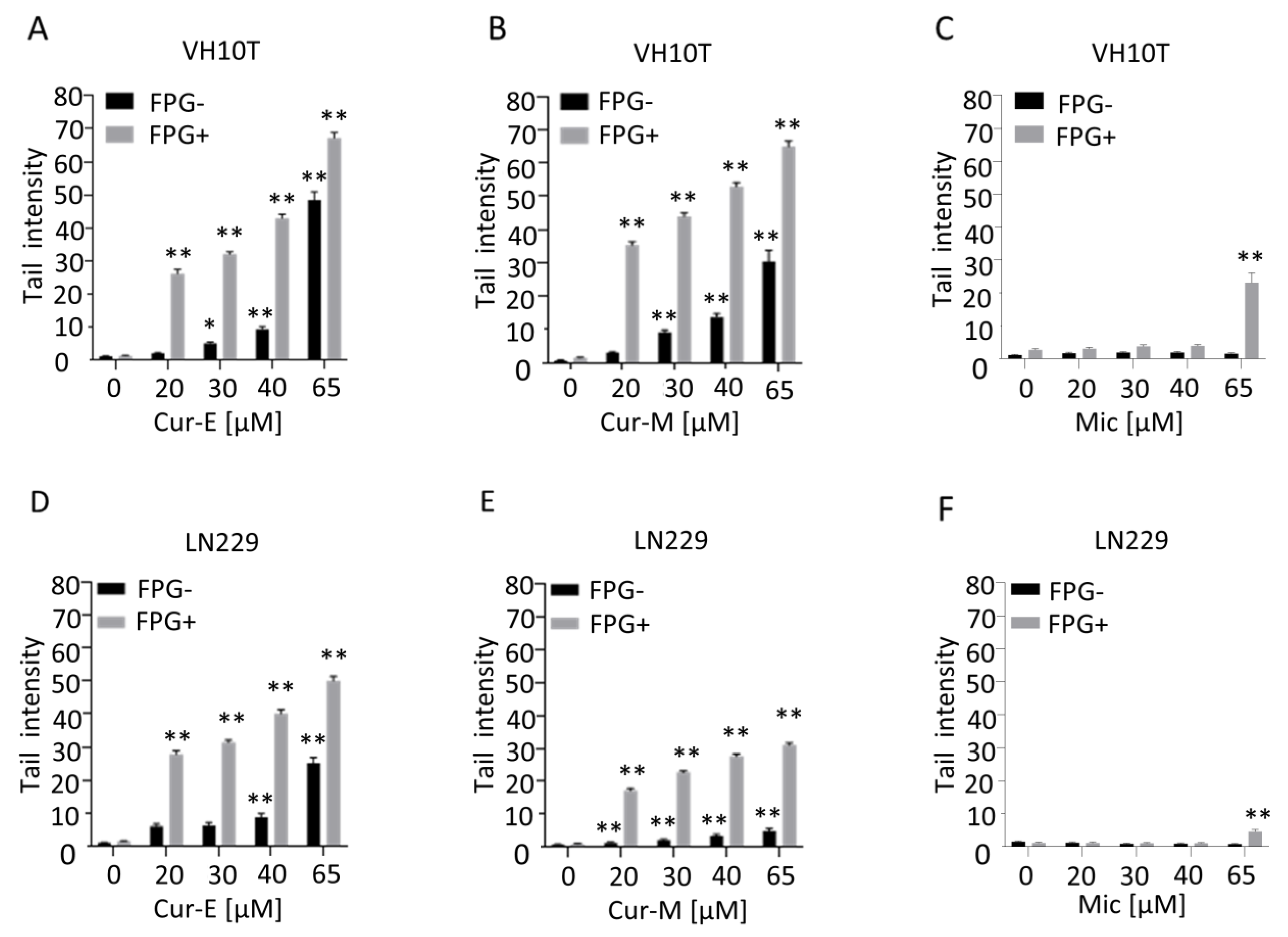
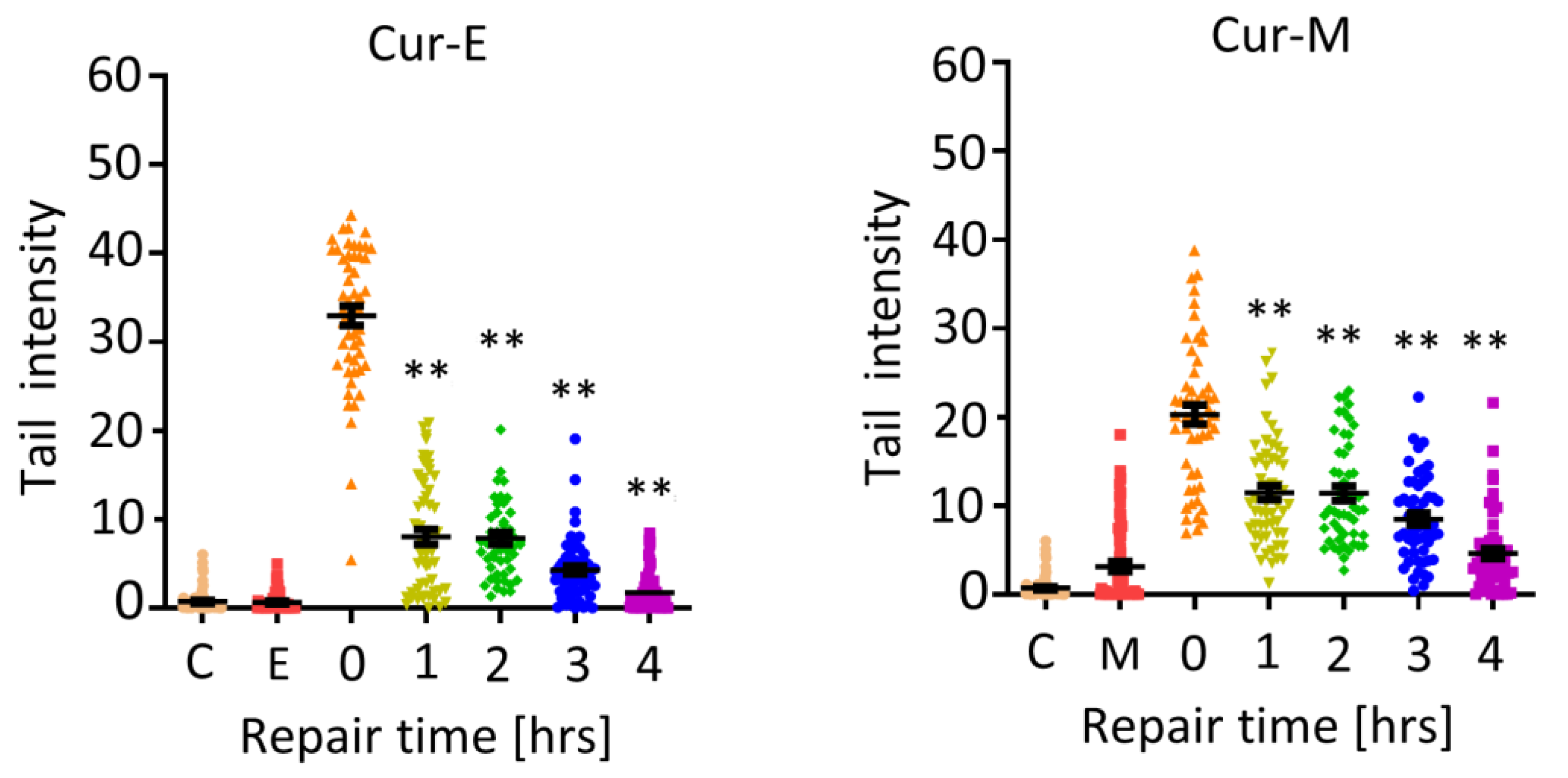
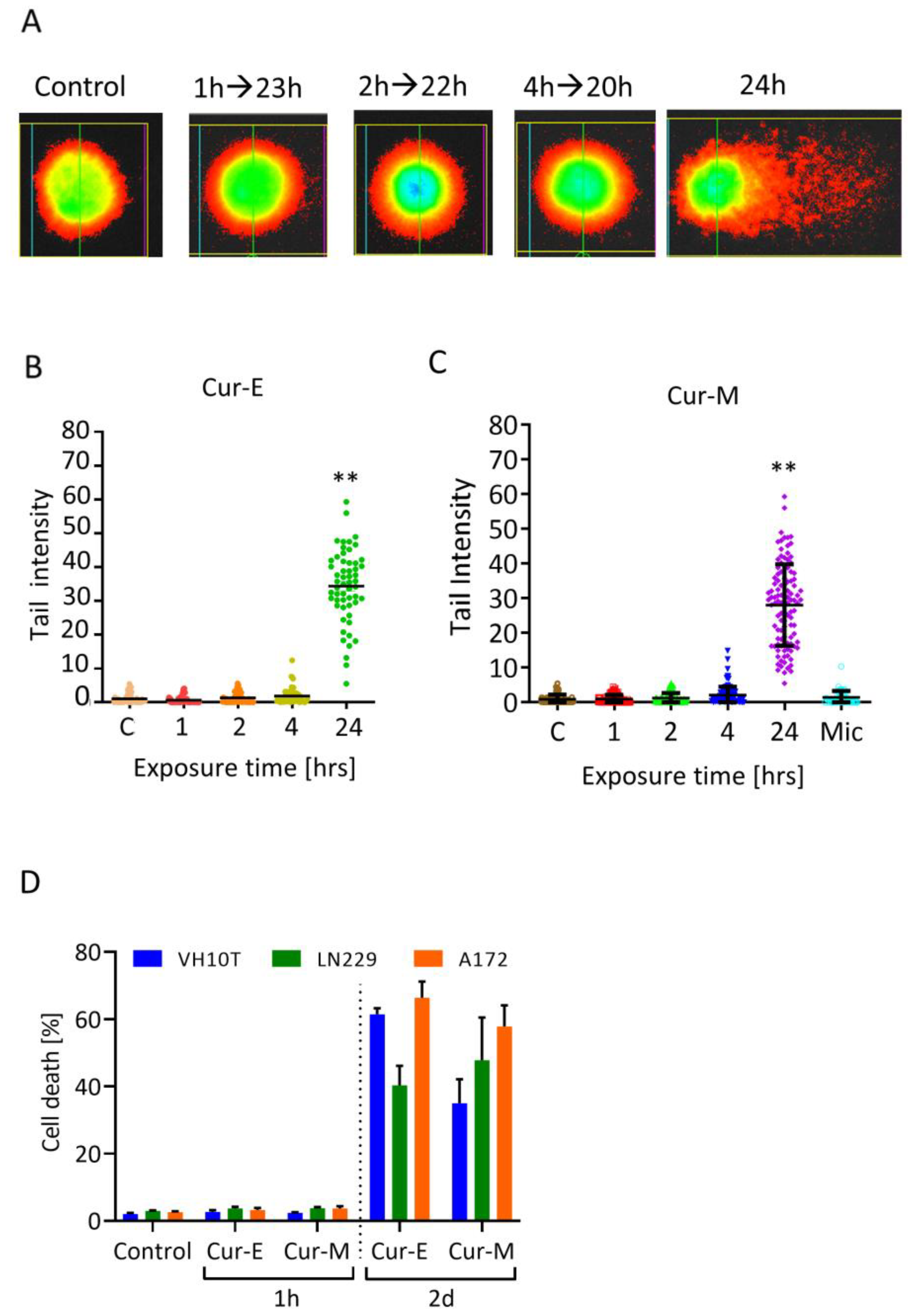
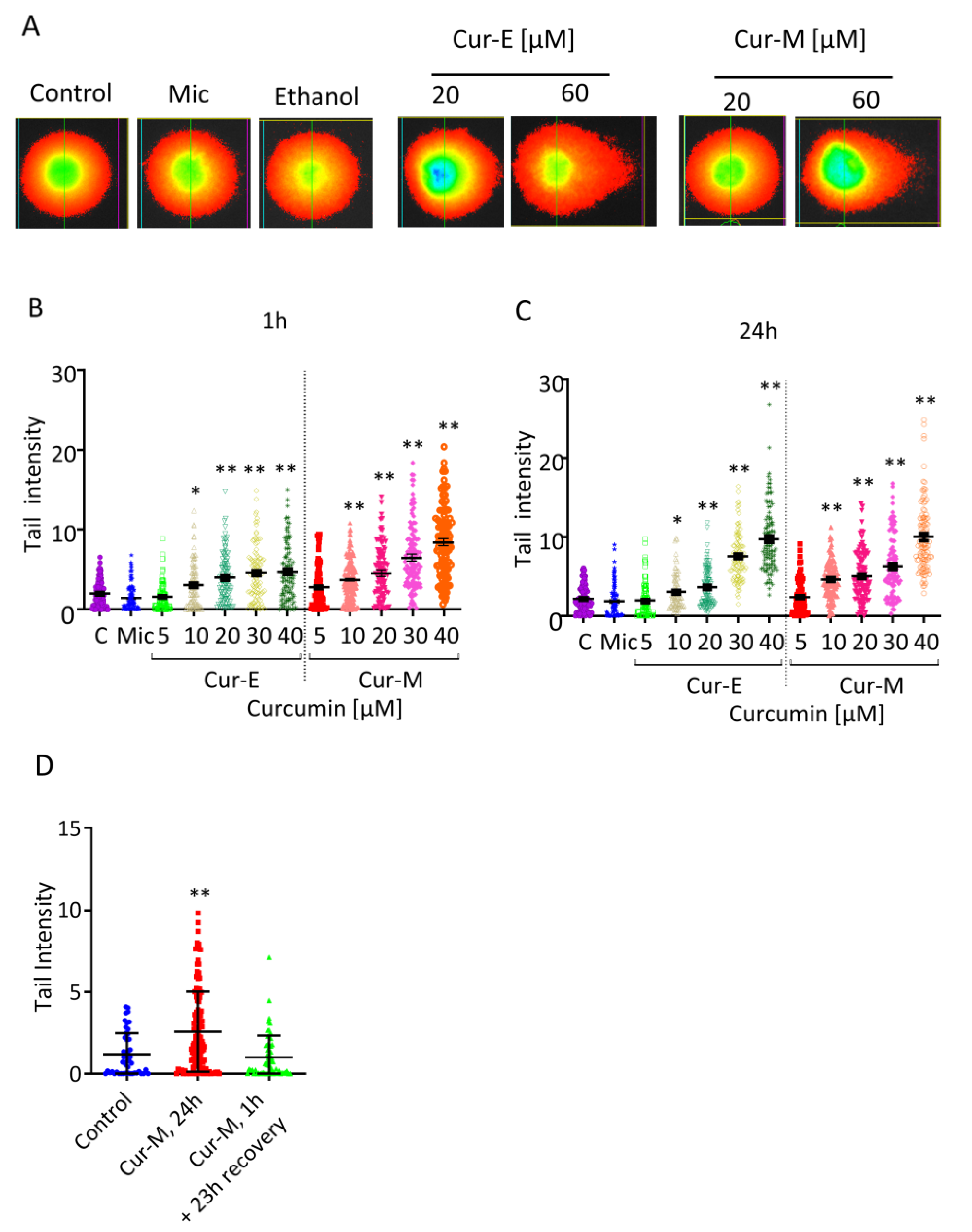
3.4. Analysis of Genotoxic Effects of Curcumin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, W.; Lu, J.; Huang, M.; Li, Y.; Chen, M.; Wu, G.; Gong, J.; Zhong, Z.; Xu, Z.; Dang, Y.; et al. Anti-cancer natural products isolated from chinese medicinal herbs. Chin. Med. 2011, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2010, 4, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Fukagawa, N.K.; Bilia, A.R.; Johnson, E.J.; Kwon, O.; Prakash, V.; Miyazawa, T.; Clifford, M.N.; Kay, C.; Crozier, A.; et al. Terms and nomenclature used for plant-derived components in nutrition and related research: Efforts toward harmonization. Nutr. Rev. 2019, 78, 451–458. [Google Scholar] [CrossRef]
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Burkard, M.; Leischner, C.; Lauer, U.M.; Busch, C.; Venturelli, S.; Frank, J. Dietary flavonoids and modulation of natural killer cells: Implications in malignant and viral diseases. J. Nutr. Biochem. 2017, 46, 1–12. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef]
- Shehzad, A.; Khan, S.; Lee, Y. Curcumin therapeutic promises and bioavailability in colorectal cancer. Drugs Today 2010, 46, 523–532. [Google Scholar] [CrossRef]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in Cancer Chemoprevention: Molecular Targets, Pharmacokinetics, Bioavailability, and Clinical Trials. Arch. Pharm. 2010, 343, 489–499. [Google Scholar] [CrossRef]
- Kasi, P.D.; Tamilselvam, R.; Skalicka-Woźniak, K.; Nabavi, S.M.; Daglia, M.; Bishayee, A.; Pazoki-Toroudi, H. Molecular targets of curcumin for cancer therapy: An updated review. Tumor Biol. 2016, 37, 13017–13028. [Google Scholar] [CrossRef]
- Bortel, N.; Armeanu-Ebinger, S.; Schmid, E.; Kirchner, B.; Frank, J.; Kocher, A. Effects of curcumin in pediatric epithelial liver tumors: Inhibition of tumor growth and alpha-fetoprotein in vitro and in vivo involving the NFkappaB- and the beta-catenin pathways. Oncotarget 2015, 6, 40680–40691. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Chuang, S.-E.; Hergenhahn, M.; Kuo, M.-L.; Lin, J.-K.; Hsieh, C.-Y.; Cheng, A.-L. Pre-clinical and early-phase clinical studies of curcumin as chemopreventive agent for endemic cancers in Taiwan. Gan Kagaku Ryoho 2002, 29 (Suppl. 1), 194–200. [Google Scholar]
- Hsu, C.-H.; Cheng, A.-L. Clinical Studies with Curcumin. Adv. Exp. Med. Biol. 2007, 595, 471–480. [Google Scholar]
- Allegra, A.; Innao, V.; Russo, S.; Gerace, D.; Alonci, A.; Musolino, C. Anticancer Activity of Curcumin and Its Analogues: Preclinical and Clinical Studies. Cancer Investig. 2017, 35, 1–22. [Google Scholar] [CrossRef]
- Aller, L.L. What about bioavailability of oral curcumin? Can. Med. Assoc. J. 2019, 191, E427. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.X.; Moist, L.; Pannu, N.; Tobe, S.; Walsh, M.; Weir, M. Bioavailability of oral curcumin. Can. Med. Assoc. J. 2019, 191, E428. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: The Golden Pigment from Golden Spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Metzler, M.; Pfeiffer, E.; Schulz, S.I.; Dempe, J.S. Curcumin uptake and metabolism. BioFactors 2012, 39, 14–20. [Google Scholar] [CrossRef]
- Tønnesen, H.H. Solubility, chemical and photochemical stability of curcumin in surfactant solutions. Studies of curcumin and curcuminoids, XXVIII. Die Pharm. 2002, 57, 820–824. [Google Scholar]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelstede, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014, 58, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Khayyal, M.T.; El-Hazek, R.M.; Elsabbagh, W.; Frank, J.; Behnam, D.; Abdel-Tawab, M. Micellar solubilisation enhances the antiinflammatory activities of curcumin and boswellic acids in rats with adjuvant-induced arthritis. Nutrition 2018, 54, 189–196. [Google Scholar] [CrossRef]
- Kocher, A.; Bohnert, L.; Schiborr, C.; Frank, J. Highly bioavailable micellar curcuminoids accumulate in blood, are safe and do not reduce blood lipids and inflammation markers in moderately hyperlipidemic individuals. Mol. Nutr. Food Res. 2016, 60, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Dützmann, S.; Schiborr, C.; Kocher, A.; Pilatus, U.; Hattingen, E.; Weissenberger, J.; Geßler, F.; Quick-Weller, J.; Franz, K.; Seifert, V.; et al. Intratumoral Concentrations and Effects of Orally Administered Micellar Curcuminoids in Glioblastoma Patients. Nutr. Cancer 2016, 68, 943–948. [Google Scholar] [CrossRef]
- Damarla, S.R.; Komma, R.; Bhatnagar, U.; Rajesh, N.; Mulla, S.M.A. An Evaluation of the Genotoxicity and Subchronic Oral Toxicity of Synthetic Curcumin. J. Toxicol. 2018, 2018, 6872753. [Google Scholar] [CrossRef]
- Decker, E.A. Phenolics: Prooxidants or antioxidants? Nutr. Rev. 1997, 55, 396–398. [Google Scholar] [CrossRef]
- Srinivasan, M.; Prasad, N.R.; Menon, V.P. Protective effect of curcumin on gamma-radiation induced DNA damage and lipid peroxidation in cultured human lymphocytes. Mutat. Res. 2006, 611, 96–103. [Google Scholar] [CrossRef]
- Seyithanoğlu, M.H.; Abdallah, A.; Kitiş, S.; Guler, E.M.; Koçyiğit, A.; Dündar, T.T.; Papaker, M.G. Investigation of cytotoxic, genotoxic, and apoptotic effects of curcumin on glioma cells. Cell. Mol. Biol. 2019, 65, 101–108. [Google Scholar] [CrossRef]
- Cao, J.; Jia, L.; Zhou, H.-M.; Liu, Y.; Zhong, L.-F. Mitochondrial and Nuclear DNA Damage Induced by Curcumin in Human Hepatoma G2 Cells. Toxicol. Sci. 2006, 91, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, L.M.; Dos Santos, G.C.; Antonucci, G.A.; Santos, A.C.; Bianchi, M.D.L.P.; Antunes, L.M.G. Evaluation of the cytotoxicity and genotoxicity of curcumin in PC12 cells. Mutat. Res. Toxicol. Environ. Mutagen. 2009, 675, 29–34. [Google Scholar] [CrossRef]
- Hendrayani, S.-F.; Al-Khalaf, H.H.; Aboussekhra, A. Curcumin Triggers p16-Dependent Senescence in Active Breast Cancer-Associated Fibroblasts and Suppresses Their Paracrine Procarcinogenic Effects. Neoplasia 2013, 15, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, W.; Kucharewicz, K.; Wnuk, M.; Lewinska, A.; Suszek, M.; Przybylska, D.; Mosieniak, G.; Sikora, E.; Bielak-Zmijewska, A. Curcumin induces senescence of primary human cells building the vasculature in a DNA damage and ATM-independent manner. AGE 2015, 37, 1–17. [Google Scholar] [CrossRef]
- He, Y.; Roos, W.P.; Wu, Q.; Hofmann, T.G.; Kaina, B. The SIAH1–HIPK2–p53ser46 Damage Response Pathway is Involved in Temozolomide-Induced Glioblastoma Cell Death. Mol. Cancer Res. 2019, 17, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Kaina, B.; Beltzig, L.; Piee-Staffa, A.; Haas, B. Cytotoxic and Senolytic Effects of Methadone in Combination with Temozolomide in Glioblastoma Cells. Int. J. Mol. Sci. 2020, 21, 7006. [Google Scholar] [CrossRef]
- Nikolova, T.; Marini, F.; Kaina, B. Genotoxicity testing: Comparison of the gammaH2AX focus assay with the alkaline and neutral comet assays. Mutat. Res. 2017, 822, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Bielak-Zmijewska, A.; Grabowska, W.; Ciolko, A.; Bojko, A.; Mosieniak, G.; Bijoch, Ł.; Sikora, E. The Role of Curcumin in the Modulation of Ageing. Int. J. Mol. Sci. 2019, 20, 1239. [Google Scholar] [CrossRef]
- Knizhnik, A.V.; Roos, W.; Nikolova, T.; Quiros, S.; Tomaszowski, K.-H.; Christmann, M.; Kaina, B. Survival and Death Strategies in Glioma Cells: Autophagy, Senescence and Apoptosis Triggered by a Single Type of Temozolomide-Induced DNA Damage. PLoS ONE 2013, 8, e55665. [Google Scholar] [CrossRef]
- He, Y.; Kaina, B. Are There Thresholds in Glioblastoma Cell Death Responses Triggered by Temozolomide? Int. J. Mol. Sci. 2019, 20, 1562. [Google Scholar] [CrossRef]
- Yosef, R.; Pilpel, N.; Tokarsky-Amiel, R.; Biran, A.; Ovadya, Y.; Cohen, S.; Vadai, E.; Dassa, L.; Shahar, E.; Condiotti, R.; et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 2016, 7, 11190. [Google Scholar] [CrossRef]
- Thayyullathil, F.; Chathoth, S.; Hago, A.; Patel, M.; Galadari, S. Rapid reactive oxygen species (ROS) generation induced by curcumin leads to caspase-dependent and -independent apoptosis in L929 cells. Free Radic. Biol. Med. 2008, 45, 1403–1412. [Google Scholar] [CrossRef]
- Collins, A.R. Measuring oxidative damage to DNA and its repair with the comet assay. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, J.; Tang, Q.; Xu, C.; Huang, Y.; Huang, D. Nano-micelles based on hydroxyethyl starch-curcumin conjugates for improved stability, antioxidant and anticancer activity of curcumin. Carbohydr. Polym. 2020, 228, 115398. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Y.; Shen, Y.; Wang, J.; Tang, J.; Zhao, Z. Enhanced oral delivery and anti-gastroesophageal reflux activity of curcumin by binary mixed micelles. Drug Dev. Ind. Pharm. 2019, 45, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Karabasz, A.; Lachowicz, D.; Karewicz, A.; Mezyk-Kopec, R.; Stalińska, K.; Werner, E.; Cierniak, A.; Dyduch, G.; Bereta, J.; Bzowska, M. Analysis of toxicity and anticancer activity of micelles of sodium alginate-curcumin. Int. J. Nanomed. 2019, 14, 7249–7262. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Schiborr, C.; Kocher, A.; Meins, J.; Behnam, D.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Transepithelial Transport of Curcumin in Caco-2 Cells Is significantly Enhanced by Micellar Solubilisation. Plant Foods Hum. Nutr. 2017, 72, 48–53. [Google Scholar] [CrossRef][Green Version]
- Seiwert, N.; Fahrer, J.; Nagel, G.; Frank, J.; Behnam, D.; Kaina, B. Curcumin Administered as Micellar Solution Suppresses Intestinal Inflammation and Colorectal Carcinogenesis. Nutr. Cancer 2021, 73, 686–693. [Google Scholar] [CrossRef]
- Jiang, M.; Yang-Yen, H.; Yen, J.J.; Lin, J. Curcumin induces apoptosis in immortalized NIH 3T3 and malignant cancer cell lines. Nutr. Cancer 1996, 26, 111–120. [Google Scholar] [CrossRef]
- Kuo, M.-L.; Huang, T.-S.; Lin, J.-K. Curcumin, an antioxidant and anti-tumor promoter, induces apoptosis in human leukemia cells. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 1996, 1317, 95–100. [Google Scholar] [CrossRef]
- Anto, R.J.; Mukhopadhyay, A.; Denning, K.; Aggarwal, B.B. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: Its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis 2002, 23, 143–150. [Google Scholar] [CrossRef]
- Kizhakkayil, J.; Thayyullathil, F.; Chathoth, S.; Hago, A.; Patel, M.; Galadari, S. Modulation of curcumin-induced Akt phosphorylation and apoptosis by PI3K inhibitor in MCF-7 cells. Biochem. Biophys. Res. Commun. 2010, 394, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, W.; Mosieniak, G.; Achtabowska, N.; Czochara, R.; Litwinienko, G.; Bojko, A.; Sikora, E.; Bielak-Zmijewska, A. Curcumin induces multiple signaling pathways leading to vascular smooth muscle cell senescence. Biogerontology 2019, 20, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, T.; Dvorak, M.; Jung, F.; Adam, I.; Kramer, E.; Gerhold-Ay, A. The gammaH2AX assay for genotoxic and nongenotoxic agents: Comparison of H2AX phosphorylation with cell death response. Toxicol. Sci. 2014, 140, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Sebastià, N.; Soriano, J.; Barquinero, J.F.; Villaescusa, J.I.; Almonacid, M.; Cervera, J.; Such, E.; Silla, M.A.; Montoro, A. In vitro cytogenetic and genotoxic effects of curcumin on human peripheral blood lymphocytes. Food Chem. Toxicol. 2012, 50, 3229–3233. [Google Scholar] [CrossRef] [PubMed]
- Ganiger, S.; Malleshappa, H.; Krishnappa, H.; Rajashekhar, G.; Rao, V.R.; Sullivan, F. A two generation reproductive toxicity study with curcumin, turmeric yellow, in Wistar rats. Food Chem. Toxicol. 2007, 45, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Unlu, A.; Nayir, E.; Kalenderoglu, M.D.; Kirca, O.; Ozdogan, M. Curcumin (Turmeric) and cancer. J. BUON 2016, 21, 1050–1060. [Google Scholar] [PubMed]
- Eckert, G.P.; Schiborr, C.; Hagl, S.; Abdel-Kader, R.; Müller, W.E.; Rimbach, G.; Frank, J. Curcumin prevents mitochondrial dysfunction in the brain of the senescence-accelerated mouse-prone 8. Neurochem. Int. 2013, 62, 595–602. [Google Scholar] [CrossRef]
- Dörsam, B.; Wu, C.-F.; Efferth, T.; Kaina, B.; Fahrer, J. The eucalyptus oil ingredient 1,8-cineol induces oxidative DNA damage. Arch. Toxicol. 2014, 89, 797–805. [Google Scholar] [CrossRef]
- Berdelle, N.; Nikolova, T.; Quiros, S.; Efferth, T.; Kaina, B. Artesunate Induces Oxidative DNA Damage, Sustained DNA Double-Strand Breaks, and the ATM/ATR Damage Response in Cancer Cells. Mol. Cancer Ther. 2011, 10, 2224–2233. [Google Scholar] [CrossRef]
- Radak, Z.; Ishihara, K.; Tekus, E.; Varga, C.; Posa, A.; Balogh, L. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol. 2017, 12, 285–290. [Google Scholar] [CrossRef]
- Boldogh, I.; Hajas, G.; Aguilera-Aguirre, L.; Hegde, M.L.; Radak, Z.; Bacsi, A.; Sur, S.; Hazra, T.K.; Mitra, S. Activation of Ras Signaling Pathway by 8-Oxoguanine DNA Glycosylase Bound to Its Excision Product, 8-Oxoguanine. J. Biol. Chem. 2012, 287, 20769–20773. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltzig, L.; Frumkina, A.; Schwarzenbach, C.; Kaina, B. Cytotoxic, Genotoxic and Senolytic Potential of Native and Micellar Curcumin. Nutrients 2021, 13, 2385. https://doi.org/10.3390/nu13072385
Beltzig L, Frumkina A, Schwarzenbach C, Kaina B. Cytotoxic, Genotoxic and Senolytic Potential of Native and Micellar Curcumin. Nutrients. 2021; 13(7):2385. https://doi.org/10.3390/nu13072385
Chicago/Turabian StyleBeltzig, Lea, Anna Frumkina, Christian Schwarzenbach, and Bernd Kaina. 2021. "Cytotoxic, Genotoxic and Senolytic Potential of Native and Micellar Curcumin" Nutrients 13, no. 7: 2385. https://doi.org/10.3390/nu13072385
APA StyleBeltzig, L., Frumkina, A., Schwarzenbach, C., & Kaina, B. (2021). Cytotoxic, Genotoxic and Senolytic Potential of Native and Micellar Curcumin. Nutrients, 13(7), 2385. https://doi.org/10.3390/nu13072385







