Replacing a Palatable High-Fat Diet with a Low-Fat Alternative Heightens κ-Opioid Receptor Control over Nucleus Accumbens Dopamine
Abstract
:1. Introduction
2. Materials and Methods
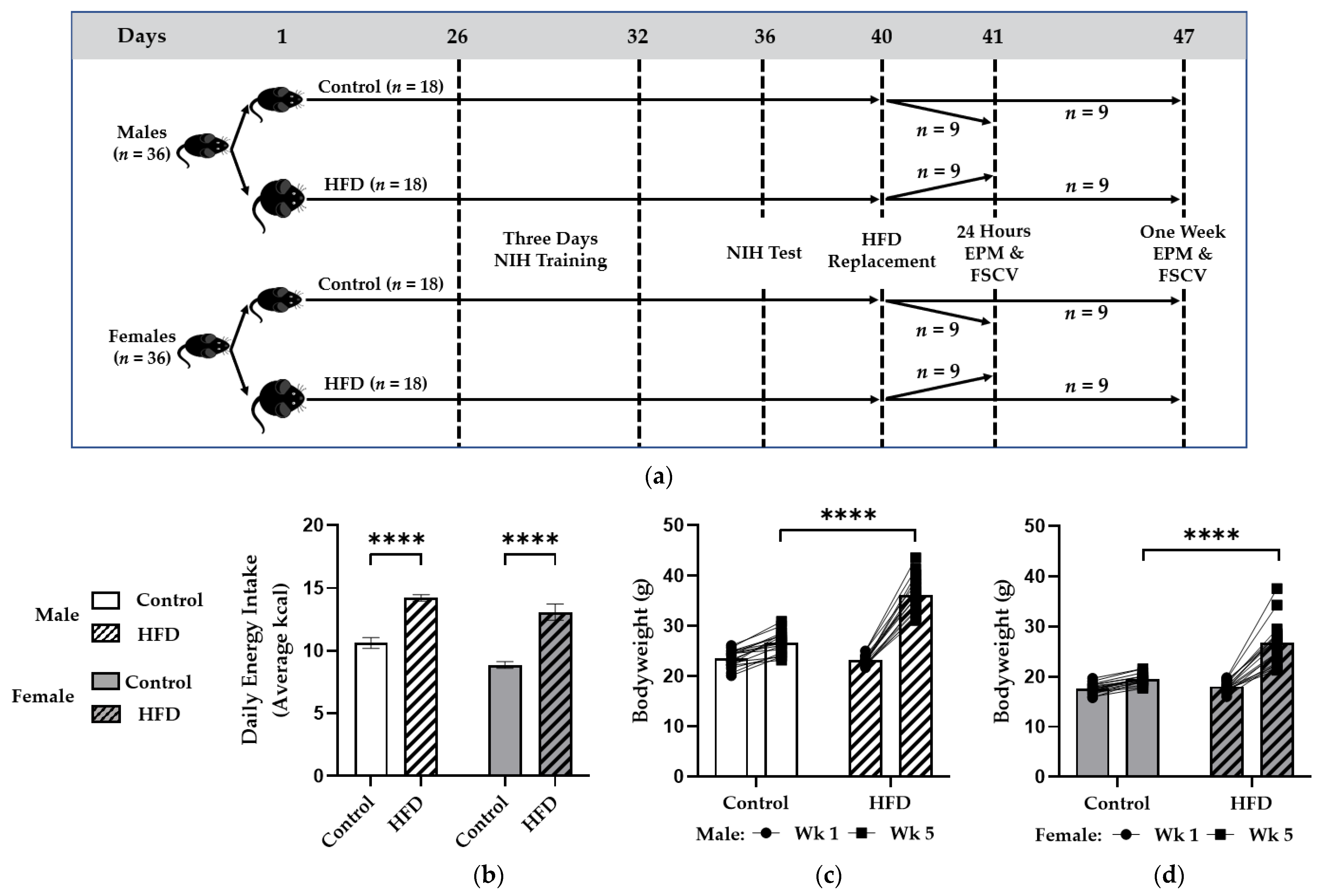
2.1. Animals, Diet, and Experimental Design
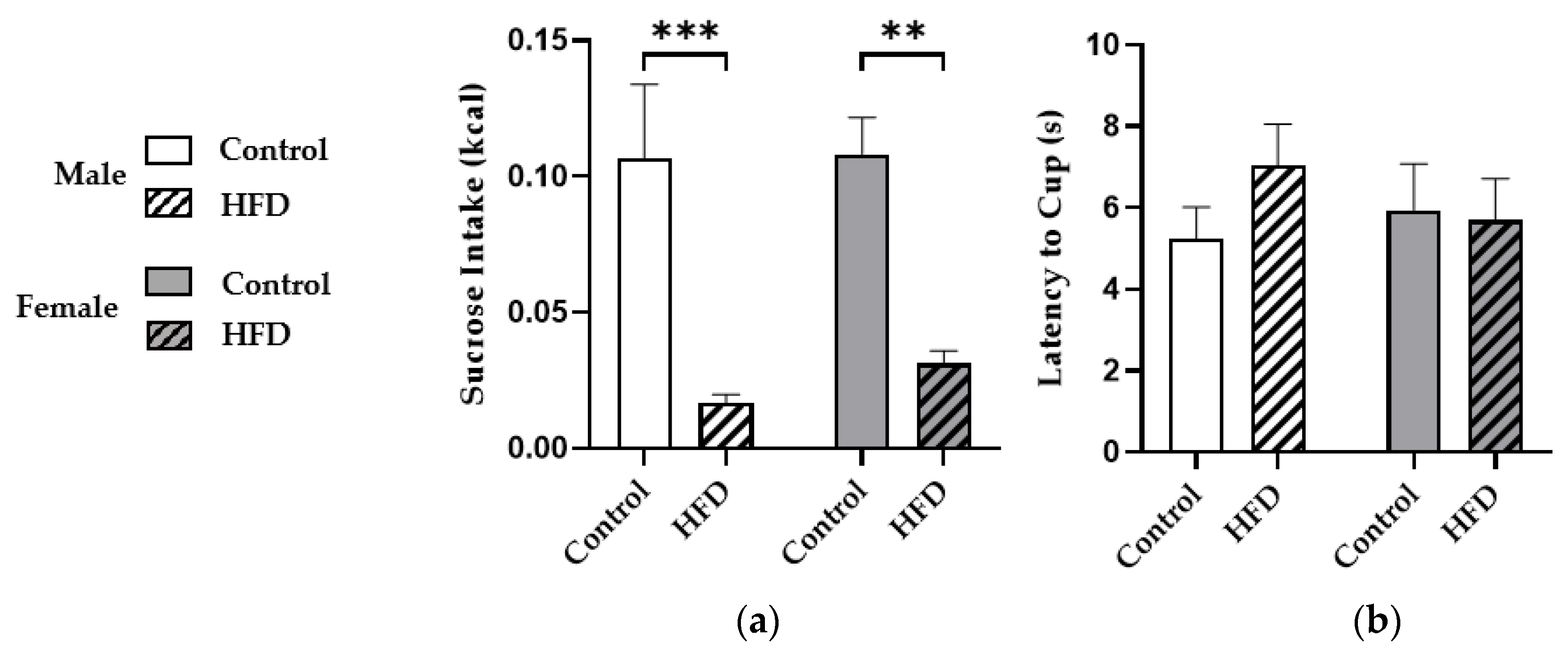
2.2. Novelty-Induced Hypophagia (NIH)
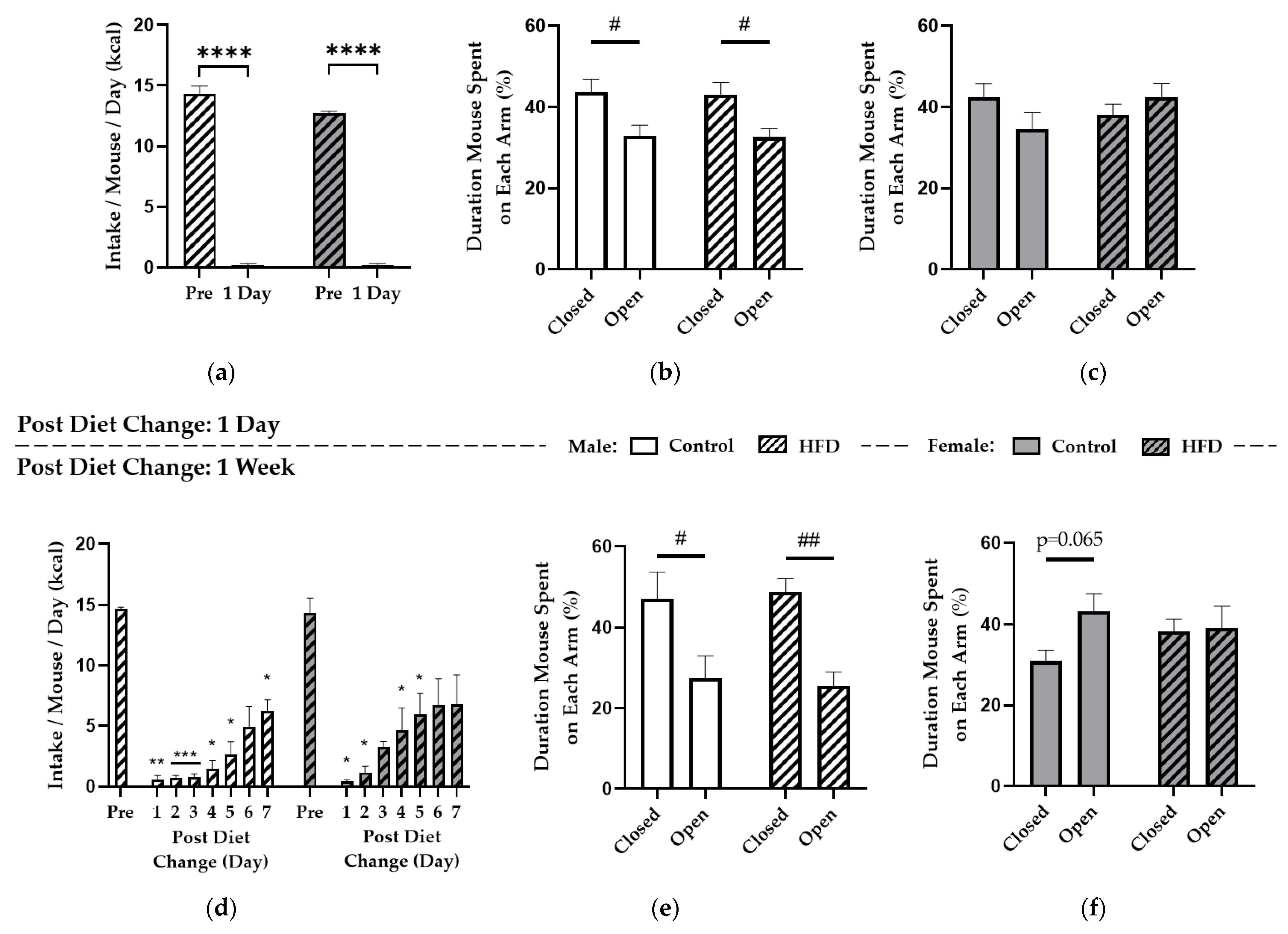
2.3. HFD Replacement and the Elevated Plus-Maze (EPM)
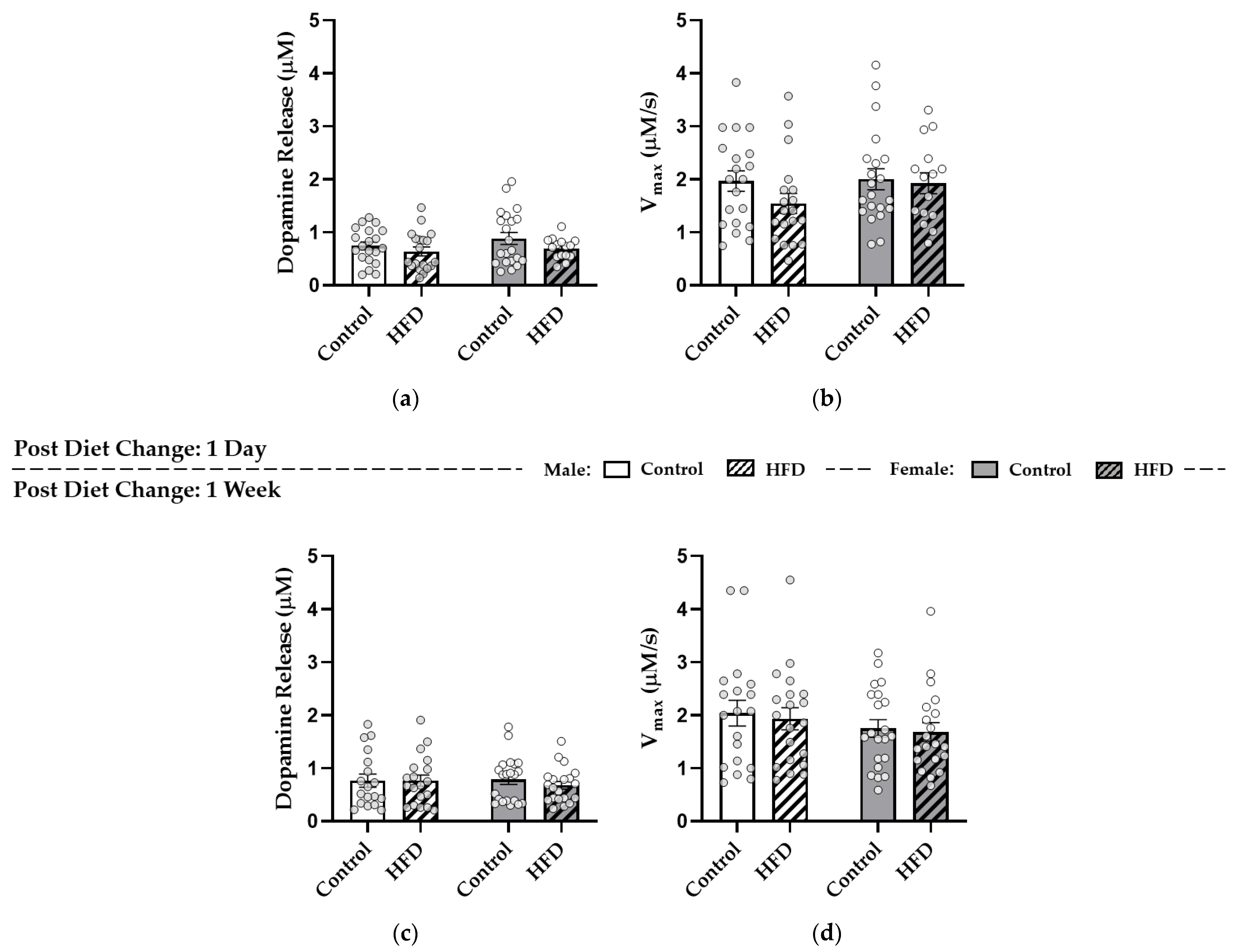
2.4. Fast-Scan Cyclic Voltammetry (FSCV)
2.5. Statistical Analysis
3. Results
3.1. Chronic HFD Feeding Promoted Caloric Intake and Weight Gain
3.2. HFD Consumption Reduced Sucrose Intake during Novelty-Induced Hypophagia
3.3. Replacing HFD with Control Diet Induced Voluntary Caloric Restriction but General Anxiety-Like Behaviors Were Similar to Controls
3.4. After HFD Replacement, Dopamine Release and Reuptake in the NAc Core Were Similar to Controls
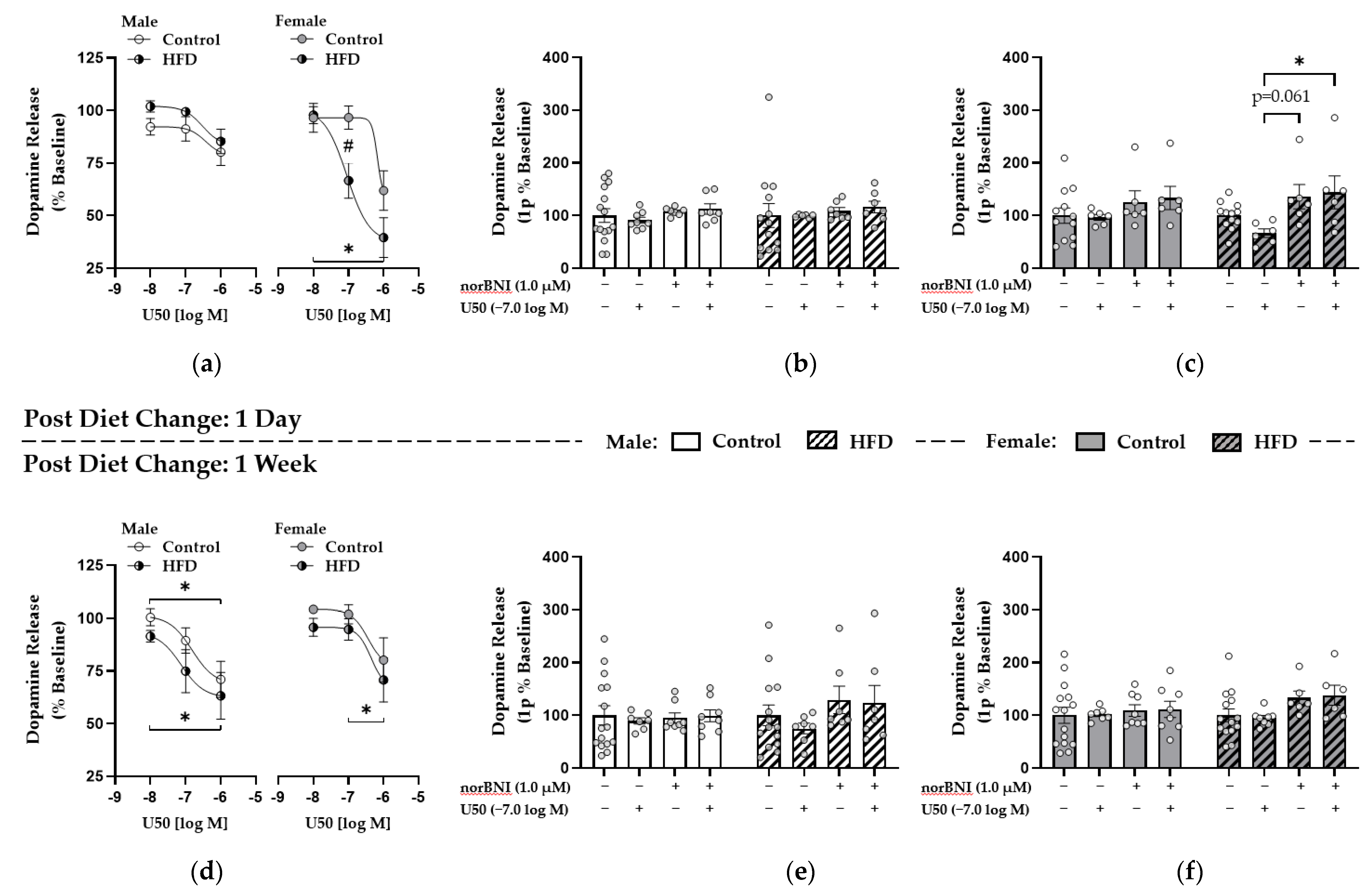
3.5. Enhanced κ-Opioid Receptor-Mediated Control over Dopamine Release in the NAc Core Was Observed One Day after HFD Replacement with Control Diet in Females
3.6. κ-. Opioid Receptor Function Was Upregulated in Males after One Week of HFD Replacement while These Effects Were Attenuated in Females
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States. NCHS Data Brief 2020, 360, 2017–2018. [Google Scholar]
- Greaves, C.; Poltawski, L.; Garside, R.; Briscoe, S. Understanding the challenge of weight loss maintenance: A systematic review and synthesis of qualitative research on weight loss maintenance. Health Psychol. Rev. 2017, 11, 145–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickering, C.; Alsiö, J.; Hulting, A.-L.; Schiöth, H.B. Withdrawal from free-choice high-fat high-sugar diet induces craving only in obesity-prone animals. Psychopharmacology 2009, 204, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Fernandes, M.F.; Fulton, S. Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int. J. Obes. 2013, 37, 1183–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajnal, A.; Smith, G.P.; Norgren, R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am. J. Physiol. Integr. Comp. Physiol. 2004, 286, R31–R37. [Google Scholar] [CrossRef]
- Rada, P.; Avena, N.M.; Barson, J.R.; Hoebel, B.G.; Leibowitz, S.F. A High-Fat Meal, or Intraperitoneal Administration of a Fat Emulsion, Increases Extracellular Dopamine in the Nucleus Accumbens. Brain Sci. 2012, 2, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Day, J.; Roitman, M.F.; Wightman, R.M.; Carelli, R.M. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat. Neurosci. 2007, 10, 1020–1028. [Google Scholar] [CrossRef]
- McCutcheon, J.E.; Beeler, J.; Roitman, M.F. Sucrose-predictive cues evoke greater phasic dopamine release than saccharin-predictive cues. Synapse 2012, 66, 346–351. [Google Scholar] [CrossRef] [Green Version]
- Barnes, C.N.; Wallace, C.W.; Jacobowitz, B.S.; Fordahl, S.C. Reduced phasic dopamine release and slowed dopamine uptake occur in the nucleus accumbens after a diet high in saturated but not unsaturated fat. Nutr. Neurosci. 2020, 1–13. [Google Scholar] [CrossRef]
- Fritz, B.M.; Munoz, B.; Yin, F.; Bauchle, C.; Atwood, B.K. A High-fat, High-sugar ‘Western’ Diet Alters Dorsal Striatal Glutamate, Opioid, and Dopamine Transmission in Mice. Neuroscience 2018, 372, 1–15. [Google Scholar] [CrossRef]
- Fordahl, S.C.; Jones, S.R. High-Fat-Diet-Induced Deficits in Dopamine Terminal Function Are Reversed by Restoring Insulin Signaling. ACS Chem. Neurosci. 2017, 8, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Hryhorczuk, C.; Florea, M.; Rodaros, D.; Poirier, I.; Daneault, C.; Des Rosiers, C.; Arvanitogiannis, A.; Alquier, T.; Fulton, S. Dampened Mesolimbic Dopamine Function and Signaling by Saturated but not Monounsaturated Dietary Lipids. Neuropsychopharmacology 2015, 41, 811–821. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Fulton, S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int. J. Obes. 2013, 37, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Karkhanis, A.N.; Holleran, K.M.; Jones, S.R. Dynorphin/Kappa Opioid Receptor Signaling in Preclinical Models of Alcohol, Drug, and Food Addiction. Int. Rev. Neurobiol. 2017, 136, 53–88. [Google Scholar] [CrossRef] [PubMed]
- Meshul, C.; McGinty, J. Kappa opioid receptor immunoreactivity in the nucleus accumbens and caudate–putamen is primarily associated with synaptic vesicles in axons. Neuroscience 2000, 96, 91–99. [Google Scholar] [CrossRef]
- Ehrich, J.M.; Messinger, D.I.; Knakal, C.R.; Kuhar, J.R.; Schattauer, S.S.; Bruchas, M.R.; Zweifel, L.; Kieffer, B.L.; Phillips, P.; Chavkin, C. Kappa Opioid Receptor-Induced Aversion Requires p38 MAPK Activation in VTA Dopamine Neurons. J. Neurosci. 2015, 35, 12917–12931. [Google Scholar] [CrossRef] [PubMed]
- Kivell, B.; Uzelac, Z.; Sundaramurthy, S.; Rajamanickam, J.; Ewald, A.; Chefer, V.; Jaligam, V.; Bolan, E.; Simonson, B.; Annamalai, B.; et al. Salvinorin A regulates dopamine transporter function via a kappa opioid receptor and ERK1/2-dependent mechanism. Neuropharmacology 2014, 86, 228–240. [Google Scholar] [CrossRef] [Green Version]
- Nash, A.I. Crosstalk between insulin and dopamine signaling: A basis for the metabolic effects of antipsychotic drugs. J. Chem. Neuroanat. 2017, 37, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Svingos, A.L.; Chavkin, C.; Colago, E.E.; Pickel, V.M. Major coexpression of κ-opioid receptors and the dopamine transporter in nucleus accumbens axonal profiles. Synapse 2001, 42, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kroemer, N.B.; Oehme, L.; Beuthien-Baumann, B.; Goschke, T.; Smolka, M.N. Lower dopamine tone in the striatum is associated with higher body mass index. Eur. Neuropsychopharmacol. 2018, 28, 719–731. [Google Scholar] [CrossRef]
- Van Bloemendaal, L.; Veltman, D.J.; Kulve, J.S.T.; Groot, P.F.; Ruhé, H.G.; Barkhof, F.; Sloan, J.H.; Diamant, M.; Ijzerman, R.G. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. Diabetes Obes. Metab. 2015, 17, 878–886. [Google Scholar] [CrossRef]
- Dulawa, S.C. Novelty-Induced Hypophagia. In Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests; Gould, T.D., Ed.; Humana Press: New York, NY, USA, 2009; pp. 247–259. [Google Scholar] [CrossRef]
- Browne, A.C.; Falcon, E.; A Robinson, S.; Berton, O.; Lucki, I. Reversal of Stress-Induced Social Interaction Deficits by Buprenorphine. Int. J. Neuropsychopharmacol. 2017, 21, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Pawlak, C.R.; Karrenbauer, B.D.; Schneider, P.; Ho, Y.-J. The Elevated Plus-Maze Test: Differential Psychopharmacology of Anxiety-Related Behavior. Emot. Rev. 2012, 4, 98–115. [Google Scholar] [CrossRef]
- Yorgason, J.T.; España, R.A.; Jones, S.R. Demon Voltammetry and Analysis software: Analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J. Neurosci. Methods 2011, 202, 158–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melchior, J.R.; Jones, S.R. Chronic ethanol exposure increases inhibition of optically targeted phasic dopamine release in the nucleus accumbens core and medial shell ex vivo. Mol. Cell. Neurosci. 2017, 85, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.H.; Karkhanis, A.N.; Steiniger-Brach, B.; Jones, S.R. Distinct Effects of Nalmefene on Dopamine Uptake Rates and Kappa Opioid Receptor Activity in the Nucleus Accumbens Following Chronic Intermittent Ethanol Exposure. Int. J. Mol. Sci. 2016, 17, 1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlin, J.; Hill-Smith, T.E.; Lucki, I.; Reyes, T.M. Reversal of dopamine system dysfunction in response to high-fat diet. Obesity 2013, 21, 2513–2521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcego, D.M.; Krolow, R.; Lampert, C.; Toniazzo, A.P.; Garcia, E.D.S.; Lazzaretti, C.; Costa, G.; Scorza, C.; Dalmaz, C. Chronic high-fat diet affects food-motivated behavior and hedonic systems in the nucleus accumbens of male rats. Appetite 2020, 153, 104739. [Google Scholar] [CrossRef] [PubMed]
- Ookuma, K.; Barton, C.; York, D.; Bray, G. Effect of Enterostatin and Kappa-Opioids on Macronutrient Selection and Consumption. Peptides 1997, 18, 785–791. [Google Scholar] [CrossRef]
- Ikeda, H.; Ardianto, C.; Yonemochi, N.; Yang, L.; Ohashi, T.; Ikegami, M.; Nagase, H.; Kamei, J. Inhibition of opioid systems in the hypothalamus as well as the mesolimbic area suppresses feeding behavior of mice. Neuroscience 2015, 311, 9–21. [Google Scholar] [CrossRef]
- Ebner, S.R.; Roitman, M.F.; Potter, D.N.; Rachlin, A.B.; Chartoff, E.H. Depressive-like effects of the kappa opioid receptor agonist salvinorin a are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology 2010, 210, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Wallace, C.; Loudermilt, M.; Fordahl, S. Effect of fasting on dopamine neurotransmission in subregions of the nucleus accumbens in male and female mice. Nutr. Neurosci. 2020, 1–12. [Google Scholar] [CrossRef]
- Patel, J.C.; Stouffer, M.A.; Mancini, M.; Nicholson, C.; Carr, K.D.; Rice, M.E. Interactions between insulin and diet on striatal dopamine uptake kinetics in rodent brain slices. Eur. J. Neurosci. 2019, 49, 794–804. [Google Scholar] [CrossRef]
- Cadoni, C.; Solinas, M.; Valentini, V.; Di Chiara, G. Selective psychostimulant sensitization by food restriction: Differential changes in accumbens shell and core dopamine. Eur. J. Neurosci. 2003, 18, 2326–2334. [Google Scholar] [CrossRef]
- Carr, K.; Tsimberg, Y.; Berman, Y.; Yamamoto, N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience 2003, 119, 1157–1167. [Google Scholar] [CrossRef]
- Tsujii, S.; Nakai, Y.; Fukata, J.; Nakaishi, S.; Takahashi, H.; Usui, T.; Imura, H. Effects of food deprivation and high fat diet on immunoreactive dynorphin A(1–8) levels in brain regions of Zucker rats. Peptides 1987, 8, 1075–1078. [Google Scholar] [CrossRef]
- Shahkarami, K.; Vousooghi, N.; Golab, F.; Mohsenzadeh, A.; Baharvand, P.; Sadat-Shirazi, M.-S.; Babhadi-Ashar, N.; Shakeri, A.; Zarrindast, M.R. Evaluation of dynorphin and kappa-opioid receptor level in the human blood lymphocytes and plasma: Possible role as a biomarker in severe opioid use disorder. Drug Alcohol Depend. 2019, 205, 107638. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.D.; Bot, G.; Li, S.; Freeman, J.C.; Reisine, T. Differential Agonist Regulation of the Human κ-Opioid Receptor. J. Neurochem. 1997, 68, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Unterwald, E.; Howells, R. Upregulation of Opioid Receptors. In Opiate Receptors and Antagonists: From Bench to Clinic; Dean, R., III, Bilsky, E., Negus, S., Eds.; Humana Press: New York, NY, USA, 2009; pp. 19–44. [Google Scholar] [CrossRef]
- Bazov, I.; Sarkisyan, D.; Kononenko, O.; Watanabe, H.; Karpyak, V.; Yakovleva, T.; Bakalkin, G. Downregulation of the neuronal opioid gene expression concomitantly with neuronal decline in dorsolateral prefrontal cortex of human alcoholics. Transl. Psychiatry 2018, 8, 122. [Google Scholar] [CrossRef] [Green Version]
- Bake, T.; Baron, J.; Duncan, J.S.; Morgan, D.G.A.; Mercer, J.G. Arcuate nucleus homeostatic systems reflect blood leptin concentration but not feeding behaviour during scheduled feeding on a high-fat diet in mice. J. Neuroendocrinol. 2017, 29, e12498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsiö, J.; Olszewski, P.; Norbäck, A.; Gunnarsson, Z.; Levine, A.; Pickering, C.; Schiöth, H. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience 2010, 171, 779–787. [Google Scholar] [CrossRef]
- Jones, K.T.; Woods, C.; Zhen, J.; Antonio, T.; Carr, K.; Reith, M.E.A. Effects of diet and insulin on dopamine transporter activity and expression in rat caudate-putamen, nucleus accumbens, and midbrain. J. Neurochem. 2017, 140, 728–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conway, S.M.; Puttick, D.; Russell, S.; Potter, D.; Roitman, M.F.; Chartoff, E.H. Females are less sensitive than males to the motivational- and dopamine-suppressing effects of kappa opioid receptor activation. Neuropharmacology 2019, 146, 231–241. [Google Scholar] [CrossRef]
- Ramos, J.; Hernandez-Casner, C.; Cruz, B.; Serafine, K.M. Sex differences in high fat diet-induced impairments to striatal Akt signaling and enhanced sensitivity to the behavioral effects of dopamine D2/D3 receptor agonist quinpirole. Physiol. Behav. 2019, 203, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Carlin, J.L.; McKee, S.E.; Hill-Smith, T.; Grissom, N.M.; George, R.; Lucki, I.; Reyes, T.M. Removal of high-fat diet after chronic exposure drives binge behavior and dopaminergic dysregulation in female mice. Neuroscience 2016, 326, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Ong, Z.Y.; Wanasuria, A.F.; Lin, M.Z.; Hiscock, J.; Muhlhausler, B.S. Chronic intake of a cafeteria diet and subsequent abstinence. Sex-specific effects on gene expression in the mesolimbic reward system. Appetite 2013, 65, 189–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ophuis, R.J.A.O.; Boender, A.J.; Van Rozen, A.J.; Adan, R.A.H. Cannabinoid, melanocortin and opioid receptor expression on DRD1 and DRD2 subpopulations in rat striatum. Front. Neuroanat. 2014, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.J.; Rasakham, K.; Huang, P.; Chudnovskaya, D.; Cowan, A.; Liu-Chen, L.Y. Sex difference in κ-Opioid Receptor (KOPR)-mediated behaviors, brain region KOPR level and KOPR-mediated guanosine 5′-O-(3-[35S]thiotriphosphate) binding in the guinea pig. J. Pharmacol. Exp. Ther. 2011, 339, 438–450. [Google Scholar] [CrossRef] [Green Version]
- Abraham, A.D.; Schattauer, S.S.; Reichard, K.L.; Cohen, J.H.; Fontaine, H.M.; Song, A.J.; Johnson, S.D.; Land, B.B.; Chavkin, C. Estrogen Regulation of GRK2 Inactivates Kappa Opioid Receptor Signaling Mediating Analgesia, But Not Aversion. J. Neurosci. 2018, 38, 8031–8043. [Google Scholar] [CrossRef] [Green Version]
- Reichard, K.L.; Newton, K.A.; Rivera, Z.M.; De Menezes, P.M.S.; Schattauer, S.S.; Land, B.B.; Chavkin, C. Regulation of kappa opioid receptor inactivation depends on sex and cellular site of antagonist action. Mol. Pharmacol. 2020, 98, 548–558. [Google Scholar] [CrossRef]
- Boukouvalas, G.; Antoniou, K.; Papalexi, E.; Kitraki, E. Post weaning high fat feeding affects rats’ behavior and hypothalamic pituitary adrenal axis at the onset of puberty in a sexually dimorphic manner. Neuroscience 2008, 153, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Lucas, L.R.; Dragisic, T.; Duwaerts, C.C.; Swiatkowski, M.; Suzuki, H. Effects of recovery from immobilization stress on striatal preprodynorphin- and kappa opioid receptor-mRNA levels of the male rat. Physiol. Behav. 2011, 104, 972–980. [Google Scholar] [CrossRef] [PubMed]
Short Biography of Authors
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallace, C.W.; Beatty, N.S.; Hutcherson, S.A.; Emmons, H.A.; Loudermilt, M.C.; Fordahl, S.C. Replacing a Palatable High-Fat Diet with a Low-Fat Alternative Heightens κ-Opioid Receptor Control over Nucleus Accumbens Dopamine. Nutrients 2021, 13, 2341. https://doi.org/10.3390/nu13072341
Wallace CW, Beatty NS, Hutcherson SA, Emmons HA, Loudermilt MC, Fordahl SC. Replacing a Palatable High-Fat Diet with a Low-Fat Alternative Heightens κ-Opioid Receptor Control over Nucleus Accumbens Dopamine. Nutrients. 2021; 13(7):2341. https://doi.org/10.3390/nu13072341
Chicago/Turabian StyleWallace, Conner W., Nari S. Beatty, Sarah A. Hutcherson, Heather A. Emmons, Madison C. Loudermilt, and Steve C. Fordahl. 2021. "Replacing a Palatable High-Fat Diet with a Low-Fat Alternative Heightens κ-Opioid Receptor Control over Nucleus Accumbens Dopamine" Nutrients 13, no. 7: 2341. https://doi.org/10.3390/nu13072341
APA StyleWallace, C. W., Beatty, N. S., Hutcherson, S. A., Emmons, H. A., Loudermilt, M. C., & Fordahl, S. C. (2021). Replacing a Palatable High-Fat Diet with a Low-Fat Alternative Heightens κ-Opioid Receptor Control over Nucleus Accumbens Dopamine. Nutrients, 13(7), 2341. https://doi.org/10.3390/nu13072341





