Do Bioactive Food Compound with Avena sativa L., Linum usitatissimum L. and Glycine max L. Supplementation with Moringa oleifera Lam. Have a Role against Nutritional Disorders? An Overview of the In Vitro and In Vivo Evidence
Abstract
1. Introduction
2. Current Status of Knowledge
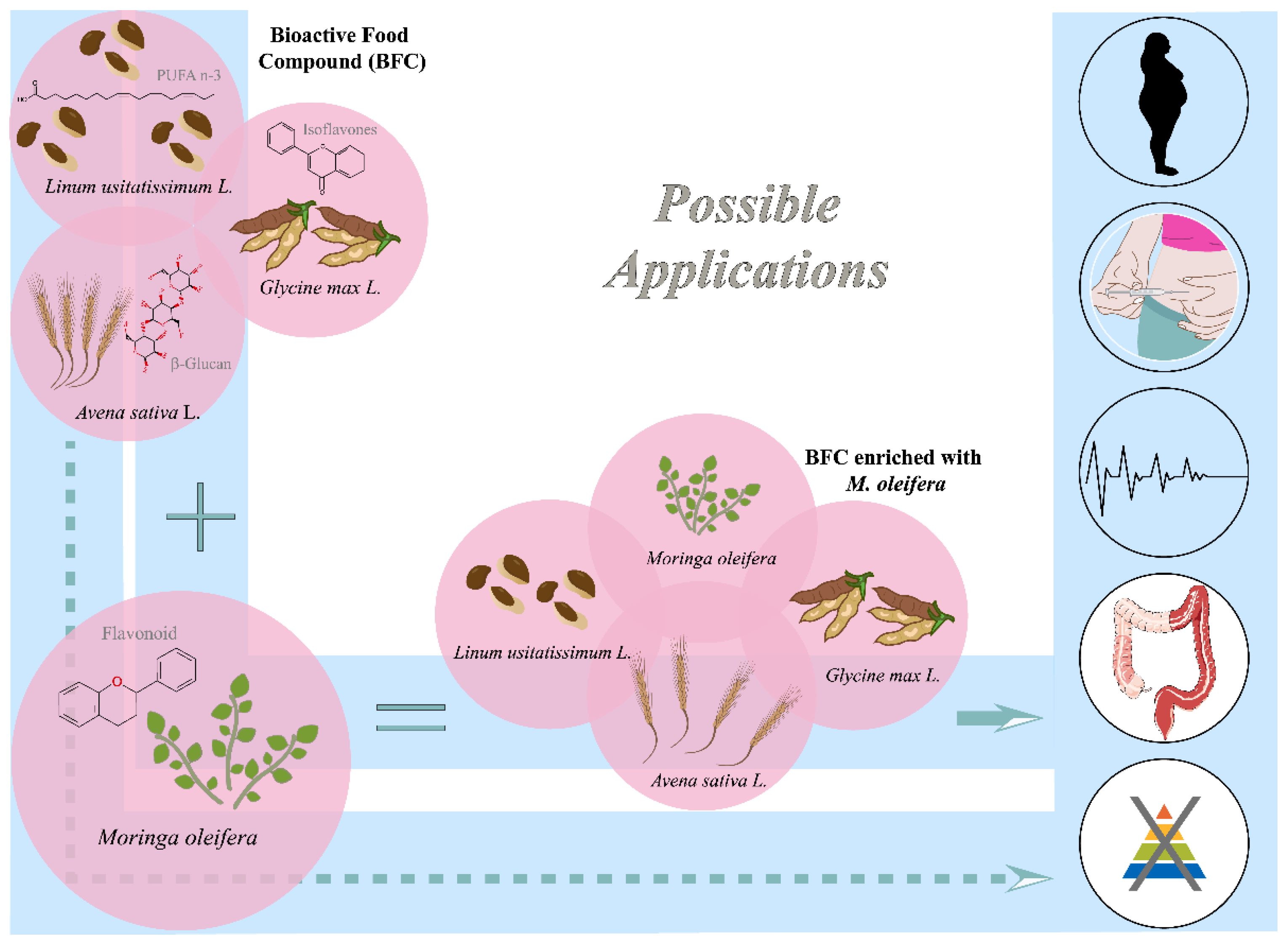
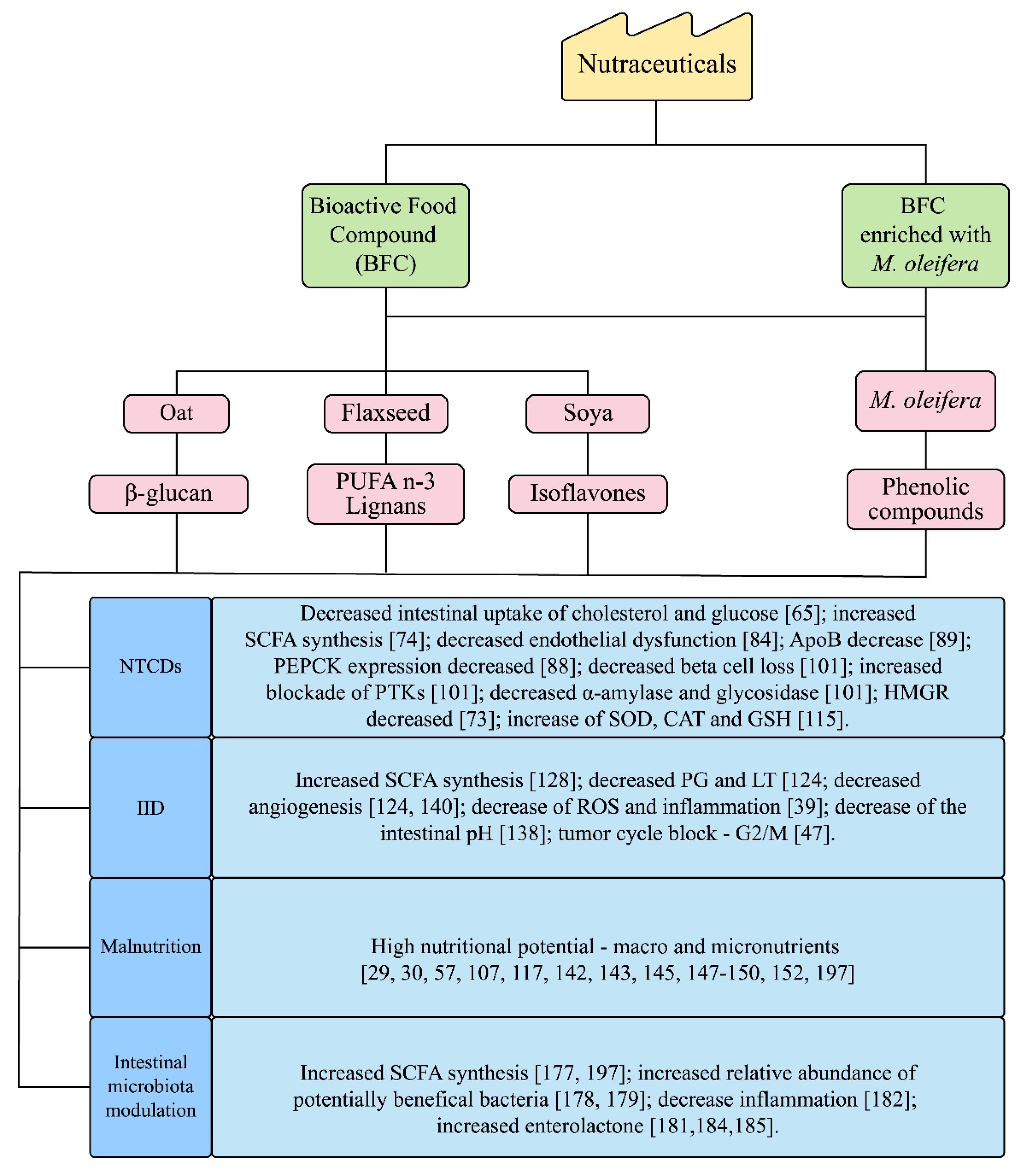
2.1. Bioactive Food Compound (BFC) and M. oleifera Nutraceuticals
2.2. Moringa oleifera Lamarck
2.3. Effect of Bioactive Food Compound (BFC) and M. oleifera on Non-Transmissible Chronic Diseases (NTCDs)/Metabolic Syndrome (MS)
2.4. Alternative Therapies and/or Prophylactics for Intestinal Inflammatory Diseases (IID): Bioactive Food Compound (BFC) and M. oleifera
2.5. Nutraceuticals for Malnutrition: Bioactive Food Compound (BFC) and M. oleifera
3. Functional Dietary Modulation of the Intestinal Microbiota from the Nutraceuticals Bioactive Food Compound (BFC) and M. oleifera
4. Concluding Remarks
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kalra, E.K. Nutraceutical definition and introduction. AAPS Pharm. Sci. 2003, 5, E25. [Google Scholar] [CrossRef]
- Adefegha, S.A. Functional foods and nutraceuticals as dietary intervention in chronic diseases; novel perspectives for health promotion and disease prevention. J. Diet. Suppl. 2018, 15, 977–1009. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Berberich, A.J.; Hegele, R.A. Nutraceuticals in 2017: Nutraceuticals endocrine disorders. Nat. Rev. Endocrinol. 2018, 14, 68–70. [Google Scholar] [CrossRef]
- Carnauba, R.A.; Baptistella, A.B.; Paschoal, V. Nutrição clínica funcional: Uma visão integrativa do paciente. Diagn Tratamento 2018, 23, 28–32. (In Portuguese) [Google Scholar]
- Naves, A.; Paschoal, V. Nutrição Clínica Funcional: Pensando por meio do Sistema ATMS. In Nutrição Clínica Funcional—dos Princípios à Prática Clínica, 2nd ed.; Paschoal, V., Naves, A., Fonseca, A.B.B.L., Eds.; Editora VP: São Paulo, Brazil, 2014; pp. 11–14. (In Portuguese) [Google Scholar]
- Souza, N.; Baptistella, A.B.; Paschoal, V.; Naves, A.; Massunaga, N.; Carnauba, R.; Hubscher, G. Nutrição funcional: Princípios e aplicação na prática clínica. Acta Port. Nutr. 2016, 7, 34–39. [Google Scholar]
- Valle, D. Genetics, individuality, and medicine in the 21st century. Am. J. Hum. Genet. 2004, 74, 374–381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Agudo, A.; Cabrera, L.; Amiano, P.; Ardanaz, E.; Barricarte, A.; Berenguer, T.; Chirlaque, M.D.; Dorronsoro, M.; Jakszyn, P.; Larranaga, N. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: Findings from the Spanish cohort of the European Prospective investigation into cancer and nutrition (EPIC-Spain). Am. J. Clin. Nutr. 2007, 85, 1634–1642. [Google Scholar] [CrossRef]
- Andlauer, W.; Fürst, P. Nutraceuticals: A piece of history, present status and outlook. Food Res. Int. 2002, 35, 171–176. [Google Scholar] [CrossRef]
- Hugenholtz, J.; Smid, E.J. Nutraceutical production with food-grade microorganisms. Curr. Opin. Biotechnol. 2002, 13, 497–507. [Google Scholar] [CrossRef]
- Roberfroid, M. Functional food concept and its application to prebiotics. Dig. Liver Dis. 2002, 34, S105–S110. [Google Scholar] [CrossRef]
- Walzem, R.L. Functional foods and health strategies. Trends Food Sci. Technol. 2004, 15, 33. [Google Scholar] [CrossRef]
- Hayes, M.; Tiwari, B.K. Bioactive carbohydrates and peptides in foods: An overview of sources, downstream processing steps and associated bioactivities. Int. J. Mol. Sci. 2015, 16, 22485–22508. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Meena, R.P.; Rai, S.K.; Pandey-Rai, S. Medicinal plants derived nutraceuticals: A re-emerging health aid. Int. J. Pharma Bio Sci. 2011, 2, 420–441. [Google Scholar]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera seeds and oil: Characteristics and uses for human health. Int. J. Mol. Sci. 2016, 17, 2141. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.d.S.; Guimarães, R.d.C.A.; Pontes, E.R.J.C.; Mendonça, L.A.B.M.; Freitas, K.d.C.; Hiane, P.A. Effectiveness of a bioactive food compound in anthropometric measures of individuals with HIV/AIDS: A nonrandomized trial. PLoS ONE 2018, 13, e0191259. [Google Scholar] [CrossRef]
- Ferreira, R.d.S.; Guimarães, R.d.C.A.; Pontes, E.R.J.C.; Nascimento, V.A.; Hiane, P.A. The effectiveness of a bioactive food compound in the lipid control of individuals with HIV/AIDS. Nutrients 2016, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.d.S.; Hiane, P.A.; Guimarães, R.d.C.A.; Ramos, M.I.L.; Demarque, D.P.; Meira, J.E.C.d. Physicochemical, microbiological and sensory evaluation of a bioactive food blend. Food Sci. Technol. 2014, 34, 609–615. [Google Scholar] [CrossRef]
- Leone, A.; Bertoli, S.; Di Lello, S.; Bassoli, A.; Ravasenghi, S.; Borgonovo, G.; Forlani, F.; Battezzati, A. Effect of Moringa oleifera leaf powder on postprandial blood glucose response: In vivo study on Saharawi people living in refugee camps. Nutrients 2018, 10, 1494. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Veronesi, M.; Strocchi, E.; Grandi, E.; Rizzoli, E.; Poli, A.; Marangoni, F.; Borghi, C. A Randomized Placebo-Controlled Clinical Trial to Evaluate the Medium-Term Effects of Oat Fibers on Human Health: The Beta-Glucan Effects on Lipid Profile, Glycemia and inTestinal Health (BELT) Study. Nutrients 2020, 12, 686. [Google Scholar] [CrossRef]
- Whitehead, A.; Beck, E.J.; Tosh, S.; Wolever, T.M.S. Cholesterol-lowering effects of oat β-glucan: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- García-Montalvo, I.A.; Méndez Díaz, S.Y.; Aguirre Guzmán, N.; Sánchez Medina, M.A.; Matías Pérez, D.; Pérez Campos, E. Increasing consumption of dietary fiber complementary to the treatment of metabolic syndrome. Nutr. Hosp. 2018, 35, 582–587. [Google Scholar] [CrossRef]
- Hendrixson, D.T.; Godbout, C.; Los, A.; Callaghan-Gillespie, M.; Mui, M.; Wegner, D.; Bryant, T.; Koroma, A.; Manary, M.J. Treatment of severe acute malnutrition with oat or standard ready-to-use therapeutic food: A triple-blind, randomised controlled clinical trial. Gut 2020, 69, 2143–2149. [Google Scholar] [CrossRef]
- Morshedzadeh, N.; Shahrokh, S.; Chaleshi, V.; Karimi, S.; Mirmiran, P.; Zali, M.R. The effects of flaxseed supplementation on gene expression and inflammation in ulcerative colitis patients: An open-labelled randomised controlled trial. Int. J. Clin. Pract. 2021, 75, e14035. [Google Scholar] [CrossRef]
- Akrami, A.; Nikaein, F.; Babajafari, S.; Faghih, S.; Yarmohammadi, H. Comparison of the effects of flaxseed oil and sunflower seed oil consumption on serum glucose, lipid profile, blood pressure, and lipid peroxidation in patients with metabolic syndrome. J. Clin. Lipidol. 2018, 12, 70–77. [Google Scholar] [CrossRef]
- Pintova, S.; Dharmupari, S.; Moshier, E.; Zubizarreta, N.; Ang, C.; Holcombe, R.F. Genistein combined with FOLFOX or FOLFOX–Bevacizumab for the treatment of metastatic colorectal cancer: Phase I/II pilot study. Cancer Chemother. Pharmacol. 2019, 84, 591–598. [Google Scholar] [CrossRef]
- Shin, A.; Lee, J.; Lee, J.; Park, M.S.; Park, J.W.; Park, S.C.; Oh, J.H.; Kim, J. Isoflavone and soyfood intake and colorectal cancer risk: A case-control study in Korea. PLoS ONE 2015, 10, e0143228. [Google Scholar] [CrossRef]
- Iuel-Brockdorf, A.-S.; Draebel, T.A.; Ritz, C.; Fabiansen, C.; Cichon, B.; Christensen, V.B.; Yameogo, C.; Oummani, R.; Briend, A.; Michaelsen, K.F. Evaluation of the acceptability of improved supplementary foods for the treatment of moderate acute malnutrition in Burkina Faso using a mixed method approach. Appetite 2016, 99, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Qi, R.; Wang, H.; Feng, C.; Leng, J. A “planting and eating soybean” project for people living with HIV/AIDS in rural Anhui—A pilot study in China. AIDS Care 2010, 22, 126–132. [Google Scholar] [CrossRef]
- Chan Sun, M.; Ruhomally, Z.B.; Boojhawon, R.; Neergheen-Bhujun, V.S. Consumption of Moringa oleifera Lam leaves lowers postprandial blood pressure. J. Am. College Nutr. 2020, 39, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Taweerutchana, R.; Lumlerdkij, N.; Vannasaeng, S.; Akarasereenont, P.; Sriwijitkamol, A. Effect of Moringa oleifera leaf capsules on glycemic control in therapy-naive type 2 diabetes patients: A randomized placebo controlled study. Evid. Based Complement. Altern. Med. 2017, 2017, 6581390. [Google Scholar] [CrossRef]
- Anthanont, P.; Lumlerdkij, N.; Akarasereenont, P.; Vannasaeng, S.; Sriwijitkamol, A. Moringa oleifera leaf increases insulin secretion after single dose administration: A preliminary study in healthy subjects. J. Med Assoc. Thail. 2016, 99, 308–313. [Google Scholar]
- El-Meidany, W.M.R.; Tayel, D.I.; El-Nawawy, A.A. Effect of Moringa oleifera water extract on Pyrexia: A case study. Can. J. Clin. Nutr. 2018, 6, 57–61. [Google Scholar] [CrossRef]
- Shija, A.E.; Rumisha, S.F. Effect of Moringa oleifera leaf powder supplementation on reducing anemia in children below two years in Kisarawe District, Tanzania. Food Sci. Nutr. 2019, 7, 2584–2594. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Rodriguez, M.; Valenzuela-Rubio, N.G.; Ochoa-Acosta, D.A.; Fierros-Valdez, J.A.; Castro-Sanchez, F.H.; Vergara-Jimenez, M. The effect of Moringa oleifera leaves in anthropometric and biochemical parameters in obese type 2 diabetes mellitus subjects. FASEB J. 2016, 30, 1176.21. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, W.; Tong, L.; Liu, X.; Zhong, K.; Liu, L.; Wang, L.; Zhou, S. Hypolipidaemic effects of oat flakes and β-glucans derived from four Chinese naked oat (Avena nuda) cultivars in Wistar–Lewis rats. J. Sci. Food Agric. 2016, 96, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Wilczak, J.; Błaszczyk, K.; Kamola, D.; Gajewska, M.; Harasym, J.P.; Jałosińska, M.; Gudej, S.; Suchecka, D.; Oczkowski, M.; Gromadzka-Ostrowska, J. The effect of low or high molecular weight oat beta-glucans on the inflammatory and oxidative stress status in the colon of rats with LPS-induced enteritis. Food Funct. 2015, 6, 590–603. [Google Scholar] [CrossRef]
- Palla, A.H.; Iqbal, N.T.; Minhas, K.; Gilani, A.H. Flaxseed extract exhibits mucosal protective effect in acetic acid induced colitis in mice by modulating cytokines, antioxidant and antiinflammatory mechanisms. Int. Immunopharmacol. 2016, 38, 153–166. [Google Scholar] [CrossRef]
- Ghule, A.E.; Jadhav, S.S.; Bodhankar, S.L. Effect of ethanolic extract of seeds of Linum usitatissimum (Linn.) in hyperglycaemia associated ROS production in PBMNCs and pancreatic tissue of alloxan induced diabetic rats. Asian Pac. J. Trop. Dis. 2012, 2, 405–410. [Google Scholar] [CrossRef]
- Dusane, M.B.; Joshi, B.N. Beneficial effect of flax seeds in streptozotocin (STZ) induced diabetic mice: Isolation of active fraction having islet regenerative and glucosidase inhibitory properties. Can. J. Physiol. Pharmacol. 2013, 91, 325–331. [Google Scholar] [CrossRef]
- Power, K.A.; Lepp, D.; Zarepoor, L.; Monk, J.M.; Wu, W.; Tsao, R.; Liu, R. Dietary flaxseed modulates the colonic microenvironment in healthy C57Bl/6 male mice which may alter susceptibility to gut-associated diseases. J. Nutr. Biochem. 2016, 28, 61–69. [Google Scholar] [CrossRef]
- Huang, H.; Krishnan, H.B.; Pham, Q.; Yu, L.L.; Wang, T.T.Y. Soy and gut microbiota: Interaction and implication for human health. J. Agric. Food Chem. 2016, 64, 8695–8709. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, P.; Sang, S. Dietary genistein inhibits methylglyoxal-induced advanced glycation end product formation in mice fed a high-fat diet. J. Nutr. 2019, 149, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, X.; Liu, Y.; Cao, H.; Han, Q.; Xie, B.; Fan, L.; Li, X.; Hu, J.; Yang, G. Effect of fermented corn-soybean meal on serum immunity, the expression of genes related to gut immunity, gut microbiota, and bacterial metabolites in grower-finisher pigs. Front. Microbiol. 2019, 10, 2620. [Google Scholar] [CrossRef] [PubMed]
- Chumark, P.; Khunawat, P.; Sanvarinda, Y.; Phornchirasilp, S.; Morales, N.P.; Phivthong-Ngam, L.; Ratanachamnong, P.; Srisawat, S.; Klai-upsorn, S.P. The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. leaves. J. Ethnopharmacol. 2008, 116, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, A.K.; Albalawi, S.M.; Athar, M.T.; Khan, A.Q.; Al-Shahrani, H.; Islam, M. Moringa oleifera as an anti-cancer agent against breast and colorectal cancer cell lines. PLoS ONE 2015, 10, e0135814. [Google Scholar] [CrossRef]
- Oboh, G.; Oyeleye, S.I.; Akintemi, O.A.; Olasehinde, T.A. Moringa oleifera supplemented diet modulates nootropic-related biomolecules in the brain of STZ-induced diabetic rats treated with acarbose. Metab. Brain Dis. 2018, 33, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Ahmed, M.A.; El Sayed, R.A. Molecular effects of Moringa leaf extract on insulin resistance and reproductive function in hyperinsulinemic male rats. J. Diabetes Metab. Disord. 2019, 18, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Jangir, R.N.; Jain, G.C. Antidiabetic and antioxidant potential of hydroalcoholic extract of Moringa oleifera leaves in streptozotocin-induced diabetic rats. Eur. J. Pharm. Med Res. 2016, 3, 438–450. [Google Scholar]
- Elabd, E.M.Y.; Morsy, S.M.; Elmalt, H.A. Investigating of Moringa oleifera role on Gut microbiota composition and inflammation associated with obesity following high fat diet feeding. Open Access Maced. J. Med Sci. 2018, 6, 1359. [Google Scholar] [CrossRef]
- Mughal, M.H.; Ali, G.; Srivastava, P.S.; Iqbal, M. Improvement of drumstick (Moringa pterygosperma Gaertn.)—A unique source of food and medicine through tissue culture. Hamdard. Med. 1999, 42, 37–42. [Google Scholar]
- Somali, M.A.; Bajneid, M.A.; Al-Fhaimani, S.S. Chemical composition and characteristics of Moringa peregrina seeds and seeds oil. J. Am. Oil Chem. Soc. 1984, 61, 85–86. [Google Scholar] [CrossRef]
- Morton, J.F. The horseradish tree, Moringa pterygosperma (Moringaceae)—A boon to arid lands? Econ. Bot. 1991, 45, 318–333. [Google Scholar] [CrossRef]
- Stohs, S.J.; Hartman, M.J. Review of the safety and efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Abdull Razis, A.F.; Ibrahim, M.D.; Kntayya, S.B. Health benefits of Moringa oleifera. Asian Pac. J. Cancer Prev. 2014, 15, 8571–8576. [Google Scholar] [CrossRef]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Promkum, C.; Kupradinun, P.; Tuntipopipat, S.; Butryee, C. Nutritive evaluation and effect of Moringa oleifera pod on clastogenic potential in the mouse. Asian Pac. J. Cancer Prev. APJCP 2010, 11, 627–632. [Google Scholar] [PubMed]
- Mendonça, L.A.B.M.; dos Santos Ferreira, R.; de Cássia Avellaneda Guimarães, R.; de Castro, A.P.; Franco, O.L.; Matias, R.; Carvalho, C.M.E. The complex puzzle of interactions among functional food, gut microbiota, and colorectal cancer. Front. Oncol. 2018, 8, 325. [Google Scholar] [CrossRef]
- Stidham, R.W.; Higgins, P.D.R. Translational research in colorectal cancer: Colorectal cancer in inflammatory bowel disease. Clin. Colon Rectal Surg. 2018, 31, 168. [Google Scholar] [PubMed]
- Velikonja, A.; Lipoglavšek, L.; Zorec, M.; Avguštin, G. Alterations in gut microbiota composition and metabolic parameters after dietary intervention with barley beta glucans in patients with high risk for metabolic syndrome development. Anaerobe 2019, 55, 67–77. [Google Scholar] [CrossRef]
- WHO. World Health Organization Malnutrition. Available online: https://www.who.int/news-room/fact-sheets/detail/malnutrition (accessed on 15 September 2020).
- Paley, C.A.; Johnson, M.I. Abdominal obesity and metabolic syndrome: Exercise as medicine? BMC Sports Sci. Med. Rehabil. 2018, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization. Obesidad y Sobrepeso. Available online: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 July 2020). (In Spanish).
- Gunness, P.; Gidley, M.J. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. 2010, 1, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.V.T.; Sievenpiper, J.L.; Zurbau, A.; Mejia, S.B.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The effect of oat β-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: A systematic review and meta-analysis of randomised-controlled trials. Br. J. Nutr. 2016, 116, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Jane, M.; McKay, J.; Pal, S. Effects of daily consumption of psyllium, oat bran and polyGlycopleX on obesity-related disease risk factors: A critical review. Nutrition 2019, 57, 84–91. [Google Scholar] [CrossRef]
- Marset, J.B.; Casas-Agustench, P.; Sánchez, N.B.; Salas-Salvadó, J. Knowledge, interest, predisposition and evaluation of functional foods in Spanish dietitians-nutritionists and experts in human nutrition and dietetics. Nutr. Hosp. 2012, 27, 632–644. [Google Scholar]
- Othman, R.A.; Moghadasian, M.H.; Jones, P.J.H. Cholesterol-lowering effects of oat β-glucan. Nutr. Rev. 2011, 69, 299–309. [Google Scholar] [CrossRef]
- Williams, P.G. The benefits of breakfast cereal consumption: A systematic review of the evidence base. Adv. Nutr. 2014, 5, 636S–673S. [Google Scholar] [CrossRef]
- Riccioni, G.; Sblendorio, V.; Gemello, E.; Di Bello, B.; Scotti, L.; Cusenza, S.; D’Orazio, N. Dietary fibers and cardiometabolic diseases. Int. J. Mol. Sci. 2012, 13, 1524–1540. [Google Scholar] [CrossRef]
- Sima, P.; VannuccI, L.; VetvicKa, V. β-glucans and cholesterol. Int. J. Mol. Med. 2018, 41, 1799–1808. [Google Scholar]
- Dias, C.B.; Moughan, P.J.; Wood, L.G.; Singh, H.; Garg, M.L. Postprandial lipemia: Factoring in lipemic response for ranking foods for their healthiness. Lipids Health Dis. 2017, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Misra, A.; Pandey, R.M. Effects of 3 g of soluble fiber from oats on lipid levels of Asian Indians-a randomized controlled, parallel arm study. Lipids Health Dis. 2017, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barroso, S.G.; das Dores, S.M.C.; Azeredo, V.B. Effects of functional foods consumption on the lipid profile and nutritional status of elderly. Int. J. Cardiovasc. Sci. 2015, 28, 400–408. [Google Scholar]
- El Rabey, H.A.; Al-Seeni, M.N.; Amer, H.M. Efficiency of barley bran and oat bran in ameliorating blood lipid profile and the adverse histological changes in hypercholesterolemic male rats. BioMed Res. Int. 2013, 2013, 263594. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, A.; Miguel, M. Dietary fiber and blood pressure control. Food Funct. 2016, 7, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Drozdowski, L.A.; Reimer, R.A.; Temelli, F.; Bell, R.C.; Vasanthan, T.; Thomson, A.B.R. β-Glucan extracts inhibit the in vitro intestinal uptake of long-chain fatty acids and cholesterol and down-regulate genes involved in lipogenesis and lipid transport in rats. J. Nutr. Biochem. 2010, 21, 695–701. [Google Scholar] [CrossRef]
- Hou, Q.; Li, Y.; Li, L.; Cheng, G.; Sun, X.; Li, S.; Tian, H. The metabolic effects of oats intake in patients with type 2 diabetes: A systematic review and meta-analysis. Nutrients 2015, 7, 10369–10387. [Google Scholar] [CrossRef]
- Li, Y.-H.; Niu, Y.-B.; Sun, Y.; Zhang, F.; Liu, C.-X.; Fan, L.; Mei, Q.-B. Role of phytochemicals in colorectal cancer prevention. World J. Gastroenterol. WJG 2015, 21, 9262. [Google Scholar] [CrossRef]
- Shen, X.L.; Zhao, T.; Zhou, Y.; Shi, X.; Zou, Y.; Zhao, G. Effect of oat β-glucan intake on glycaemic control and insulin sensitivity of diabetic patients: A meta-analysis of randomized controlled trials. Nutrients 2016, 8, 39. [Google Scholar] [CrossRef]
- Paschoal, V.; Naves, A.; da Fonseca, A. Nutrição Clínica Funcional: Suplementação Nutricional; VP Editora: São Paulo, Brazil, 2013. [Google Scholar]
- Machado, A.M.; de Paula, H.; Cardoso, L.D.; Costa, N.M.B. Effects of brown and golden flaxseed on the lipid profile, glycemia, inflammatory biomarkers, blood pressure and body composition in overweight adolescents. Nutrition 2015, 31, 90–96. [Google Scholar] [CrossRef]
- Haghighatsiar, N.; Askari, G.; Saraf-Bank, S.; Feizi, A.; Keshmiri, H. Effect of flaxseed powder on cardiovascular risk factor in dyslipidemic and hypertensive patients. Int. J. Prev. Med. 2019, 10, 218. [Google Scholar]
- Khan, S. Potential role of Escherichia coli DNA mismatch repair proteins in colon cancer. Crit. Rev. Oncol. Hematol. 2015, 96, 475–482. [Google Scholar] [CrossRef]
- Venkatachalam, S.; Ponnuswamy, T.K.; Bindu, G.; Devi, Y.; Arvind, E. Comparison of the hypoglycemic effect of flaxseed and metformin in streptozotocin induced diabetic rats. Eur. J. Pharm. Med Res. 2015, 2, 594–603. [Google Scholar]
- Ali, A.A.; Abd Al Haleem, E.N.; Khaleel, S.A.-H.; Sallam, A.S. Protective effect of cardamonin against acetic acid-induced ulcerative colitis in rats. Pharmacol. Rep. 2017, 69, 268–275. [Google Scholar] [CrossRef]
- Mohammadi-Sartang, M.; Sohrabi, Z.; Barati-Boldaji, R.; Raeisi-Dehkordi, H.; Mazloom, Z. Flaxseed supplementation on glucose control and insulin sensitivity: A systematic review and meta-analysis of 25 randomized, placebo-controlled trials. Nutr. Res. 2018, 76, 125–139. [Google Scholar] [CrossRef]
- Pizzini, A.; Lunger, L.; Demetz, E.; Hilbe, R.; Weiss, G.; Ebenbichler, C.; Tancevski, I. The role of omega-3 fatty acids in reverse cholesterol transport: A review. Nutrients 2017, 9, 1099. [Google Scholar] [CrossRef] [PubMed]
- Hasiewicz-Derkacz, K.; Kulma, A.; Czuj, T.; Prescha, A.; Żuk, M.; Grajzer, M.; Łukaszewicz, M.; Szopa, J. Natural phenolics greatly increase flax (Linum usitatissimum) oil stability. BMC Biotechnol. 2015, 15, 1–14. [Google Scholar] [CrossRef]
- Tavarini, S.; Castagna, A.; Conte, G.; Foschi, L.; Sanmartin, C.; Incrocci, L.; Ranieri, A.; Serra, A.; Angelini, L.G. Evaluation of chemical composition of two linseed varieties as sources of health-beneficial substances. Molecules 2019, 24, 3729. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Kong, Y.; Hu, X.; Li, K.; Guo, X.; Liu, C. Defatted flaxseed flour improves weight loss and lipid profile in overweight and obese adults: A randomized controlled trial. Food Funct. 2020, 11, 8237–8247. [Google Scholar] [CrossRef]
- Karamali, M.; Kashanian, M.; Alaeinasab, S.; Asemi, Z. The effect of dietary soy intake on weight loss, glycaemic control, lipid profiles and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: A randomised clinical trial. J. Hum. Nutr. Diet. 2018, 31, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Asemi, Z. The effect of soy intake on metabolic profiles of women with gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 2015, 100, 4654–4661. [Google Scholar] [CrossRef] [PubMed]
- McGraw, N.J.; Krul, E.S.; Grunz-Borgmann, E.; Parrish, A.R. Soy-based renoprotection. World J. Nephrol. 2016, 5, 233. [Google Scholar] [CrossRef]
- Wang, X.-L.; Chiang, T.-Y.; Roux, N.; Hao, G.; Ge, X.-J. Genetic diversity of wild banana (Musa balbisiana Colla) in China as revealed by AFLP markers. Genet. Resour. Crop Evol. 2007, 54, 1125–1132. [Google Scholar] [CrossRef]
- Kusunoki, M.; Sato, D.; Tsutsumi, K.; Tsutsui, H.; Nakamura, T.; Oshida, Y. Black soybean extract improves lipid profiles in fenofibrate-treated type 2 diabetics with postprandial hyperlipidemia. J. Med. Food 2015, 18, 615–618. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Zare, M.; Nouripour, F. Effect of soy and soy isoflavones on obesity-related anthropometric measures: A systematic review and meta-analysis of randomized controlled clinical trials. Adv. Nutr. 2017, 8, 705–717. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C.-W. Soybean bioactive peptides and their functional properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef]
- Mosallanezhad, Z.; Mahmoodi, M.; Ranjbar, S.; Hosseini, R.; Clark, C.C.T.; Carson-Chahhoud, K.; Norouzi, Z.; Abbasian, A.; Sohrabi, Z.; Jalali, M. Soy intake is associated with lowering blood pressure in adults: A systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Complement Ther. Med. 2021, 59, 102692. [Google Scholar] [CrossRef]
- Mejia, S.B.; Messina, M.; Li, S.S.; Viguiliouk, E.; Chiavaroli, L.; Khan, T.A.; Srichaikul, K.; Mirrahimi, A.; Sievenpiper, J.L.; Kris-Etherton, P. A meta-analysis of 46 studies identified by the FDA demonstrates that soy protein decreases circulating LDL and total cholesterol concentrations in adults. J. Nutr. 2019, 149, 968–981. [Google Scholar]
- Ramdath, D.D.; Padhi, E.M.T.; Sarfaraz, S.; Renwick, S.; Duncan, A.M. Beyond the cholesterol-lowering effect of soy protein: A review of the effects of dietary soy and its constituents on risk factors for cardiovascular disease. Nutrients 2017, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.S.; Kamili, A.; Wat, E.; Chung, R.W.; Tandy, S. Reduction in intestinal cholesterol absorption by various food components: Mechanisms and implications. Atheroscler. Suppl. 2010, 11, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zou, S.; Xu, H.; Li, M.; Tong, Z.; Xu, M.; Xu, X. Hypoglycemic activity of the Baker’s yeast β-glucan in obese/type 2 diabetic mice and the underlying mechanism. Mol. Nutr. Food Res. 2016, 60, 2678–2690. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Goyal, R.; Barwal, S. Domestic processing effects on physicochemical, nutritional and anti-nutritional attributes in soybean (Glycine max L. Merill). Int. Food Res. J. 2013, 20, 3203–3209. [Google Scholar]
- Soltanian, N.; Janghorbani, M. Effect of flaxseed or psyllium vs. placebo on management of constipation, weight, glycemia, and lipids: A randomized trial in constipated patients with type 2 diabetes. Clin. Nutr. ESPEN 2019, 29, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Tabassi, Z.; Reiner, Ž.; Panahandeh, I.; Naderi, F.; Aghadavod, E.; Amirani, E.; Taghizadeh, M.; Shafabakhsh, R.; Satari, M.; et al. The effects of n -3 fatty acids from flaxseed oil on genetic and metabolic profiles in patients with gestational diabetes mellitus: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2020, 123, 792–799. [Google Scholar] [CrossRef]
- Fernandes, Â.; Bancessi, A.; Pinela, J.; Dias, M.I.; Liberal, Â.; Calhelha, R.C.; Ćirić, A.; Soković, M.; Catarino, L.; Ferreira, I.C.F.R. Nutritional and phytochemical profiles and biological activities of Moringa oleifera Lam. edible parts from Guinea-Bissau (West Africa). Food Chem. 2021, 341, 128229. [Google Scholar] [CrossRef] [PubMed]
- Dixit, K.; Kamath, D.V.; Alluri, K.V.; Davis, B.A. Efficacy of a novel herbal formulation for weight loss demonstrated in a 16-week randomized, double-blind, placebo-controlled clinical trial with healthy overweight adults. Diabetes Obes. Metab. 2018, 20, 2633–2641. [Google Scholar] [CrossRef]
- Mbikay, M. Therapeutic potential of Moringa oleifera leaves in chronic hyperglycemia and dyslipidemia: A review. Front. Pharmacol. 2012, 3, 24. [Google Scholar] [CrossRef]
- Metwally, F.M.; Rashad, H.M.; Ahmed, H.H.; Mahmoud, A.A.; Abdol Raouf, E.R.; Abdalla, A.M. Molecular mechanisms of the anti-obesity potential effect of Moringa oleifera in the experimental model. Asian Pac. J. Trop. Biomed. 2017, 7, 214–221. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef]
- Jain, P.G.; Patil, S.D.; Haswani, N.G.; Girase, M.V.; Surana, S.J. Hypolipidemic activity of Moringa oleifera Lam., Moringaceae, on high fat diet induced hyperlipidemia in albino rats. Rev. Bras. Farmacogn. 2010, 20, 969–973. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Tiwari, P.; Sahu, P.K.; Kumar, S. A Review of the phytochemical and pharmacological characteristics of Moringa oleifera. J. Pharm. Bioallied Sci. 2018, 10, 181–191. [Google Scholar] [CrossRef]
- Abd Rani, N.Z.; Husain, K.; Kumolosasi, E. Moringa genus: A review of phytochemistry and pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef]
- Omodanisi, E.I.; Aboua, Y.G.; Oguntibeju, O.O. Assessment of the anti-hyperglycaemic, anti-inflammatory and antioxidant activities of the methanol extract of Moringa oleifera in diabetes-induced nephrotoxic male wistar rats. Molecules 2017, 22, 439. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, D.; Rai, P.K.; Mehta, S.; Chatterji, S.; Shukla, S.; Rai, D.K.; Sharma, G.; Sharma, B.; Watal, G. Role of Moringa oleifera in regulation of diabetes-induced oxidative stress. Asian Pac. J. Trop. Med. 2013, 6, 426–432. [Google Scholar] [CrossRef]
- Singh, R.; Devi, S.; Gollen, R. Role of free radical in atherosclerosis, diabetes and dyslipidaemia: Larger-than-life. Diabetes Metab. Res. Rev. 2015, 31, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-B.; Chen, G.-L.; Guo, M.-Q. Antioxidant and anti-inflammatory activities of the crude extracts of Moringa oleifera from Kenya and their correlations with flavonoids. Antioxidants 2019, 8, 296. [Google Scholar] [CrossRef]
- Aju, B.Y.; Rajalakshmi, R.; Mini, S. Protective role of Moringa oleifera leaf extract on cardiac antioxidant status and lipid peroxidation in streptozotocin induced diabetic rats. Heliyon 2019, 5, e02935. [Google Scholar] [CrossRef]
- Yamashita, Y.; Nakamura, A.; Nanba, F.; Saito, S.; Toda, T. Black soybean improves vascular function and blood pressure: A randomized, placebo controlled, crossover trial in humans. Nutrients 2020, 12, 2755. [Google Scholar] [CrossRef]
- Khan, M.I.; Anjum, F.M.; Sohaib, M.; Sameen, A. Tackling metabolic syndrome by functional foods. Rev. Endocr. Metab. Disord. 2013, 14, 287–297. [Google Scholar] [CrossRef]
- Irfan, H.M.; Khan, N.A.K.; Asmawi, M.Z. Moringa oleifera Lam. leaf extracts reverse metabolic syndrome in Sprague Dawley rats fed high-fructose high fat diet for 60-days. Arch. Physiol. Biochem. 2020, 15, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Li, Y.-Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. WJG 2014, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; De Silva, P.; Korzenik, J.R.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 2013, 145, 970–977. [Google Scholar] [CrossRef]
- Thies, F.; Masson, L.F.; Boffetta, P.; Kris-Etherton, P. Oats and bowel disease: A systematic literature review. Br. J. Nutr. 2014, 112, S31–S43. [Google Scholar] [CrossRef]
- Aaltonen, K.; Laurikka, P.; Huhtala, H.; Mäki, M.; Kaukinen, K.; Kurppa, K. The long-term consumption of oats in celiac disease patients is safe: A large cross-sectional study. Nutrients 2017, 9, 611. [Google Scholar] [CrossRef] [PubMed]
- Gilissen, L.J.W.J.; Van der Meer, I.M.; Smulders, M.J.M. Why oats are safe and healthy for celiac disease patients. Med. Sci. 2016, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Comino Montilla, I.M.; Moreno Amador, M.d.L.; Sousa Martín, C. Role of oats in celiac disease. World J. Gastroenterol. 2015, 21, 11825–11831. [Google Scholar] [CrossRef]
- Scaioli, E.; Liverani, E.; Belluzzi, A. The imbalance between n-6/n-3 polyunsaturated fatty acids and inflammatory bowel disease: A comprehensive review and future therapeutic perspectives. Int. J. Mol. Sci. 2017, 18, 2619. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 1–30. [Google Scholar]
- Palla, A.H.; Gilani, A.-U.-H.; Bashir, S.; Ur Rehman, N. Multiple mechanisms of flaxseed: Effectiveness in inflammatory bowel disease. Evid. Based Complement. Altern. Med. 2020, 2020, 7974835. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Cheng, D.; Huang, C.; Li, Y.; Lao, C.; Xia, Y.; Liu, W.; Gong, X.; Hu, D.; Li, B. Improvement of colonic immune function with soy isoflavones in high-fat diet-induced obese rats. Molecules 2019, 24, 1139. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Sang, L.-X.; Chang, B. Isoflavones and inflammatory bowel disease. World J. Clin. Cases 2020, 8, 2081. [Google Scholar] [CrossRef]
- Głąbska, D.; Guzek, D.; Grudzińska, D.; Lech, G. Influence of dietary isoflavone intake on gastrointestinal symptoms in ulcerative colitis individuals in remission. World J. Gastroenterol. 2017, 23, 5356. [Google Scholar] [CrossRef]
- Minaiyan, M.; Asghari, G.; Taheri, D.; Saeidi, M.; Nasr-Esfahani, S. Anti-inflammatory effect of Moringa oleifera Lam. seeds on acetic acid-induced acute colitis in rats. Avicenna J. Phytomed. 2014, 4, 127–136. [Google Scholar]
- Jaja-Chimedza, A.; Graf, B.L.; Simmler, C.; Kim, Y.; Kuhn, P.; Pauli, G.F.; Raskin, I. Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. PLoS ONE 2017, 12, e0182658. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Carreiro, D.M. O Ecossistema Intestinal na Saúde e na Doença; Ed. Metha: São Paulo, Brazil, 2016. (In Portuguese) [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Ko, K.-P.; Yeo, Y.; Yoon, J.-H.; Kim, C.-S.; Tokudome, S.; Koriyama, C.; Lim, Y.-K.; Chang, S.-H.; Shin, H.-R.; Kang, D. Plasma phytoestrogens concentration and risk of colorectal cancer in two different Asian populations. Clin. Nutr. 2018, 37, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Núñez, M.L.; Loarca-Piña, G.; Berhow, M.; Gonzalez de Mejia, E. Glucosinolate-rich hydrolyzed extract from Moringa oleifera leaves decreased the production of TNF-α and IL-1β cytokines and induced ROS and apoptosis in human colon cancer cells. J. Funct. Foods 2020, 75, 104270. [Google Scholar] [CrossRef]
- Sturtzel, B.; Dietrich, A.; Wagner, K.H.; Gisinger, C.; Elmadfa, I. The status of vitamins B6, B12, folate, and of homocysteine in geriatric home residents receiving laxatives or dietary fiber. J. Nutr. Health Aging 2010, 14, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Tsikritzi, R.; Moynihan, P.J.; Gosney, M.A.; Allen, V.J.; Methven, L. The effect of macro-and micro-nutrient fortification of biscuits on their sensory properties and on hedonic liking of older people. J. Sci. Food Agric. 2014, 94, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [PubMed]
- Feres, N.H.; de Lima Reis, S.R.; Veloso, R.V.; Arantes, V.C.; Souza, L.M.I.; Carneiro, E.M.; Boschero, A.C.; Reis, M.A.B.; Latorraca, M.Q. Soybean diet alters the insulin-signaling pathway in the liver of rats recovering from early-life malnutrition. Nutrition 2010, 26, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ronoh, A.K.; Were, G.M.; Wakhu-Wamunga, F.; Wamunga, J.B. Effect of soybean fortified porridges on the nutritional status of pre-school children 3–5 years old from Western Kenya. J. Food Nutr. Sci. 2017, 5, 155. [Google Scholar]
- Akomo, P.; Bahwere, P.; Murakami, H.; Banda, C.; Maganga, E.; Kathumba, S.; Sadler, K.; Collins, S. Soya, maize and sorghum ready-to-use therapeutic foods are more effective in correcting anaemia and iron deficiency than the standard ready-to-use therapeutic food: Randomized controlled trial. BMC Public Health 2019, 19, 806. [Google Scholar] [CrossRef]
- Cruz, R.J.; Uy, E.; De Leon, M.N. Randomized controlled trial on the effect of 10 grams Moringa oleifera powder leaves on the level of hemoglobin and hematocrit on infants age 6–9 months. Eur. J. Pediatrics 2016, 175, 1647–1648. [Google Scholar]
- Amaglo, N.K.; Bennett, R.N.; Curto, R.B.L.; Rosa, E.A.S.; Turco, V.L.; Giuffrida, A.; Curto, A.L.; Crea, F.; Timpo, G.M. Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringa oleifera L., grown in Ghana. Food Chem. 2010, 122, 1047–1054. [Google Scholar] [CrossRef]
- Glover-Amengor, M.; Aryeetey, R.; Afari, E.; Nyarko, A. Micronutrient composition and acceptability of Moringa oleifera leaf-fortified dishes by children in Ada-East district, Ghana. Food Sci. Nutr. 2017, 5, 317–323. [Google Scholar] [CrossRef]
- Natsir, H.; Wahab, A.W.; Budi, P.; Dali, S.; Arif, A.R. Amino acid and mineral composition of Moringa oleivera leaves extract and its bioactivity as antioxidant. J. Phys. Conf. Ser. 2019, 1317, 012030. [Google Scholar] [CrossRef]
- Alain Mune Mune, M.; Nyobe, E.C.; Bakwo Bassogog, C.; Minka, S.R. A comparison on the nutritional quality of proteins from Moringa oleifera leaves and seeds. Cogent Food Agric. 2016, 2, 1213618. [Google Scholar] [CrossRef]
- Kou, X.; Li, B.; Olayanju, J.B.; Drake, J.M.; Chen, N. Nutraceutical or pharmacological potential of Moringa oleifera Lam. Nutrients 2018, 10, 343. [Google Scholar] [CrossRef]
- Tshingani, K.; Donnen, P.; Mukumbi, H.; Duez, P.; Dramaix-Wilmet, M. Impact of Moringa oleifera Lam. Leaf powder supplementation versus nutritional counseling on the body mass index and immune response of HIV patients on antiretroviral therapy: A single-blind randomized control trial. BMC Complement. Altern. Med. 2017, 17, 1–13. [Google Scholar] [CrossRef]
- Barichella, M.; Pezzoli, G.; Faierman, S.A.; Raspini, B.; Rimoldi, M.; Cassani, E.; Bertoli, S.; Battezzati, A.; Leone, A.; Iorio, L. Nutritional characterisation of Zambian Moringa oleifera: Acceptability and safety of short-term daily supplementation in a group of malnourished girls. Int. J. Food Sci. Nutr. 2019, 70, 107–115. [Google Scholar] [CrossRef]
- Sultana, S. Nutritional and functional properties of Moringa oleifera. Metabolism Open 2020, 8, 100061. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Ling, K.H.; El-Nezami, H.; Wang, M.F. Influence of functional food components on gut health. Crit. Rev. Food Sci. Nutr. 2019, 59, 1927–1936. [Google Scholar] [CrossRef]
- Greiner, T.; Bäckhed, F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol. Metab. 2011, 22, 117–123. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Consortium, M. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Novelo, L.L.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; Hyöty, H.; et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Brown, J.M.; Hazen, S.L. The gut microbial endocrine organ: Bacterially derived signals driving cardiometabolic diseases. Annu. Rev. Med. 2015, 66, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Carroll, I.M.; Ringel-Kulka, T.; Keku, T.O.; Chang, Y.-H.; Packey, C.D.; Sartor, R.B.; Ringel, Y. Molecular analysis of the luminal-and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G799–G807. [Google Scholar] [CrossRef]
- Palm, N.W.; De Zoete, M.R.; Cullen, T.W.; Barry, N.A.; Stefanowski, J.; Hao, L.; Degnan, P.H.; Hu, J.; Peter, I.; Zhang, W. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 2014, 158, 1000–1010. [Google Scholar] [CrossRef]
- Saulnier, D.M.; Riehle, K.; Mistretta, T.A.; Diaz, M.A.; Mandal, D.; Raza, S.; Weidler, E.M.; Qin, X.; Coarfa, C.; Milosavljevic, A.; et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1782–1791. [Google Scholar] [CrossRef]
- Sobhani, I.; Tap, J.; Roudot-Thoraval, F.; Roperch, J.P.; Letulle, S.; Langella, P.; Corthier, G.; Van Nhieu, J.T.; Furet, J.P. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS ONE 2011, 6, e16393. [Google Scholar] [CrossRef]
- Zackular, J.P.; Rogers, M.A.; Ruffin, M.T.t.; Schloss, P.D. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev. Res. 2014, 7, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Huq, S.; Yatsunenko, T.; Haque, R.; Mahfuz, M.; Alam, M.A.; Benezra, A.; DeStefano, J.; Meier, M.F.; Muegge, B.D. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 2014, 510, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tremaroli, V.; Bäckhed, F. Linking microbiota to human diseases: A systems biology perspective. Trends Endocrinol. Metab. 2015, 26, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Meijnikman, A.S.; Gerdes, V.E.; Nieuwdorp, M.; Herrema, H. Evaluating causality of gut microbiota in obesity and diabetes in humans. Endocr. Rev. 2018, 39, 133–153. [Google Scholar] [CrossRef]
- Clemens, R.; van Klinken, B.J.-W. The future of oats in the food and health continuum. Br. J. Nutr. 2014, 112, S75–S79. [Google Scholar] [CrossRef] [PubMed]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary flaxseed as a strategy for improving human health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Rose, D.J. Impact of whole grains on the gut microbiota: The next frontier for oats? Br. J. Nutr. 2014, 112, S44–S49. [Google Scholar] [CrossRef]
- Clemens, R.; van Klinken, B.J.-W. Oats, more than just a whole grain: An introduction. Br. J. Nutr. 2014, 112, S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Martoni, C.J.; Prakash, S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: A randomized controlled trial. Eur. J. Clin. Nutr. 2012, 66, 1234–1241. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Eichholzer, M.; Richard, A.; Nicastro, H.L.; Platz, E.A.; Linseisen, J.; Rohrmann, S. Urinary lignans and inflammatory markers in the US National Health and Nutrition Examination Survey (NHANES) 1999–2004 and 2005–2008. Cancer Causes Control 2014, 25, 395–403. [Google Scholar] [CrossRef]
- Senizza, A.; Rocchetti, G.; Mosele, J.I.; Patrone, V.; Callegari, M.L.; Morelli, L.; Lucini, L. Lignans and gut microbiota: An interplay revealing potential health implications. Molecules 2020, 25, 5709. [Google Scholar] [CrossRef]
- Lemos, J.R.N.; de Alencastro, M.G.; Konrath, A.V.; Cargnin, M.; Manfro, R.C. Flaxseed oil supplementation decreases C-reactive protein levels in chronic hemodialysis patients. Nutr. Res. 2012, 32, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Gaya, P.; Medina, M.; Sánchez-Jiménez, A.; Landete, J.M. Phytoestrogen metabolism by adult human gut microbiota. Molecules 2016, 21, 1034. [Google Scholar] [CrossRef]
- Kezimana, P.; Dmitriev, A.A.; Kudryavtseva, A.V.; Romanova, E.V.; Melnikova, N.V. Secoisolariciresinol diglucoside of flaxseed and its metabolites: Biosynthesis and potential for nutraceuticals. Front. Genet. 2018, 9, 641. [Google Scholar] [CrossRef]
- Quartieri, A.; García-Villalba, R.; Amaretti, A.; Raimondi, S.; Leonardi, A.; Rossi, M.; Tomàs-Barberàn, F. Detection of novel metabolites of flaxseed lignans in vitro and in vivo. Mol. Nutr. Food Res. 2016, 60, 1590–1601. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Ma, X.-Q.; Yang, D.-H.; Guo, Z.-R.; Liu, G.-R.; Zhao, G.-X.; Tang, J.; Zhang, Y.-N.; Ma, M.; Cai, S.-Q. Production of enterodiol from defatted flaxseeds through biotransformation by human intestinal bacteria. BMC Microbiol. 2010, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lagkouvardos, I.; Kläring, K.; Heinzmann, S.S.; Platz, S.; Scholz, B.; Engel, K.H.; Schmitt-Kopplin, P.; Haller, D.; Rohn, S.; Skurk, T. Gut metabolites and bacterial community networks during a pilot intervention study with flaxseeds in healthy adult men. Molecular Nutr. Food Res. 2015, 59, 1614–1628. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, O.; Fuentealba, C.; Ampuero, D.; Figuerola, F.; Estévez, A.M. The effect of Lactobacillus acidophilus and Lactobacillus casei on the in vitro bioaccessibility of flaxseed lignans (Linum usitatissimum L.). Food Funct. 2018, 9, 2426–2432. [Google Scholar] [CrossRef]
- Inoguchi, S.; Ohashi, Y.; Narai-Kanayama, A.; Aso, K.; Nakagaki, T.; Fujisawa, T. Effects of non-fermented and fermented soybean milk intake on faecal microbiota and faecal metabolites in humans. Int. J. Food Sci. Nutr. 2012, 63, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lu, X.; Yang, X. Stachyose-enriched α-galacto-oligosaccharides regulate gut microbiota and relieve constipation in mice. J. Agric. Food Chem. 2013, 61, 11825–11831. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Kuda, T.; Yazaki, T.; Takahashi, H.; Kimura, B. Caecal fermentation, putrefaction and microbiotas in rats fed milk casein, soy protein or fish meal. Appl. Microbiol. Biotechnol. 2014, 98, 2779–2787. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Dersjant-Li, Y.; Peisker, M. The impact of soy oligosaccharides on digestion and intestinal health in weaning piglets. Livest. Sci. 2010, 134, 187–189. [Google Scholar] [CrossRef]
- Miao, S.; Zhao, C.; Zhu, J.; Hu, J.; Dong, X.; Sun, L. Dietary soybean meal affects intestinal homoeostasis by altering the microbiota, morphology and inflammatory cytokine gene expression in northern snakehead. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Umu, Ö.C.O.; Mydland, L.T.; Øverland, M.; Press, C.M.; Sørum, H. Rapeseed-based diet modulates the imputed functions of gut microbiome in growing-finishing pigs. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Boué, S.; Fortgang, I.; Levy, R.J., Jr.; Bhatnagar, D.; Burow, M.; Fahey, G.; Heiman, M.L. A novel gastrointestinal microbiome modulator from soy pods reduces absorption of dietary fat in mice. Obesity 2016, 24, 87–95. [Google Scholar] [CrossRef] [PubMed]
| Physiological and Metabolic Effects | Population/Treatment Time/Applied Dose | References |
|---|---|---|
| Oat | ||
| β-glucans reduced mean LDL-C and TC levels | 83 participants, 8 weeks, boxes containing 28 sachets, 3 g β-glucans | [21] |
| Reduced LDL and TC levels | 28 RCTs, ≥3 g/day, food products, ≥2 weeks | [22] |
| Reduced glucose, TC, triglycerides and BMI and waist-hip ratio | 30 participants with MS, 37.26 years/8 weeks/15 g/day fiber intake oat bran | [23] |
| Improved nutritional status | 1406 children between 6 and 59 months of age with uncomplicated SAM/12 weeks/30 g oat-RUTF | [24] |
| Flaxseed | ||
| GF and FO attenuated systemic inflammation | 75 patients with UC, GF (30 g/day) and FO (10 g/day), 12 weeks | [25] |
| Patients with mild to moderately severe UC | 18 years and over, phase 2, FLC, 300 mg FLC, 12 week | NCT02201758 |
| Effective in amelioration of some symptoms of MS and decrease BP and lipid peroxidation | 60 participants/aged 30 to 60 years/ 25 mL/d FO/7 week | [26] |
| Soya | ||
| Add genistein FOLFOX or FOLFOX-Bevacizumab. Efficacy results are notable | 13 participants metastatic colorectal cancer, 7 days, 2 weeks | [27] |
| Reduced risk for overall colorectal cancer | 901 participants with colorectal cancer, 2669 participants as control, high intake of total soy products or dietary isoflavones | [28] |
| Added genistein to the regimens of FOLFOX or FOLFOX-Avastin | Participants with metastatic (stage IV) colorectal cancer, Phase 2 | NCT01985763 |
| Soybean fortified meal improved the nutritional status of the children malnutrition | 1546 children aged 6–23 months/1 month of supplementation/3 meals per day with 40g CSB per meal | [29] |
| Nutrition in the management of HIV/AIDS. Higher total blood protein, blood white protein, level of blood hemoglobin and higher CD4 cell count | 47 PLWHAs/Nutritional and intervention Education Program/100 g soybean/day | [30] |
| Moringa oleifera | ||
| Decreased postprandial glucose response. M. oleifera leaf powder could be a hypoglycemic herbal drug | 17 Saharawi diabetics and 10 healthy subjects, 20 g of M. oleifera leaf powder | [20] |
| M. oleifera infusion effect in hot water twice daily on the blood glucose plasma lipids level and blood anti-oxidant status. Comparison of the lipid profiles of both healthy and hyperglycemic participants | RCS, 18–65 years (adult, older adult), 103 participants—M. oleifera leaves infused | NCT04314258 |
| Investigation of the effects of M. oleifera supplementation on the levels of inflammatory markers, specifically the hsCRP, hgbA1c level and clinical outcome in diabetic patients through a cohort study | 56 participants, supplementation of M. oleifera | NCT02308683 |
| Potential lowering effect on both SBP and DBP, postprandial follow-up | 41 healthy participants, 120 g of cooked M. oleifera leaves | [31] |
| Glycemic control and no adverse effects in T2DM. Tendency on blood pressure reduction in T2DM | Therapy-naïve T2DM with the duration of diabetes of less than 5 years, 20–70 years, 8 g, day, 40 days of M. oleifera leaf capsules | [32] |
| Increased insulin secretion, potential agent in the treatment of type 2 diabetes | 10 healthy subjects, 24–34 years, 4 g capsules M. oleifera leaf powder | [33] |
| Antipyretic effects | A CS, an 18-month-old girl, 40 mL of warm water extract of 5 g | [34] |
| Investigated weight gain and hemoglobin and vitamin A status | Adolescent girls, 150 gm of Sajna shak/bora (Moringa) 5 days/week/6 months | NCT04156321 |
| Reduction in anemia cases | 95 anemic children/200 g/m/M. oleifera leaf powder/6 months | [35] |
| M. oleifera improved parameters associated with obese-DM2 | 24 obese DM2/17 women and 7 men, 20–60y/22w M. oleifera leaves | [36] |
| To evaluate the in vivo bioavailability of key nutrients and bioactives and biological activities of the leaves, malnourishment prevention | 10 participants/18 Years and older (Adult, Older Adult)/Healthy males or females/corn Moringa Diet | NCT04092517 |
| Physiological and Metabolic Effects | Population/Treatment Time/Applied Dose | References |
|---|---|---|
| Oat | ||
| Hypolipidemic | Wistar–Lewis male rats—30 days/oat flake powders: dose of 5 g kg−1 body weight per day. β-glucan extracted and purified dose of 0.3 g kg−1 body weight per day | [37] |
| Improved values of antioxidative potential markers; positive effect on the colon tissue of healthy rats with LPS-induced enteritis | 72 old male Sprague–Dawley rats: Male Sprague—Dawley rats/6 weeks/84.0% Low molecular weight oat Beta-glucan | [38] |
| Flaxseed | ||
| Reducing goblet cell depletion, scavenging oxygen-derived free radicals, reduce neutrophil infiltration that may be attributed due to decreasing IFN-γ and TNF-α and increasing IL-17 levels | BALB/c mice induced colitis/6 days/150, 300 and 500 mg/kg/day | [39] |
| Antihyperglycemic effect mediated through inhibition of ROS | Male Wistar rats/21 days/200 and 400 mg/kg/EELU | [40] |
| Improved glucose utilization; increased glucose-6-phosphate dehydrogenase; reduction of PPHG | Male Swiss mice/21 days/2, 1 mg flaxseed powder | [41] |
| Alters the baseline colonic microenvironment of healthy mice, which may modify subsequent mucosal microbial defense and injury-repair responses leading to altered susceptibility to different gut-associated diseases | C57Bl/6 male mice/3 weeks/10% flaxseed | [42] |
| Soya | ||
| Decreased body weight and the plasma TG and LDL concentrations. Decreased in activity of mTORC1. Suppressed lipogenesis and adipogenesis, potential mechanism of soy isoflavones regulating lipid homeostasis. | 64 Male rats/4 weeks/Basal diets + 50 mg/kg; 150 mg/kg; 400 mg/kg doses of soy isoflavones | [43] |
| Reduced the body weight gain and related biomarkers. Fat deposits, dyslipidemia, hyperglycemia and fatty liver were ameliorated by dietary genistein. | Male C57BL/6J mice/(n = 15, 16 weeks) 0.25% genistein (Study 1) and (n = 75, 18 weeks) 0.2% and 0.067% (Study 2) dose-response effect of genistein | [44] |
| Altered the microbial composition and modulated the metabolic pathway of the microbial metabolism in the colon. Serum levels of IgG and IgM were significantly increased in FF group pigs (p < 0.05). FF significantly decreased the abundances of Bacteroides and Verrucomicrobia in the duodenum and decreased the abundances of Bacteroides, Proteobacteria and Verrucomicrobiain in the colon and significantly increased the abundances of Firmicutes and Actinobacteria (p < 0.05). Serum immunity and expression of genes related to gut immunity were associated with bacterial strains at the family level | 48 growing barrow pigs/2 feeding groups (n = 24 each, UF and FF) | [45] |
| Moringa oleifera | ||
| Antioxidant, hypolipidemic and antiatherosclerotic activities, (p < 0.05) lowered the cholesterol levels and reduced the atherosclerotic plaque formation to about 50% and 86%, respectively | Rabbit/12 weeks/M. oleifera leaf extract | [46] |
| Anti-cancer activity/MDA-MB-231 and HCT-8 cancer cell lines | In vitro/250 mg of extracts were dissolved in 1.0 mL of ethanol | [47] |
| Prevention of cognitive damage due to chronic hyperglycemia and oxidative stress | 88 Wistar rats/14 days/2 e 4% de ML/MS. | [48] |
| Moringa leaf extract reversed hepatic insulin insensitivity, up-regulation of genes involved in insulin receptors and glucose uptake in the liver | 10 hyperinsulinemic male rats/4 weeks/300 mg aqueous extract of M. oleifera leaves/kg body weight. | [49] |
| Reduction in blood glucose and HbA1c levels and an elevation in serum insulin and hepatic glycogen levels. | Wistar rats/60 days/70% M. oleifera leaf extract (100, 250 and 500 mg/kg b.wt./day) | [50] |
| Regulation of weight gain and inflammation associated with high-fat-induced-obesity through gut bacteria modulation. | 45 Swiss albino mice/3 months/(200 mg/Kg M. oleifera leaf extract | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, R.d.S.; Mendonça, L.A.B.M.; Santos, C.d.; Hiane, P.A.; Matias, R.; Franco, O.L.; de Oliveira, A.K.M.; do Nascimento, V.A.; Pott, A.; Carvalho, C.M.E.; et al. Do Bioactive Food Compound with Avena sativa L., Linum usitatissimum L. and Glycine max L. Supplementation with Moringa oleifera Lam. Have a Role against Nutritional Disorders? An Overview of the In Vitro and In Vivo Evidence. Nutrients 2021, 13, 2294. https://doi.org/10.3390/nu13072294
Ferreira RdS, Mendonça LABM, Santos Cd, Hiane PA, Matias R, Franco OL, de Oliveira AKM, do Nascimento VA, Pott A, Carvalho CME, et al. Do Bioactive Food Compound with Avena sativa L., Linum usitatissimum L. and Glycine max L. Supplementation with Moringa oleifera Lam. Have a Role against Nutritional Disorders? An Overview of the In Vitro and In Vivo Evidence. Nutrients. 2021; 13(7):2294. https://doi.org/10.3390/nu13072294
Chicago/Turabian StyleFerreira, Rosângela dos Santos, Lígia Aurélio Bezerra Maranhão Mendonça, Cristiane dos Santos, Priscila Aiko Hiane, Rosemary Matias, Octávio Luiz Franco, Ademir Kleber Morbeck de Oliveira, Valter Aragão do Nascimento, Arnildo Pott, Cristiano Marcelo Espinola Carvalho, and et al. 2021. "Do Bioactive Food Compound with Avena sativa L., Linum usitatissimum L. and Glycine max L. Supplementation with Moringa oleifera Lam. Have a Role against Nutritional Disorders? An Overview of the In Vitro and In Vivo Evidence" Nutrients 13, no. 7: 2294. https://doi.org/10.3390/nu13072294
APA StyleFerreira, R. d. S., Mendonça, L. A. B. M., Santos, C. d., Hiane, P. A., Matias, R., Franco, O. L., de Oliveira, A. K. M., do Nascimento, V. A., Pott, A., Carvalho, C. M. E., & Guimarães, R. d. C. A. (2021). Do Bioactive Food Compound with Avena sativa L., Linum usitatissimum L. and Glycine max L. Supplementation with Moringa oleifera Lam. Have a Role against Nutritional Disorders? An Overview of the In Vitro and In Vivo Evidence. Nutrients, 13(7), 2294. https://doi.org/10.3390/nu13072294








